
1
Genetics
Introduction:
Common with 2% of live-born babies having a significant congenital malformation and
about 5% a genetic disorder.
Burdensome to the affected individual, family and society, as many are associated with
severe and permanent disability.
Genetically determined diseases:
Single gene mutations (mendelian disorders) 6%.
Chromosomal disorders 7.5%.
Multifactorially inherited conditions 20%.
Disorders that show an unusual pattern of inheritance 2-3%.
Teratogenically caused conditions 6%.
Mendelian inheritance:
Disorders with these patterns of inheritance, described by Mendel in 1865, are rare
individually, but collectively numerous, with over 15 000 single gene traits or disorders
described.
The hallmark of a single-gene disorder is that the phenotype is overwhelmingly
determined by changes that affect an individual gene (quantity or function).
The phenotypes associated with single-gene disorders can vary from one patient to
another based on the severity of the change affecting the gene and additional
modifications caused by genetic, environmental, and/or stochastic factors. This feature
of genetic disease is termed variable expressivity.
There are three classic forms of genetic inheritance: autosomal dominant, autosomal
recessive, and X-linked.
Autosomal dominant inheritance:
This is the most common mode of Mendelian inheritance.
Autosomal dominant inheritance is determined by the presence of one abnormal gene
on one of the autosomes (chromosomes 1–22).
Ibnlatef
Notes
Pediatrics
2
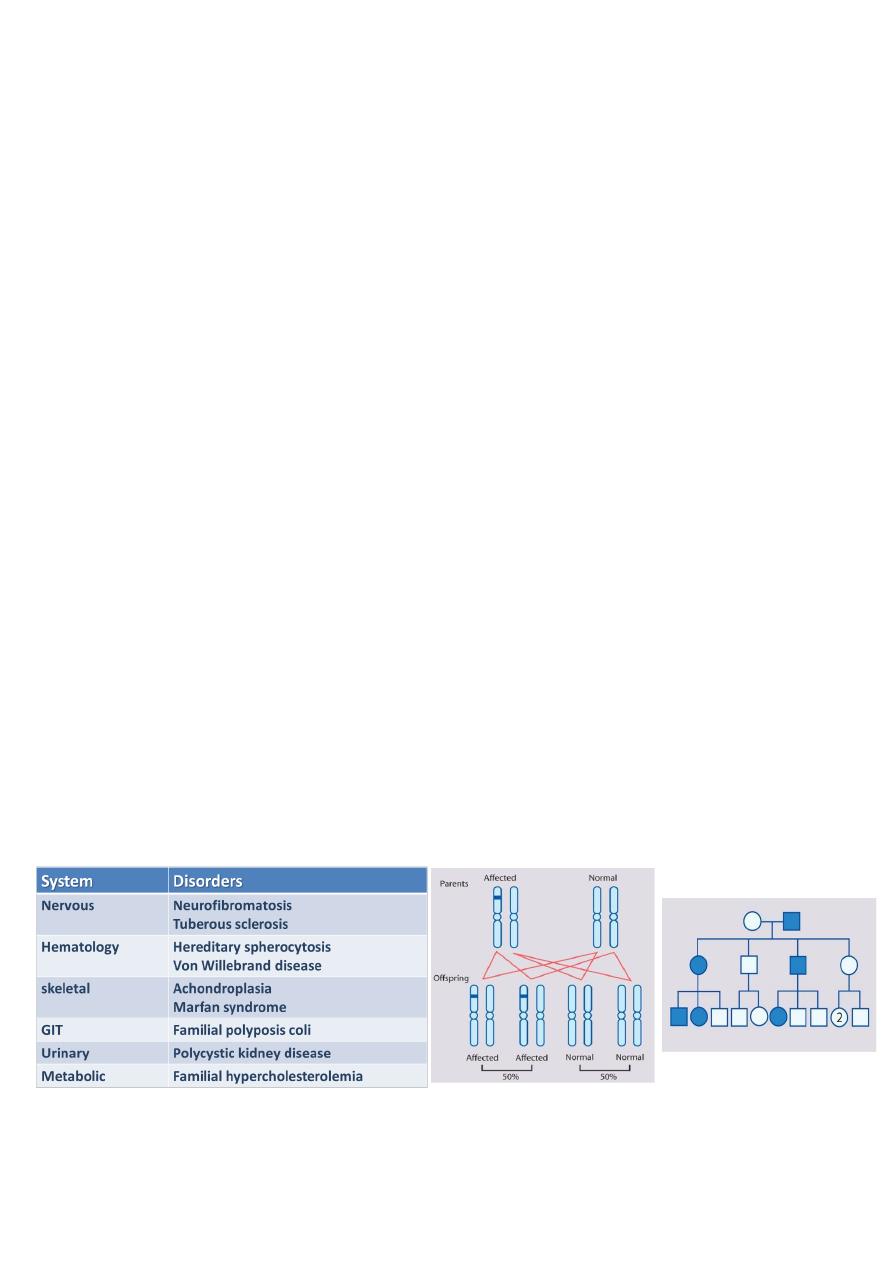
Male and female offspring each have a 1 in 2 (50%) chance of inheriting the abnormal
gene from an affected parent (in each pregnancy).
Autosomal dominant diseases:
Tuberous sclerosis.
Marfan syndrome (part of neuro-cutaneous syndrome).
Neurofibromatosis (part of neuro-cutaneous syndrome).
Von Hippel Lindau (part of neuro-cutaneous syndrome).
Huntington's disease.
Retinoblastoma.
Waardenburg syndrome.
Myotonic dystrophy.
Familial hypercholestrolemia (LDL receptor defect Type IIa).
Adult polycystic kidney disease.
Familial adenomatous polyposis and Peutz Jeghers Syndrome.
