Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
1
Digestion and Absorption of Proteins
Proteins are large polypeptide molecules coiled by bonds in their tertiary
structure,
Proteins are most important constituent of cell membranes and
cytoplasm. Muscle and blood plasma also contain certain specific
proteins. protein is the most important biological molecules in building
up and maintenances of the structure of body, giving as much energy as
carbohydrates in the course of
metabolism in the body
. the digestion of
proteins involves the gradual breakdown of this polypeptide by enzymatic
hydrolysis in to amino acid molecules which are absorbed in the blood
stream.
The protein load received by the gut is derived from two sources 70-100g
dietary protein which is required daily and 35 -200g endogenous protein
(secreted enzymes and proteins in the gut or from intestinal epithelia cell
turnover)Only 1-2g of nitrogen equivalent to 6-12g of proteins are lost in
the feces on a daily basis.
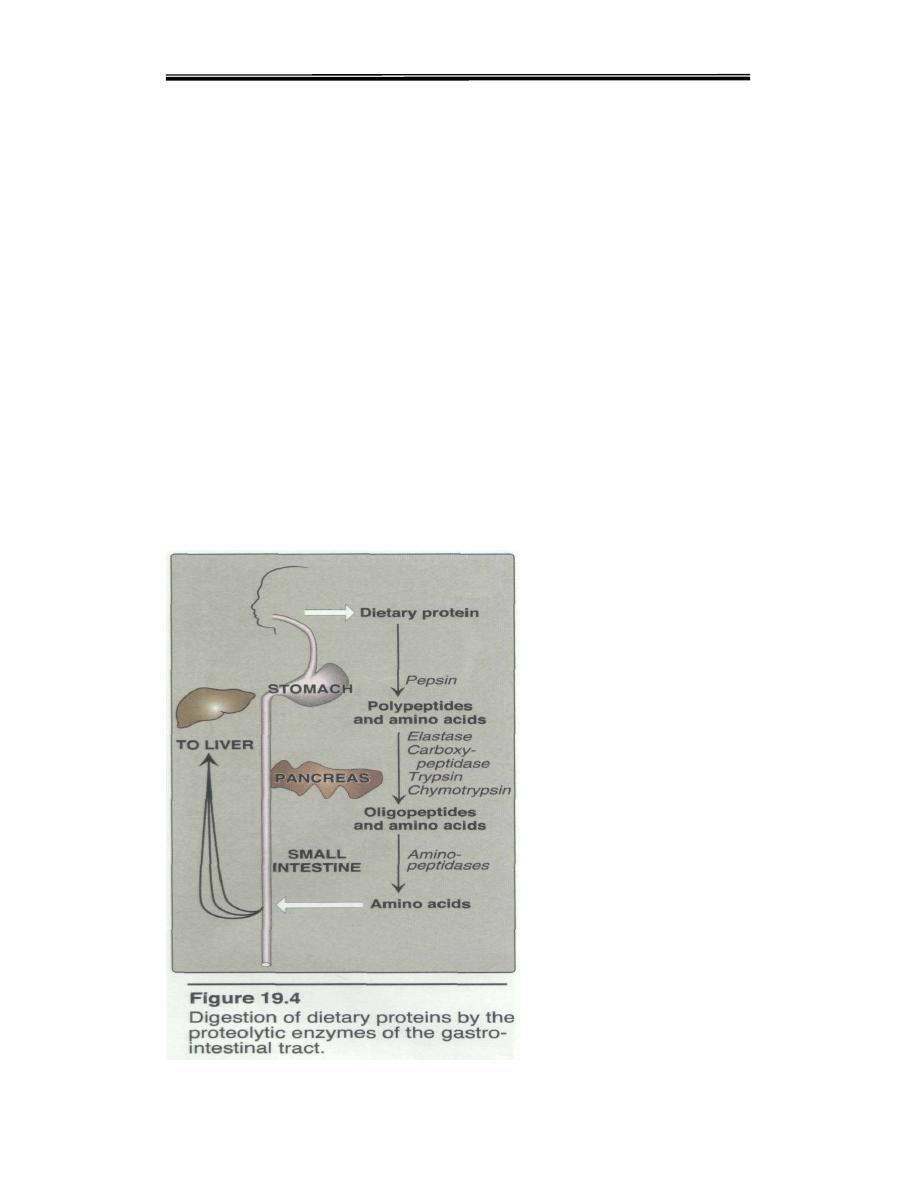
Proteolytic enzymes responsible for degrading proteins are produced by
three different organs: the stomach, the pancreas, and the small intestine
(Figure 19.4).

Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
2
The process of protein digestion can be divided, depending on the sources
of peptidases.
A. Gastric Digestion
The digestion of proteins begins in the stomach, which secretes gastric juice
a unique solution containing hydrochloric acid and the proenzyme,
pepsinogen: Entry of a protein in to stomach stimulates the gastric mucosa to
secrete a hormone gastrin which in turn stimulates the secretion of Hcl by
the parietal cells of the gastric glands and pepsinogen by the chief cells.
THE Gastric Digestion role are included the following;
1. Hydrochloric acid: Stomach acid is too dilute pH 2 to 3) to
hydrolyze proteins. The acid functions instead to kill some bacteria and
to denature proteins, thus making them more susceptible to subsequent
hydrolysis by proteases.
2. Pepsin: This acid-stable endopeptidase is secreted by the serous
cells of the stomach as an inactive zymogen (or proenzyme),
pepsinogen. In general, zymogens contain extra amino acids in their
sequences, which prevent them from being catalytically active. [Note:
Removal of these amino acids permits the proper folding required for an
active enzyme.] Pepsinogen is activated to pepsin, either by HCI ,or
autocatalytically by other pepsin molecules that have already been
activated. This active pepsin cleaves the ingested protein at their amino
terminus of aromatic amino acids (Phe, Tyr, and Trp.) The major
products of pepsin action are large peptide fragments and some free
