Clinical biochemistry second stage lecture 5 Dr.Thana Alsewedy
1
BIOSYNTHESIS OF NONESSENTIAL AMINO ACID
Nonessential amino acids are synthesized from intermediates of
metabolism or, as in the case of tyrosine and cysteine, from essential
amino acids. Two amino acids—histidine and arginine—are generally
classified as nonessential. However, their normal concentrations are
limiitin, and, during periods of tissue growth (for example, in children or
in individuals recovering from wasting diseases), histidine and arginine
need to be supplemented in the diet. [Note: Some amino acids found in
proteins, such as hydroxyproline and hydroxylysine are modified after
their incorporation into the protein .
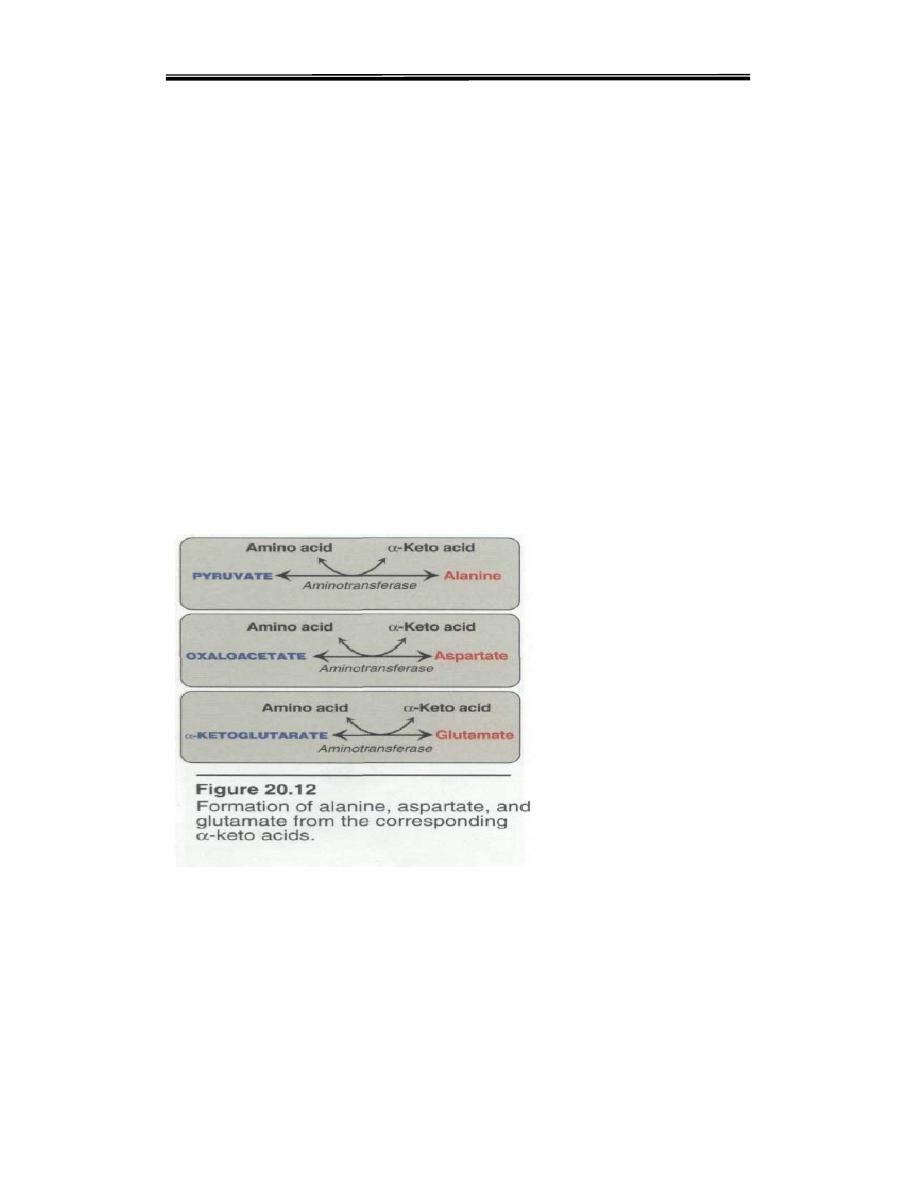
A. Synthesis from α-keto acids. Alanine, aspartate, and
glutamate are
synthesized by transfer of an amino group to the α-keto acids, pyruvate,
oxaloacetate, and α-keto-glutarate, respectively. These transamination
reactions are the most direct of the biosynthetic pathways. Glutamate is
unusual in that it can also be synthesized by the reverse of oxidative
deamination
,
catalyzed by glutamate dehydrogenase
Pyridoxal Phosphate: In a transamination reaction, i.e. reactions
involving the transfer of the α -amino group of an amino acid to the α -
carbon of a keto acid, thereby forming a new amino acid and a new keto
acid. Pyridoxal phosphate (vitamin B6) acts as an intermediate carrier of
the amino group that is being transferred.