Hereditory spherocytosis.
Achondroplasia.
Ehlor's Danlos (vascular type).
Acute intermittent porphyria.
Hypertrophic Obstructive Cardiomyopathy (HOCM).
Von Willebrand Disease.
Polydactyly.
Osteogenesis Imperfecta (Except Type VII).
Hereditary hemorrhagic telengiactasia (Osler-weber-rendu syndrome).
Osteopetrosis Type II (Adult type).
Hypokalemic Periodic Paralysis.

3
Characteristics of autosomal dominant:
Variation in expression:
o
Within a family, some affected individuals may manifest the disorder mildly and
others more severely.
o
For example, a parent with tuberous sclerosis may have mild skin abnormalities
only, but his or her affected child may have, in addition, epilepsy and learning
difficulties.
Non-penetrance:
o
Refers to the lack of clinical signs and symptoms in an
individual who must have inherited the abnormal gene.
o
An example of this is otosclerosis, in which only about 40% of
gene carriers develop deafness.
No family history of the disorder:
o
A new mutation in one of the gametes leading to the conception of the affected
person.
o
This is the most common reason for absence of a family history in dominant
disorders, e.g. >80% of individuals with achondroplasia have normal parents.
Homozygosity:
o
In the rare situation where both parents are affected by the same autosomal
dominant disorder, there is a 1 in 4 risk that a child will be homozygous for the
altered gene.
o
This usually causes a more severe phenotype which may be lethal, as with
achondroplasia.
Rules of autosomal dominant inheritance:
Trait appears in every generation.
Each child of an affected parent has a 1 in 2 chance of being affected.
Males and females are equally affected.
Male-to-male transmission occurs.
Traits generally involve mutations in genes that code for regulatory or structural
proteins (collagen).
Examples of some autosomal dominant disorders:
Achondroplasia:
o
Achondroplasia is the most common skeletal dysplasia in humans.
o
The bony abnormalities in achondroplasia lead to short stature, macrocephaly, a
flat midface with a prominent forehead, and rhizomelic shortening of the
limbs.
