
All pediatrics
seminars
For
6
th
stage

Content
Topics:
Page:
Acute Diarrhoea in Children
3
The edematous child
18
Pyrexia of unknown origin
27
Vaccination
30
Acute respiratory infection
51
ARI control and prevention
57
Neonatal emergencies
62

Seminar1
: Acute Diarrhoea in Children
Welcome to the module on Management of Acute Diarrhoea (AD) in Children!
Diarrhoeal disease remains a leading cause of morbidity and mortality amongst children in
low and middle income countries.
Most deaths result from the associated shock, dehydration and electrolyte imbalance.
In malnutrition, the risk of AD, its complications and mortality are increased.
How to use this module:
This module aims to address deficiencies in the management of AD and dehydration in
children that we identified during a clinical audit.
We suggest that you start with the learning objectives and try to keep these in mind as
you go through the module slide by slide, in order and at your own pace.
Print-out the diarrhoea SDL answer sheet. Write your answers to the questions (Q1, Q2
etc.) on the sheet as best you can before looking at the answers.
Repeat the module until you have achieved a mark of >20 (>80%).
You should research any issues that you are unsure about. Look in your textbooks,
access the on-line resources indicated at the end of the module and discuss with your
peers and teachers.
Finally, enjoy your learning! We hope that this module will be enjoyable to study and
complement your learning about AD from other sources.
Learning Outcomes:
By the end of this module, you should be competent in the management of acute diarrhoea
/ dehydration.
In particular you should be able to:
Describe when to use oral and parenteral fluids and what solutions to use
Identify the malnourished child and adjust management accordingly
Describe when antibiotic treatment is indicated and the adverse effects of the overuse
of antibiotics
Describe the use of zinc in AD
Definition of AD:
There is a wide range of normal stool patterns in children which makes the precise
definition of AD difficult
According to the World Health Organization (WHO), AD is the passage of loose* or
watery stools, three times or more in a 24 hour period for upto14 days
In the breastfed infant, the diagnosis is based on a change in usual stool frequency and
consistency as reported by the mother
AD must be differentiated from persistent diarrhoea which is of >14 days duration and
may begin acutely. Typically, this occurs in association with malnutrition and/or HIV
infection and may be complicated by dehydration
*Takes the shape of the container
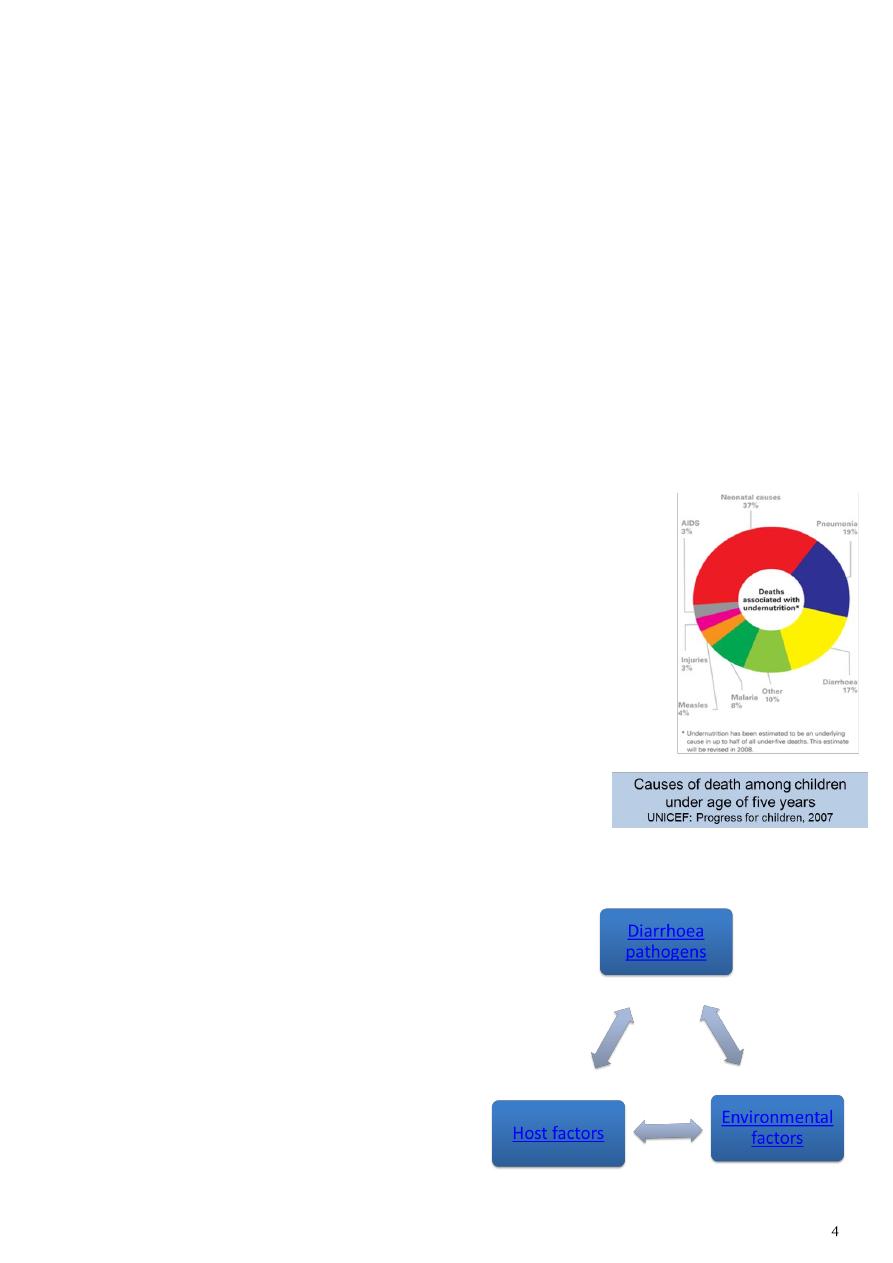
The burden of diarrhoeal disease:
Despite the fact that diarrhoea can be prevented, about 2
billion cases of diarrhoea occur globally every year in
children under 5 years
About 2 million child deaths occur due to diarrhoea every
year
More than 80% of these deaths are in Africa and South Asia
Diarrhoea is the third most common cause of death (see
diagram)
In Nigeria, diarrhoea causes 151,700 deaths of children
under five every year,* the second highest rate in the world
after India
* UNICEF/WHO, Diarrhoea: Why children are still dying and
what can be done, 2009
Causes and risk factors for AD:
Microbial, host and environmental factors
interact to cause AD
Click on the boxes to find out more