Clinical biochemistry second stage lecture 5 Dr.Thana Alsewedy
2
B Synthesis by amidation
1.Glutamine:.
This amino acid, which contains an amide linkage with ammonia at the γ-
carboxyl, is formed from glutamate by glutamine synthetasThe reaction is
driven by the hydrolysis of ATP. In addition to producing glutamine for
protein synthesis, the reaction also serves as a major mechanism for the
detoxification of ammonia in brain and liver
2. Asparagine: This amino acid, which contains an amide linkage with
ammonia at the β-carboxyl, is formed from aspartate by asparagine
synthetase, using glutamine
as the amide donor. The reaction requires ATP, and, like the synthesis of
glutamine, has an equilibrium far in the direction of asparagine synthesis.
C. Proline
Glutamate is converted to proline by cyclization and reduction reaction
D. Serine, glycine, and cysteine
1. Serine arises from 3-phosphoglycerate an intermediate in glycolysis,
which is first oxidized to phosphopyruvate, and then transaminated to 3-
phosphoserine. Serine is formed by hydrolysis of the phosphate ester. Serine
can also be formed from glycine through transfer of hydroxymethyl group
Clinical biochemistry second stage lecture 5 Dr.Thana Alsewedy
3
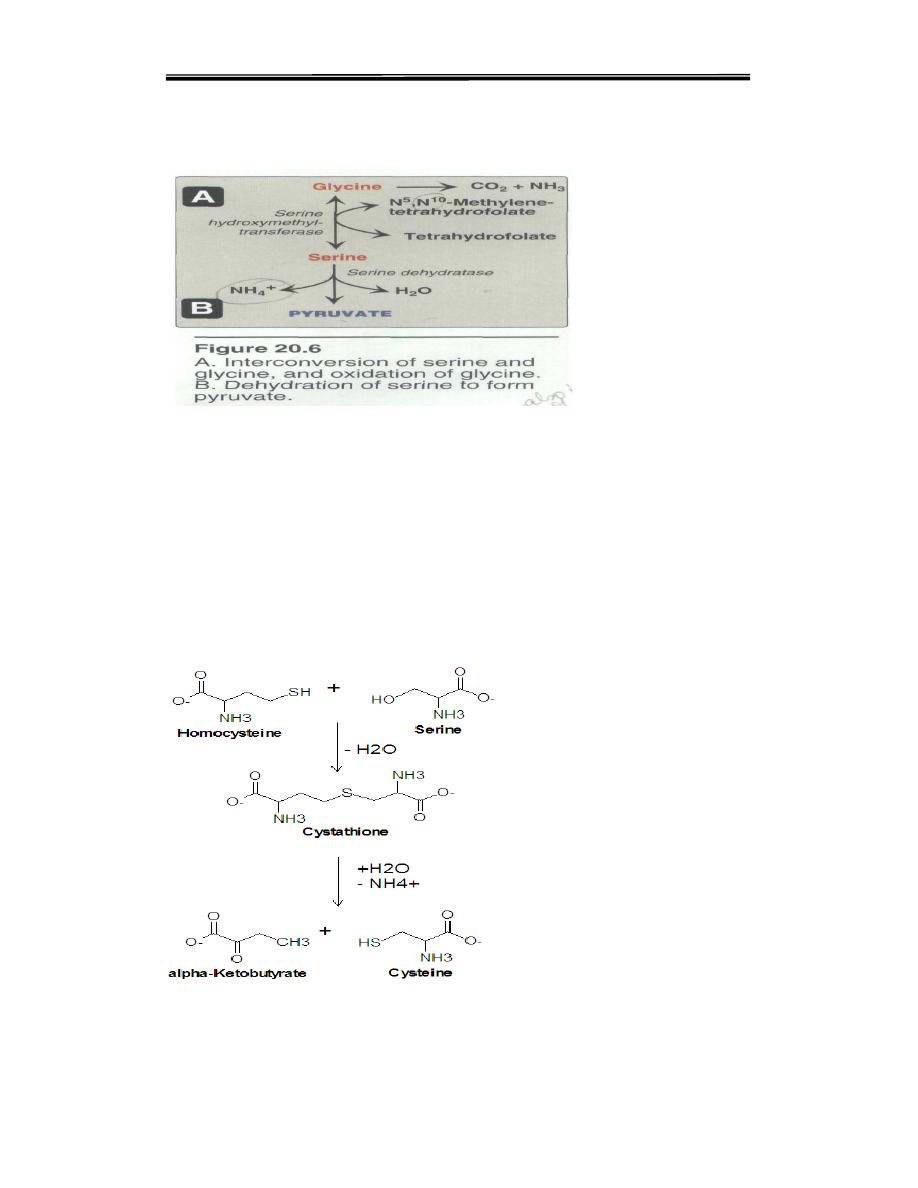
2. Glycine is synthesized from serine by removal of a hydroxy-methyl
group, also by serine hydroxymethyl transferase (see Figure 20.6A).