4
Neurofibromatosis Type 1:
Marfan Syndrome:
o
Clinical symptoms mostly involve three systems: cardiac, musculoskeletal, and
ophthalmologic.
o
Musculoskeletal findings dolichostenomelia (a tall, thin body habitus),
arachnodactyly (spider-like fingers and toes), abnormalities of the sternum (pectus
excavatum or carinatum), kyphoscoliosis, pes planus, and joint laxity.
o
Eye findings high myopia, which eventually can lead to vitreoretinal
degeneration; an abnormal suspensory ligament of the lens, which can lead to
ectopia lentis (dislocation of the lens; in Marfan syndrome, the lens usually
dislocates upward and outward); and cataracts.
o
Cardiac findings a weakened aortic wall, which leads to progressive dilation of
the aortic root, aortic insufficiency followed ultimately by aortic dissection is a
common complication of this disorder.
Autosomal recessive inheritance:
Many hundred disorders resulting from this type of
inheritance are known.
An affected individual is homozygous for the abnormal
gene, having inherited an abnormal allele from each
parent, both of whom are unaffected heterozygous
carriers.
For two carrier parents, the risk of each child, male or
female, being affected is 1 in 4 (25%).
All offspring of affected individuals will be carriers.
AD
heterozygous, AR
homozygous.
AD more than AR.
AD
risk in each pregnancy is 50%.
AR
risk in each pregnancy is 25%.
AD
affect structural proteins.
AR
affect metabolic pathway.

5
Consanguinity:
It is thought that we all carry at least one abnormal
recessive gene.
Fortunately, our partners usually carry a different one.
Marrying a cousin or other relative increases the chance
of both partners carrying the same abnormal autosomal
recessive gene, inherited from a common ancestor.
A couple who are cousins therefore have a small increase
in the risk of having a child with a recessive disorder.
Racial factor:
Recessive gene frequencies may vary between racial groups.
Cystic fibrosis is common in north Europeans.
Sickle cell disease in black Africans and Americans.
Thalassaemias in Mediterranean or Asian ethnicity.
Tay-Sachs disease in Ashkenazi Jews.
Rules of autosomal recessive inheritance:
Affected individuals are homozygous for the abnormal gene; each unaffected parent
will be a heterozygous carrier.
Two carrier parents have a 1 in 4 risk of having an affected child.
All offspring of affected individuals will be carriers.
Males and females are likely to be affected equally.
Risk of these disorders is increased by consanguinity and within specific populations.
Autosomal recessive disorders often affect metabolic pathways, whereas autosomal
dominant disorders often affect structural proteins.
Autosomal recessive diseases:

6
Inborn errors of metabolism:
Although individually rare, inborn errors of metabolism are an important cause of
paediatric morbidity and mortality.
The specialized nature of the diagnostic tests and subsequent management often
means that these patients are managed in specialist centres.
However, as the prognosis for most patients depends upon the speed of diagnosis, all
doctors need to be familiar with their variable presentation and diagnosis.
Presentation of inborn errors of metabolism:
An inborn error of metabolism may be suspected before birth from a positive family
history or previous unexplained deaths in the family.
After birth, inborn errors of metabolism usually, but not invariably, present in one of
five ways:
1.
As a result of newborn screening, e.g. phenylketonuria (PKU), or family screening,
e.g. familial hypercholesterolaemia
2.
After a short period of apparent normality, with a severe neonatal illness with poor
feeding, vomiting, encephalopathy, acidosis, coma and death, e.g. organic acid or
urea cycle disorders.
3.
As an infant or older child with an illness similar to that described above but with
hypoglycaemia as a prominent feature or as an ALTE (acute life-threatening
episode) or near-miss 'cot death', e.g. a fat oxidation defect such as medium-chain
acyl-CoA dehydrogenase deficiency (MCADD).