amino acids.
B. Pancreatic Digestion
Pancreatic zymogens proceed digestion as the acidic stomach contents pass
in to the small intestine, A low pH triggers the secretion of hormone
Secretin in the blood. Secretin stimulates the pancreas to secrete HCO-3
(bicarbonate), which in the small intestine neutralizes the gastric HCL and
abruptly change the pH to 7.0.The entry of large peptide fragments and
some free amino acids in the upper part of the small intestine (Duodenum),
excites the release of a hormone cholecytokinin (CCK).CCK function:
1) stimulates gall bladder contraction.
2) stimulate secretion of several pancreatic enzymes whose activity is
between pH 7and 8 in proenzyme forms.
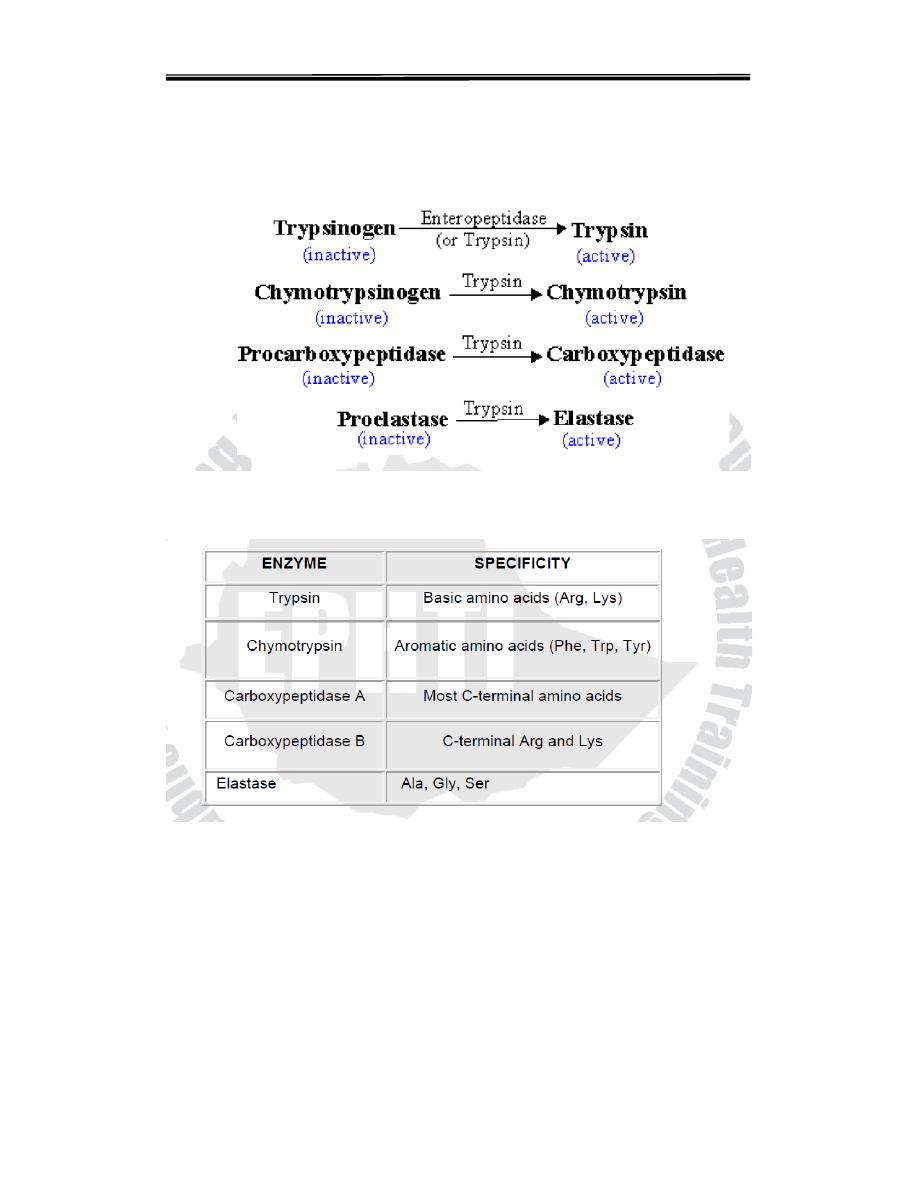
Three of these pro-enzyme are trypsinogen, chymotrypsinogen and
procarboxy peptidase, localized in the exocrine cells.Synthesis of these
enzymes as inactive precursors protects the exocrine cells from destructive
proteolytic attack.When the proenzyme reach the lumen of the small
intestine, initially the enteropeptidase (oldname Enterokinase) a protease
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
3
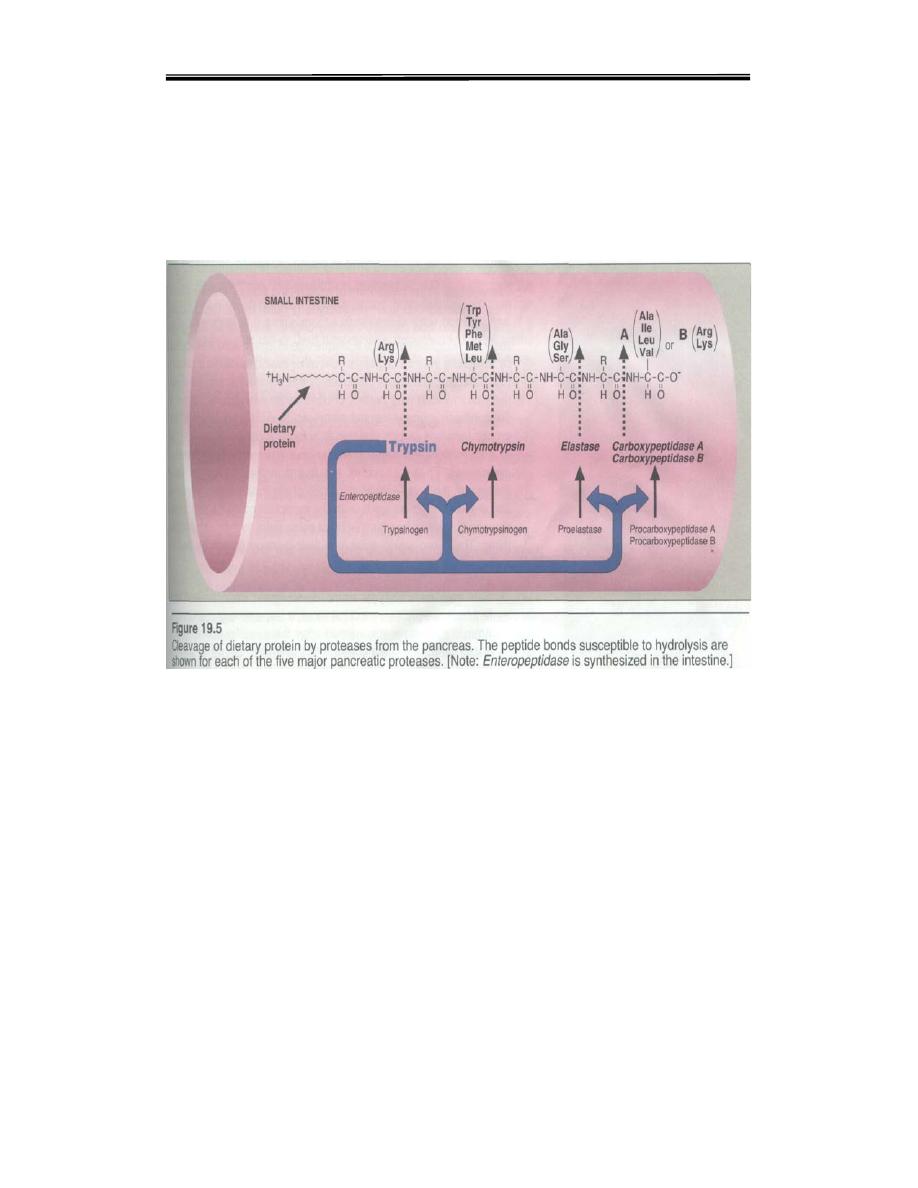
produced by duodenal epithelial cells, activates pancreatic trypsinogen to
trypsin by the removal of a hexapeptide from NH2 – terminus Trypsin in
turn auto catalytically activates more trypsinogen to trypsin and other
proenzymes and liberating chymotrypsin, elastas, and carboxypeptidase’s
By the sequential action of these proteolytic enzymes and peptides
ingested proteins are hydrolyzed to yield a mixture of free amino acids
which can be transported across the epithelial lining of the small intestine
Table 5.4: Digestive enzymes and their specificity
C. Intestinal Digestion
Since pancreatic juice does not contain appreciable aminopeptidase
activity final digestion of dia and oligopeptides depends on the small
intestinal enzymes.
The lumenal surface of epithelial cells is rich in endopeptidase
,dipeptidase and aminopeptidase activity, the end products of the cell
surface digestion are free amino acids and di and tripeptides.These are
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
4
passed in to the interior of the epithelial cell where other specific
peptidases convert almost all of them to a single amino acids that are
transported to the blood stream by the opposite side of the cell membrane
and carried to liver (primarily) and other tissues for oxidative
degradation. This process complete the absorption of 99% of digested
proteins. The whole scheme is as shown in the figure below.
II. Transport of Amino Acids in to Intestinal Epithelial cells.
The absorption of amino acids occurs mainly in the small intestine. It is
Active transport mechanism and energy requiring process. (For L-aas
and dipeptides): there are mainly two mechanism
1- Carrier protein transport system
( sodium – amino acid carrier system ).