Host factors:
Biological factors increase susceptibility to AD:
Age: The incidence of AD peaks at around age 6-11 months, remains high through 24
months and then decreases
Failure to get immunised against rotavirus
Failure of measles vaccination; measles predisposes to diarrhoea by damage to the
intestinal epithelium and immune suppression
Malnutrition is associated with an increased incidence, severity and duration of diarrhea
Behavioral factors increase the risk of AD:
Not breastfeeding exclusively for 6 months
Using infant feeding bottles: they easily become contaminated with diarrhoea
pathogens and are difficult to clean
Not washing hands after defecation, handling faeces or before handling food
Environmental factors:
These include:
Seasonality: The incidence of AD has seasonal variation in many regions
o In temperate climates, viral diarrhoea peaks during winter whereas bacterial
diarhoea occurs more frequently during the warm season
o In tropical areas, viral and bacterial diarrhoeal occur throughout the year with
increased frequency during drier, cooler months.
Poor domestic and environmental sanitation especially unsafe water
Poverty
Common causes of AD:
More than 20 viruses, bacteria and parasites have been associated with acute diarhoea
Worldwide, rotavirus is the commonest cause of severe dehydrating diarrhoea causing
0.6 million deaths annually, 90% of which occur in developing countries
The incidence of specific pathogens varies between developed and developing countries
In developed countries, about 40% of AD cases are due to rotavirus and only 10-20% are
of bacterial origin while in developing countries, 50-60% are caused by bacteria while
15-25% are due to rotavirus
Clinical types of AD:
There are 2 main clinical types of AD
Each is a reflection of the underlying pathology and altered physiology
Answer to Q1a
This statement is True.
The incidence of diarrhoea is highest in age group 6-11 months. This is likely to be
associated with declining levels of antibodies acquired from the mother, lack of active
immunity in the infant and the introduction of complementary foods that may be
contaminated with diarrhoeal pathogens.
Answer to Q1b
This statement is True
Diarrhoea that begins acutely and lasts less than 14 days is called acute diarrhoea