4.
In a subacute way, after a period of normal development, with regression,
organomegaly and coarse facies, e.g. mucopolysaccharide disease or other
lysosomal storage disorder or with enlargement of the liver and/or spleen alone,
with or without accompanying biochemical upset such as hypoglycaemia, e.g.
glycogen storage disease.
5.
As a dysmorphic syndrome.
Examples of inborn errors of metabolism:
Phenylketonuria:
o
It is either due to a deficiency of the enzyme phenylalanine hydroxylase (classical
PKU) or in the synthesis or recycling of the biopterin cofactor for this enzyme.
o
Untreated, it usually presents with developmental delay at 6-12 months of age.
o
There may be a musty odour.
o
Many affected children are fair-haired and blue-eyed and some develop eczema
and seizures.

7
o
Fortunately, most affected children are detected through the national biochemical
screening programme (Guthrie test).
o
Treatment of classical PKU is with restriction of dietary phenylalanine, whilst
ensuring there is sufficient for optimal physical and neurological growth.
o
The blood plasma phenylalanine is monitored regularly.
o
The current recommendation is to maintain the diet throughout life.
o
This is particularly important during pregnancy, when high maternal phenylalanine
levels may damage the fetus.
o
Maternal hyperphenylalaninemia requires rigorous management before conception
and throughout pregnancy to prevent fetal brain damage, congenital heart disease,
and microcephaly.
Galactosemia:
o
This rare, recessively inherited carbohydrate metabolic disorder results from
deficiency of the enzyme galactose-1-phosphate uridyl transferase, which is
essential for galactose metabolism.
o
The neonatal screening test must have a rapid because affected infants may die in
the first week of life.
o
Manifestations are most striking in a neonate who, when fed milk, generally
exhibits evidence of:
Liver failure hyperbilirubinemia, coagulation defect, and hypoglycemia.
Disordered renal tubular function acidosis, glycosuria, and aminoaciduria.
Cataracts.
Affected infants are at increased risk for severe neonatal Escherichia coli sepsis.
Older children have learning disorders.
o
Investigations:
Depend on dietary galactose intake.
When galactose is ingested (as lactose): levels of plasma galactose increase.
Erythrocyte galactose-1-phosphate are elevated.
Hypoglycemia is frequent.
Albuminuria is present.
Galactose frequently is present in the urine (Clinitest +ve).
DNA testing for the mutations in galactose-1-phosphate uridyltransferase.
Renal tubular dysfunction may be evidenced by a normal anion gap
hyperchloremic metabolic acidosis.
o
Management is with a lactose- and galactose-free diet for life.
o
Even if treated early, there are moderate learning difficulties (adult IQ 60-80).
Glycogen storage disorders:
o
These mostly recessively inherited disorders have specific enzyme defects which
prevent mobilization of glucose from glycogen, resulting in an abnormal storage of
glycogen in liver and/or muscle.
o
There are nine main enzyme defects.

8
o
Glycogen storage diseases fall into the following four categories:
Diseases that predominantly affect the liver and have a direct influence on blood
glucose (types I, VI, and VIII).
Diseases that predominantly involve muscles and affect the ability to do
anaerobic work (types V and VII).
Diseases that can affect the liver and muscles and directly influence blood
glucose and muscle metabolism (type III).
Diseases that affect various tissues but have no direct effect on blood glucose or
on the ability to do anaerobic work (types II and IV). Type II (Pompe's disease)
the heart is severely affected, leading to death from cardiomyopathy.
o
Management is to maintain blood glucose by frequent feeds or by carbohydrate
infusion via a gastrostomy or nasogastric tube in infancy.
o
In older children, glucose levels can be maintained using slow-release
oligosaccharides (corn starch).
Notes:
o
Phenylketonuria occur is 2 months or more.
o
Galactosemia occur at the early life.
o
Tendon mass spectrometer detect inborn error of metabolism.