3. Cysteine is synthesized by two consecutive reactions in which
homocysteine combines with serine, forming cystathionine, which, in
turn, is hydrolyzed to α-ketobutyrate and cysteine (see Figure 20.8).
Homocysteine is derived from methionine. Because methionine is an
essential amino acid, cysteine synthesis can be sustained only if the
dietary intake of methionine is adequate
Clinical biochemistry second stage lecture 5 Dr.Thana Alsewedy
4
E. Tyrosine
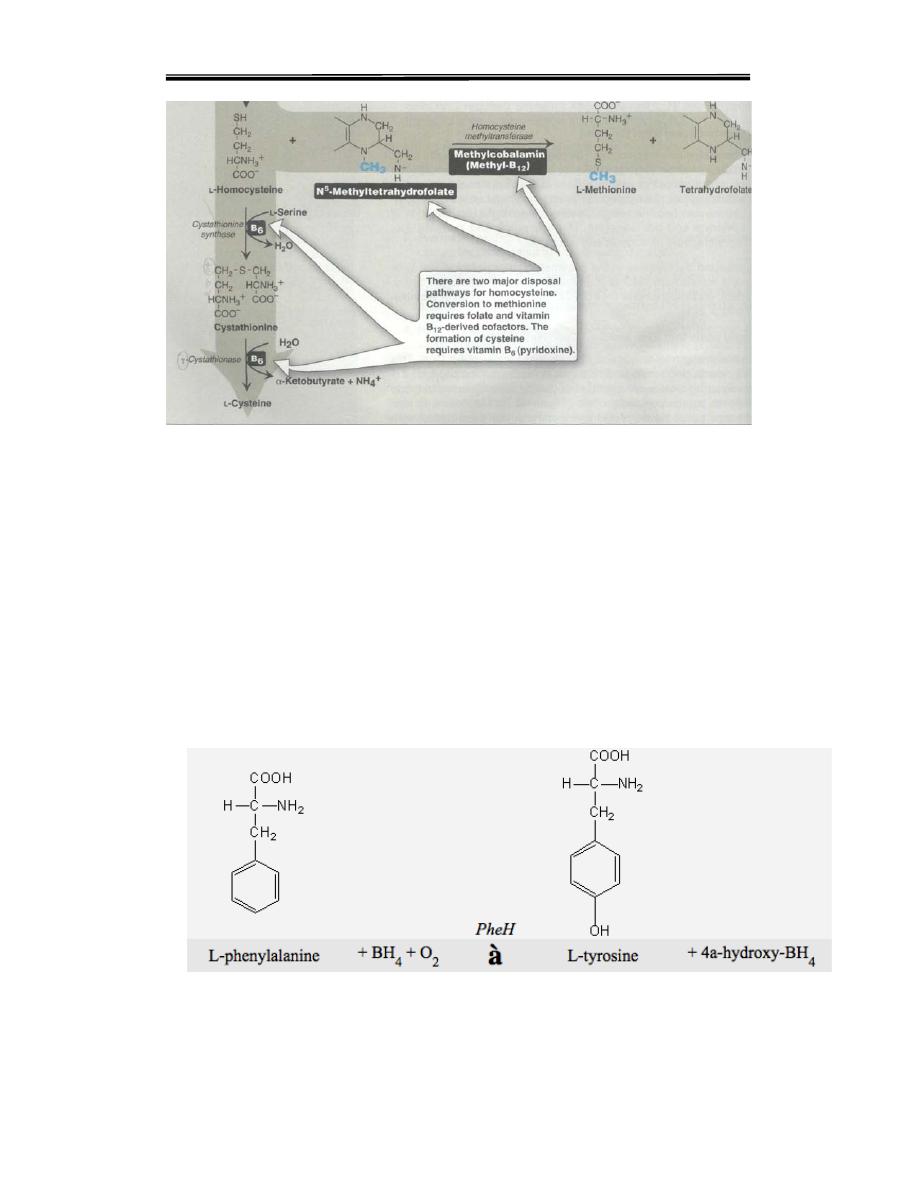
Tyrosine is formed from phenylalanine by phenylalanine hydroxylase.
The reaction requires molecular oxygen and the coenzyme
tetrahydrobiopterin,. One atom of molecular oxygen becomes the
hydroxyl group of tyrosine, and the other atom is reduced to water.
During the reaction, tetrahydrobiopterin is oxidized to dihydrobiopterin.
Tetrahydrobiopterin is regenerated from dihydrobiopterin in a separate
reaction requiring NADPH. Tyrosine, like cysteine, is formed from an
essential amino acid and, is therefore, nonessential only in the presence of
adequate dietary phenylalanine
.