2-H- depend trasport system
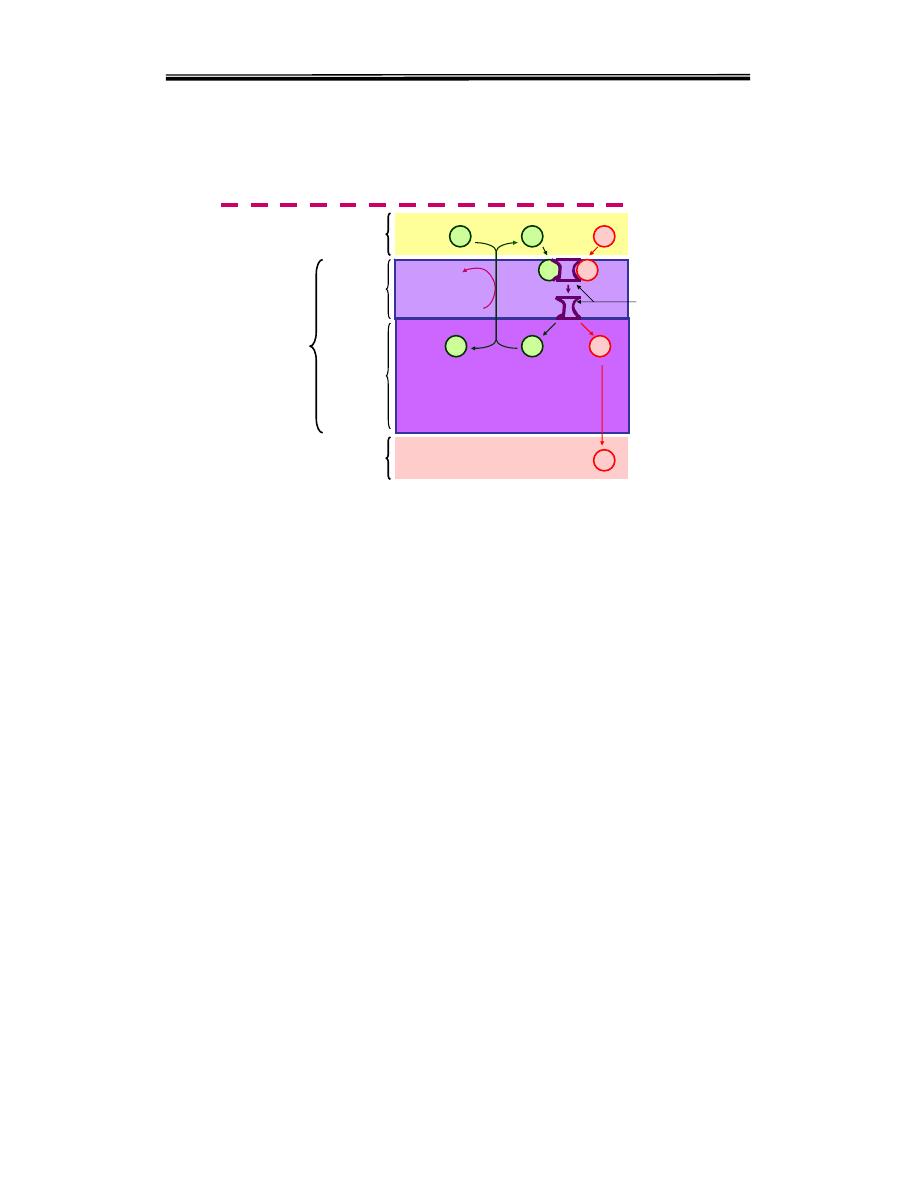
The mechanism of active transport of amino acids are similar with that of
glucose uptake at the brush - border membrane the
Na+ - depend
symporters
a.as are absorbed by specific carrier protein in the cell
membrane of the small intestinal cells.This carrier protein has one site for
the a.a. and another site for the Na+. It transports them from the intestinal
lumen across the cell membrane to the cytoplasm. Then the a.a. passes to
the blood down its conc. Gradient, while the Na+ is pumped out from the
cell to the intestinal lumen by Na/K+ pump utilizing ATP as a source of
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
5
energy
derived
from
Na/K+
pump.
Carrier protein transport system
( sodium – amino acid carrier system )
a.a.
a.a.
Na+
Na+
K+
ATP
ADP+Pi
Na/K ATPase
Cell membrane
Cytoplasm
Intestinal cell
Portal blood
a.a.
K+
Na+
a.a.
Carrier
Intestinal lumen
A similar H+ dependent symport
is present on the brush border
surface of di and tripeptides active transport in to the cell.
There are at least six specific symporter systems have been identified for
the uptake of L-amino acids from the intestinal lumen.
1. Neutral amino acid symporters with short or polar side chains.
Ser, Thr, Ala,
2. Neutral amino acid symporter for aromatic or hydrophobic side chains.
Phe, Tyr,
3. lmino acid symporter Pro,and OH – Pro
4. Basic amino acid symporter Lys, Arg and Cys.
5. Acidic amino acid symporter. Asp, Glu
6. β amino acid symporter β-Ala,.