X-linked recessive inheritance:
Over 400 disorders have been described in which an
abnormal recessive gene is carried on the X
chromosome.
Males are more commonly and more severely affected
than females.
Occasionally a female carrier shows mild signs of the
disease (manifesting carrier).
Each son of a female carrier has a 1 in 2 (50%) risk of
being affected.
Each daughter of a female carrier has a 1 in 2 (50%)
risk of being a carrier.
Daughters of affected males will all be carriers.
Sons of affected males will not be affected, since a
man passes a Y chromosome to his son.
Males are affected; females can be carriers but are
usually healthy or have mild disease.
Male-to-male transmission excludes X-linkage but is seen with autosomal dominant
and Y-linked inheritance.
Family history may be negative - new mutations and gonadal mosaicism.
Identifying female carriers is important to be able to provide genetic counselling.

9
Affected female in X-linked recessive diseases:
Turner’s syndrome.
Manifesting carrier.
Lyon hypothesis (inactivation of other chromosome).
X-linked recessive diseases:
Note: Fragile X syndrome second most common cause of mental retardation (1st is Down).
X-linked dominant inheritance:
Only few X linked dominant disorders have been
described.
Both male and female affected, but female have less
severe symptoms due to X- chromosome inactivation,
e.g. hypophsphatemic rickets.
In which female carriers typically manifest abnormal
findings.
An affected man will have only affected daughters and unaffected sons, and half of the
offspring of an affected woman will be affected.
Some X-linked dominant conditions are lethal in males. An example is incontinentia
pigmenti.
In X-linked dominant or recessive there is no male to male transmission.
Y-linked inheritance:
Y-linked traits are extremely rare.
Y-linked inheritance would result in only males being affected, with transmission from
an affected father to all his sons.
Y-linked genes determine sexual differentiation and spermatogenesis, and mutations
are associated with infertility and so are rarely transmitted.
Chromosomal abnormalities:
Chromosomal abnormalities are either numerical or structural.
They occur in approximately 10% of spermatozoa and 25% of mature oocytes.
10
They are a very common cause of early spontaneous miscarriage.
The estimated incidence of chromosomal abnormalities in live-born infants is about 1
in 150 and these usually, but not always, cause multiple congenital anomalies and
learning difficulties.
Acquired chromosomal changes play a significant role in carcinogenesis and tumor
progression.
50% of spontaneous abortions have chromosomal abnormalities.
In newborns and older children, many features suggest the presence of a chromosome
anomaly, including LBW (SGA), FTT, developmental delay, and the presence of three or
more congenital malformations.
The diagnosis is confirmed by chromosome analysis.
Multifactorial inheritance:
Multifactorial inheritance refers to traits that are caused by a combination of inherited
and environmental factors.
Characteristics of multifactorial inheritance:
The risk of recurrence is related to the incidence of the disease.
Some disorders have a sex predilection Pyloric stenosis is more common in males,
whereas congenital dislocation of the hips is more common in females
The likelihood that both identical twins will be affected with the same
malformation is less than 100% but much greater than the chance that both members
of a nonidentical twin pair will be affected, this is in contrast with the pattern seen in
mendelian inheritance, in which identical twins almost always share fully penetrant
genetic disorders.
The risk of recurrence is increased when multiple family members are affected a
simple example is that the risk of recurrence for unilateral cleft lip and palate is 4% for
a couple with 1 affected child and increases to 9% with 2 affected children.
The risk of recurrence may be greater when the disorder is more severe an infant
who has long-segment Hirschsprung disease has a greater chance of having an affected
sibling than the infant who has short-segment Hirschsprung disease.
Conditions with multifactorial inheritance:
Congenital malformations:
o
Neural tube defects (anencephaly and spina bifida).
o
Congenital heart disease.
o
Cleft lip and palate.
o
Pyloric stenosis.

11
o
Congenital dislocation of the hip.
o
Talipes equinovarus.
o
Hypospadias.