Diarrhoea lasting longer than 14 days is persistent diarrhoea
Answer to Q1c
This statement is False
Bacterial pathogens cause most cases of diarrhoea in developing countries
Bacteria are responsible for 50-60% of cases of AD while rotavirus is responsible for 15-
25% cases
Answer to Q1d
This statement is True.
Undernourished children are at higher risk of suffering more frequent, severe and
prolonged episodes of diarrhoea
Answer to Q1e
This statement is False
East Asia and Pacific, South Asia and Africa are home to 9%, 38% and 46% respectively of
child deaths from diarrhoea
The rest of the world contributes only 7%
Clinical scenarios:
You will now work through a series of cases of AD
You will learn how to assess and manage children according to the latest WHO
guidelines
Start with scenario A. Try to answer the questions yourself before clicking on the
answers

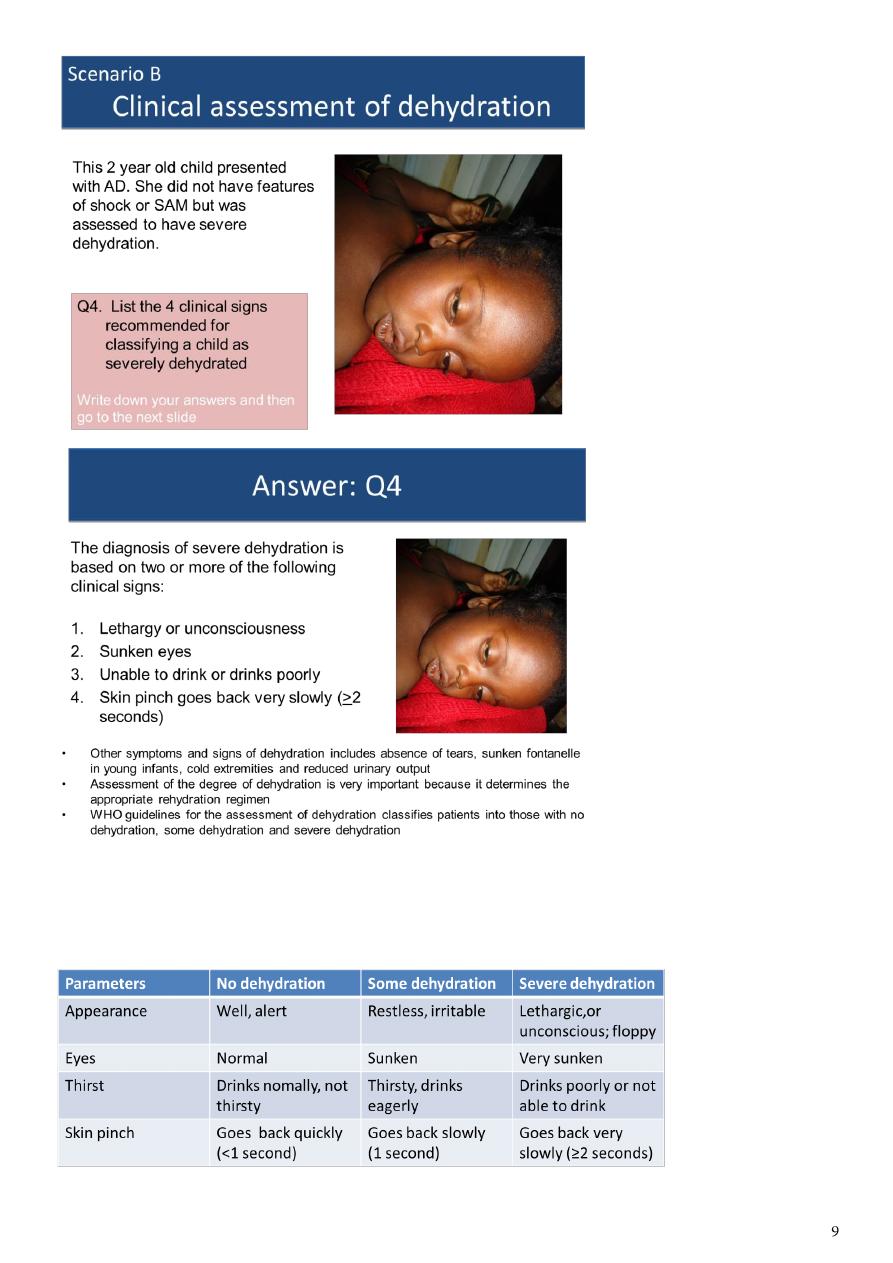
WHO guideline for the classification of dehydration:

There are other established guidelines. Click here to see details
Other guidelines used to assess dehydration due to AD:
National Institute for Health and Clinical Excellence guidelines (NICE/UK)
ESPGHAN guidelines
These classify patients into
o minimal or no dehydration
o mild to moderate dehydration
o severe dehydration
o AAP guideline classifies patients as mild (3-5%), moderate (6-9%) and severe (>10%)
dehydration
Various scoring systems (Fortini et al., Gorelick et al.) proposed for assessment of child
with dehydration, but there is limited evidence to support their use particularly in
developing countries
Treatment of severe dehydration:
Q5: Children with severe dehydration require rapid IV rehydration followed by oral
rehydration therapy
Q6: For IV rehydration, Ringer’s lactate (also called Hartmann’s solution) is
recommended. If not available, normal saline can be used
Q7: Give 100ml/kg of fluid as shown below:
aRepeat if the radial pulse is still very weak or not detectable


Answer: Q10a
This statement is False. The appropriate treatment is use of oral rehydration fluid. IV
infusion is only recommended for children with shock or severe dehydration. Even when a
child with some dehydration can not tolerate oral fluids, it is advisable to give oral fluids
through a nasogastric tube.
Answer: Q10b
This statement is True. WHO/UNICEF recommends the new improved oral rehydration
solution which has reduced concentration of sodium and glucose (LO-ORS). LO-ORS reduces
the risk of hypertonicity, reduces stool output, shortens the duration of diarrhoea and
reduces the need for intravenous fluids.
– Give the child 75ml/kg of ORS in the first 4 hours
– Show the mother how to give ORS solution, a teaspoonful every 1-2 minutes for child
under 2 years
– If the child vomits, wait 10 minutes, then resume giving ORS solution more slowly
– Monitor the child to be sure child is taking ORS solution
– Check child’s eyelids; if they become puffy, stop ORS solution
– Reassess the child after 4 hours, checking for signs of dehydration
– Teach the mother how to prepare ORS solution at home
– Advise on breastfeeding, for those still breastfeeding, and adequate feeding
– If no dehydration, teach the mother the rules of home treatment

Answers: Scenario D
Q11:
This child has acute bloody diarrhoea also called dysentery
Most episodes are due to Shigella spp
The diagnostic signs of dysentery are frequent loose stools with visible red blood
Other findings in the history or on examination may include
– Abdominal pain
– Fever
– Convulsions
– Lethargy
– Dehydration
– Rectal prolapse
Q12:
All children with severe dysentery require antibiotic treatment for 5 days
– Give an oal antibiotic to which most strains of shigella in your localiity are
sensitive
– Examples of antibiotics to which shigella strains can be sensitive are ciprofloxacin
and other fluoroquinolones
Also manage any dehydration
Ensure breastfeeding is continued for childen still breastfeeding and normal diet for older
childen
Follow-up the child

Answer: scenario E - Fluid management in children with SAM
Q13: The child has severe acute malnutrition: SAM
Q14: No. Dehydration is difficult to diagnose in SAM and it is often over diagnosed. The
doctor’s choice of IV normal saline, amount of fluid and rapidity of given IV fluid were all
incorrect and may have caused the child’s deterioration
Q15: The pathophysiological mechanisms that affect fluid management are:
• Although plasma sodium may be very low, total body sodium is often increased due
to
– increased sodium inside cells
– additional sodium in extracellular fluid if there is nutritional oedema
– reduced excretion of sodium by the kidneys
• Cardiac function is impaired in SAM
This explains why treatment with IV fluids can result in death from sodium overload and
heart failure.
• The correct management is reduced sodium oral rehydration fluid (ORF; e.g.
ReSoMal) given by mouth or naso-gastric tube if necessary. The volume and rate of
ORF are much less for malnourished than well-nourished children (see next slide)
IV fluids should be used only to treat shock in children with SAM who are also lethargic or
have lost consciousness!