These transporter systems are also present in the renal tubules and defects
in their constituent protein structure can lead to disease called Hartnup
disease( Hartnup disease is an autosomal recessive disorder caused by the
defective transport of amino acids in the small intestine and the kidneys).
Intracellular Protein Degradation
Protein degradation:
All proteins in the body are constantly being
degraded. Half life(t 1/2) of a protein is the time taken to lower its
concentration to half of the initial value. There are two major enzyme
systems responsible for degrading damaged or
unneeded proteins: the
1 -Energy-dependent ubiquitin-proteasome mechanism,
2-The non-energy-dependent degradative enzymes of the lysosomes.
Proteasomes mainly degrade endogenous proteins, that is, proteins that
were synthesized within the cell.
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
6
Lysosomes primarily degrade extracellular proteins, such as plasma
proteins that are taken into the cell by endocytosis, and cell-surface
membrane proteins that are used in receptor-mediated endocytosis.
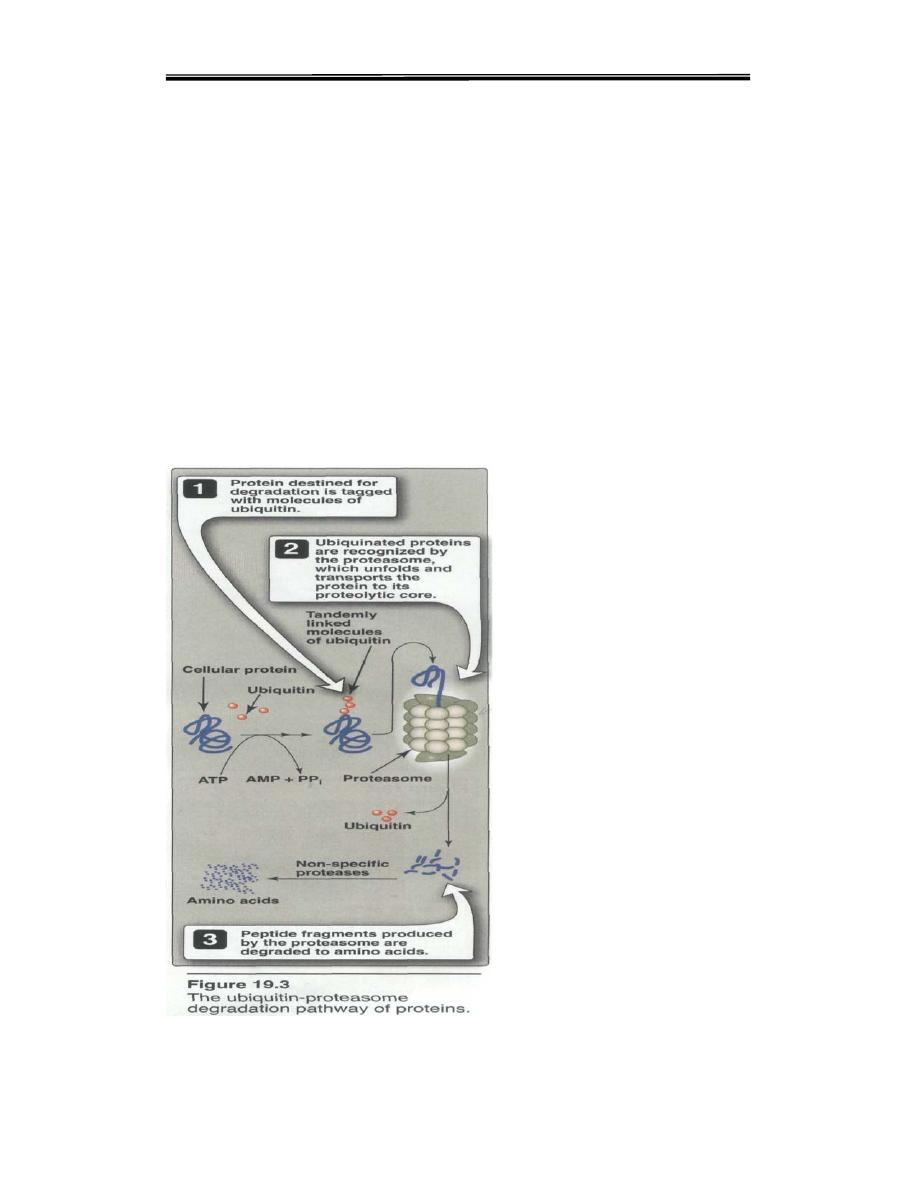
1. Ubiquitin-proteasome proteolytic pathway: Proteins destined for
degradation by the ubiquitin-proteasome mechanism are first covalently
attached to ubiquitin, a small, globular protein. Ubiquitination of the
target substrate occurs through linkage of the α-carboxyl glycine of
ubiquitin to a lysine e-amino group on the protein substrate. The
consecutive addition of ubiquitinmoieties generates a polyubiquitin
chain. Proteins tagged with ubiquitin are then recognized by a large,
barrel-shaped,proteolytic molecule called proteasome, a which functions
like a garbage disposal (Figure 19.3). The proteosome cuts the target
protein into fragments that are then further degraded to amino acids,
which enter the amino acid pool. Degradation of proteins by the
ubiquitinproteosome
complex
(unlike
simple
hydrolysis
by
proteolyticenzymes) requires ATP, that is, it is energy-dependent.

Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
7
b. Chemical signals for protein degradation: Because proteins have
different half-lives, it is clear that protein degradation cannot be random, but