Childhood:
o
Atopy (especially asthma and eczema).
o
Epilepsy.
o
Diabetes mellitus type 1.
Adult life:
o
Atherosclerosis and coronary artery disease.
o
Diabetes mellitus type 2.
o
Alzheimer’s disease.
o
Malignancy (especially the common cancers, e.g. breast and colorectal cancer).
o
Hypertension.
o
Cerebrovascular disease (especially stroke).
Unusual genetic mechanisms:
Trinucleotide repeat expansion mutations:
o This is a class of unstable mutations caused by unstable expansions of trinucleotide
repeat sequences inherited in Mendelian fashion.
o Fragile X syndrome and myotonic dystrophy were among the first disorders found
to be due to such mutations.
o Other disorders
Huntington disease, spinocerebellar ataxia and Friedreich’s
ataxia.
o These disorders follow different patterns of inheritance but share certain unusual
properties due to the nature of the underlying mutation.
o Clinical anticipation is often seen, with the disorders presenting at an earlier age
and becoming more severe in successive generations of a family as the triplet
expands, and with entirely new mutations being exceptionally rare.
Mitochondrial or cytoplasmic inheritance:
o Each cell contains thousands of copies of the mitochondrial genome.
o Inherited disorders of the mitochondria may result from mutations in the nuclear
genome (the chromosomal genome of the cell nucleus) or in the mitochondria’s
own genome.
o In disorders of the mtDNA, the mutation may be present in all or only some of the
mitochondria, so that the tissues affected and the severity of the condition can be
highly variable.
o Large deletions of the mtDNA can only be present in a proportion of the
mitochondria as they would otherwise be lethal to the cell.

12
o Mutations in mtDNA cause overlapping clusters of disease phenotypes (Leber
hereditary optic neuropathy and various mitochondrial myopathies and
encephalopathies, MERFF, MELAS, NARP).
o Mitochondrial DNA mutations show only maternal transmission, since only the egg
contributes mitochondria to the zygote.
Imprinting and uniparental disomy:
o In the past, it was assumed that the activity of a gene is the same regardless of
whether it is inherited from the mother or father.
o It has been shown that the expression of some genes is influenced by the sex of the
parent who had transmitted it.
o This phenomenon is called ‘imprinting’.
o An example involves Prader–Willi syndrome (PWS) (hypotonia, developmental
delay, hyperphagia and obesity).
o The PWS chromosomal region is found at 15q11–13 (at bands 11–13 on the long
arm of chromosome 15).
o The paternal copy of this chromosomal region has to function for normal
development, in its absence, a child will develop PWS.
o Failure to inherit a functioning maternal copy of this chromosomal region results in
an entirely different condition, Angelman syndrome (AS) (causing severe cognitive
impairment, a characteristic facial appearance, ataxia and epilepsy), because only
the maternal copy of one particular gene in this region is able to function (the
paternal copy is inactive because of imprinting).
o There are two main ways that a child can develop one or other condition:
De novo deletion Parental chromosomes are normal, and a deletion occurs as
a new mutation in the child. If the deletion occurs on the paternal chromosome
15, the child has Prader–Willi syndrome. If the deletion affects the maternal
chromosome 15, the child has Angelman syndrome.
Uniparental disomy This is when a childinherits two copies of a chromosome
from one parent and none from the other parent. In Prader–Willi syndrome the
affected child has no paternal (but two maternal) copies of chromosome 15q11–
13. In Angelman syndrome, the affected child has no maternal (but two
paternal) copies of chromosome 15q11–13. This can be detected with DNA
analysis.
o There exist other mechanisms that can lead to these conditions.
Note:
Imprinting is the unusual property of some genes that express only the copy derived from the parent
of a given sex.
----------------------------------------------------------------------------------------------
www.facebook.com/ibnlatef
https://goo.gl/RpvNsl