End of clinical scenarios
The next few slides are on how to assess nutritional status, indications for laboratory
investigations, rational use of antibiotics and usage of zinc
Assessment of nutritional status:
• Assessment of nutritional status is important in children with
diarhoeal disease to identify those with severe acute malnutrition
(SAM)
• This is because abnormal physiological processes in SAM markedly
affect the distribution of sodium and therefore directly affect
clinical management
• In patients with SAM, although plasma sodium may be very low,
total body sodium is often increased due to:
– increased sodium inside cells as a result of decrease activity of sodium pumps
– additional sodium in extracellular fluid if there is nutritional oedema
– reduced excretion of sodium by the kidneys
Methods of nutritional assessment:
Nutritional assessment can be done by:
• Looking for visible signs of severe wasting such as
muscle wasting and reduced subcutaneous fat
• Looking for other signs of malnutrition: angular
stomatitis, conjuctival and palmar pallor, sparse and
brittle hair, hypo- and hyperpigmentation of the skin
• Looking for nutritional oedema (pitting oedema of both feet)
• Use of anthropometry such as Weight-for-Height z-score (WHZ; < -3.0) or Mid-Upper
Arm Circumference (MUAC < 11.5cm in children aged 6-60 months)
MUAC: recommended for nutritional assessment in dehydration:
• MUAC is widely used in community screening of malnutrition because it is easy to
perform, accurate and quick

• MUAC is measured using Shakir’s strip or an inelastic
tape measure placed on the upper arm midway between
acromion process and olecranon
• Dehydration reduces weight; MUAC was less affected by
dehydration than WFLz score in a recent study*
Laboratory investigations:
AD is usually self-limiting and investigations to identify the
infectious agent are
not required
A. Indications for stool microscopy, culture and sensitivity
• Blood and mucus in the stool
• High fever
• Suspected septicaemic illness
• Diagnosis of AD is uncertain
B. Indications for measurement of Urea and Electrolytes
• Severe dehydration or shock
• Children on IV fluid
• Children with severe malnutrition
• Suspected cases of hypernatreamic dehydration
Rational use of antibiotics:
• Even though bacterial pathogens are the commonest cause of AD in developing
countries, there should be cautious and rational use of antibiotics to discourage
development of microbial resistance, avoid side effects and reduce cost
• Antibiotics should be used for:
– Severe invasive bacterial diarrhoea eg Shigellosis
– Cholera
– Girdiasis
– Suspected or proven sepsis
– Immunocompromised children

Zinc and diarrhoea:
• Zinc deficiency is common in developing countries and zinc is lost during diarrhoea
• Zinc deficiency is associated with impaired electrolyte and water absorption,
decreased brush border enzyme activity and impaired cellular and humoral immunity
• Treatment with zinc reduces the duration and severity of AD and also reduces the
frequency of further episodes during the subsequent 2-3 months
• WHO recommends that children from developing countries with diarrhoea be given
zinc for 10-14 days
– 10mg daily for children <6 months
– 20 mg daily for children >6 months
How can we prevent diarrhoeal disease?
This involves intervention at two levels:
• Primary prevention (to reduce disease transmission)
– Rotavirus and measles vaccines
– Handwashing with soap
– Providing adequate and safe drinking water
– Environmental sanitation
• Secondary prevention (to reduce disease severity)
– Promote breastfeeding
– Vitamin A supplementation
– Treatment of episodes of AD with zinc