rather is influenced by some structural aspect of the protein. For example,
some proteins that have been chemically altered by oxidation or tagged with
ubiquitin are preferentially degraded. The half-life of a protein is influenced by
the nature of the N-terminal residue. For example ,proteins that have serine
as the N-terminal amino acid are long-lived, with a half-life of more than
twenty hours. contrast, proteins with aspartate as the N-terminal amino acid
have a half-life of only three minutes.
Nitrogen Balance:
A healthy adult eating a varied and plentiful diet is generally in “Normal
Nitrogen Balance” a state where the amount of nitrogen ingested each day
is balanced by the amount excreted resulting no net change in the amount
of the body Nitrogn and this occure in a well fed condition, excreted
nitrogen comes from digestion of excess protein or from normal turnover
Protein turnover (Synthesis and degradation) .Under some conditions the
Nitrogen imbalances include
is either in negative or positive nitrogen
balance.
In negative nitrogen balance more nitrogen is excreted than ingested.
This occurs in starvation and certain diseases.During starvation the carbon
skeleton of most amino acids from proteins fed in to gluconeogenesis to
maintain the blood glucose level ; in this process ammonia is released and
excreted mostly as urea and is not reincorporated in to protein.
A diet deficient in an essential amino acid also leads to a negative
nitrogen balance since body proteins are degraded to provide the deficient
essential amino acid.
Positive nitrogen balance occurs in pregnancy and during feeding after
starvation and occurs in growing children who are increasing their body
weight
Amino acid pool
and protein turnover.
Amino acid catabolism is part of the larger process of whole body
nitrogen metabolism. Nitrogen enters the body in a variety of compounds
present in food, the most important being amino acids contained in
dietary protein. Nitrogen leaves the body as urea, ammonia, and other
products derived from amino acid metabolism. The role of body proteins
in these transformations involves two important concepts: the amino acid
pool and protein turnover.
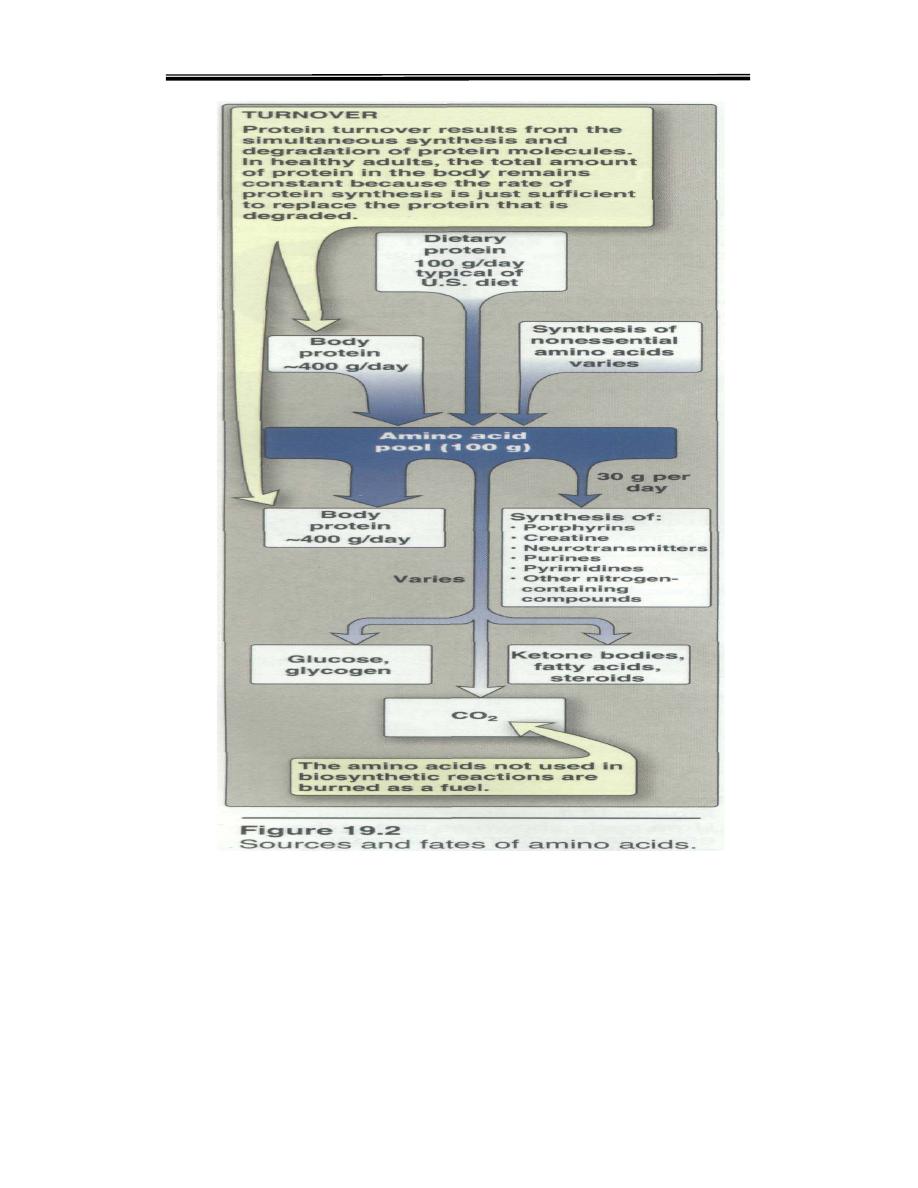
A. Amino acid pool
The entire collection of free amino acids in the body called Amino
acid pool.Amino acids released by hydrolysis of dietary or tissue
protein, or synthesized denova mix with other free amino acids
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
8
distributed throughout the body. Collectively, they constitute the
amino acid pool (Figure 19.2). The amino acid pool, containing about
100g of amino acids, is small in comparison with the amount of
protein in the body (about 12kg in a 70 kg man).
B. Protein turnover
Protein turnover is the balance between
protein synthesis
and
protein
degradation
.Most proteins in the body are constantly being synthesized
and then degraded, permitting the removal of abnormal or unneeded
proteins. For many proteins, regulation of synthesis determines the
concentration of protein in the cell .in healthy adults, the total amount of
protein in the body remains constant, because the rate of protein synthesis
is just sufficient to replace the protein that is degraded. The process,
protein turnover, leads to the hydrolysis and resynthesis of 300 to 400 g
of body protein each day. The rate of protein turnover varies widely for
individual proteins. Short-lived proteins (for example, many regulatory
proteins and misfolding proteins) are rapidly degraded, having half-lives
measured in minutes or hours. Long-lived proteins, with half-lives of
days to weeks, constitute the majority of proteins in the cell. Structural
proteins, such as collagen
Clinical biochemistry second stage lecture 1 Dr.Thanaa Alsewedy
9