Seminar2
: The edematous child
Oedema is a common presenting problem in paediatrics. It is defined as accumulation of
excess interstitial fluid and could be localised or generalised. Oedema results from either
excess salt and water retention of from increased transfer of fluid across the capillary
membranes. As such, it could be a presentation of mild conditions such as insect bite
reaction to more serious conditions such as glomerulonephritis, hepatic and cardiac
disease. Understanding the pathophysiology of oedema is important in the clinical
approach and management of this condition in children.
Contents
– Causes of oedema
– Clinical approach
– Investigations
– Management
CAUSES OF OEDEMA
Oedema results when there is:
1) Increased hydrostatic pressure
• acute nephritic syndrome, acute tubular necrosis, cardiac failure
2) Decreased plasma oncotic pressure (hypoproteinaemic states)
• nephrotic syndrome, chronic liver failure, protein losing enteropathy, protein
• caloric malnutrition
3) Increased capillary leakage
• insect bite, trauma, allergy, sepsis, angio-oedema
4) Impaired lymphatic flow
• lymphatic obstruction (tumour), congenital lymphoedema
5) Impaired venous flow
• hepatic venous outflow obstruction, superior/inferior vena cava obstruction
Mechanism of oedema formation in renal disease
Oedema formation between nephritic and nephrotic syndrome is markedly different.
Understanding oedema formation in these two conditions is important to differentiate the
two, as their management is entirely different
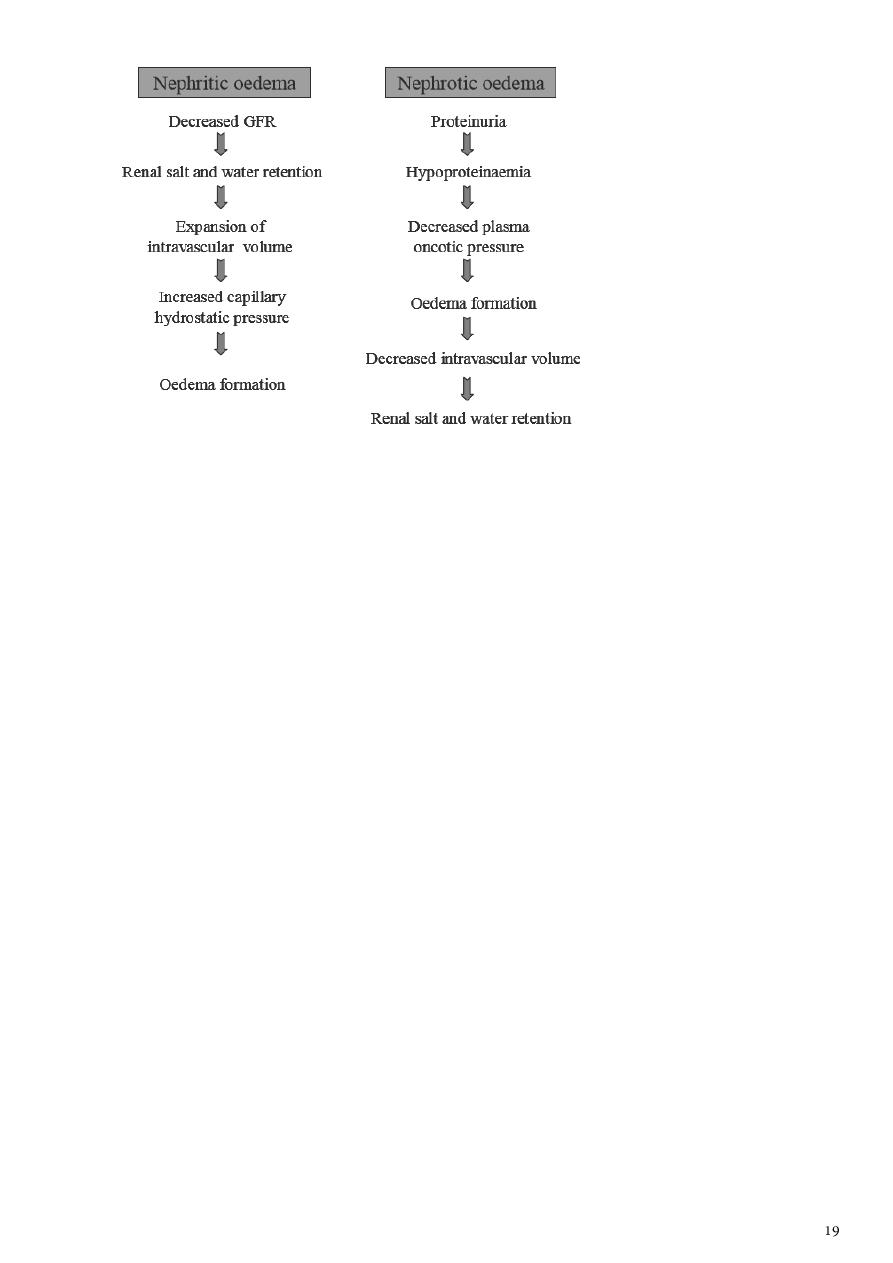
As such, a child with nephritic oedema will have symptoms and signs of
intravascular fluid overload such as orthopnoea, cardiomegaly, raised jugular venous
pressure, pulmonary congestion and hepatomegaly.
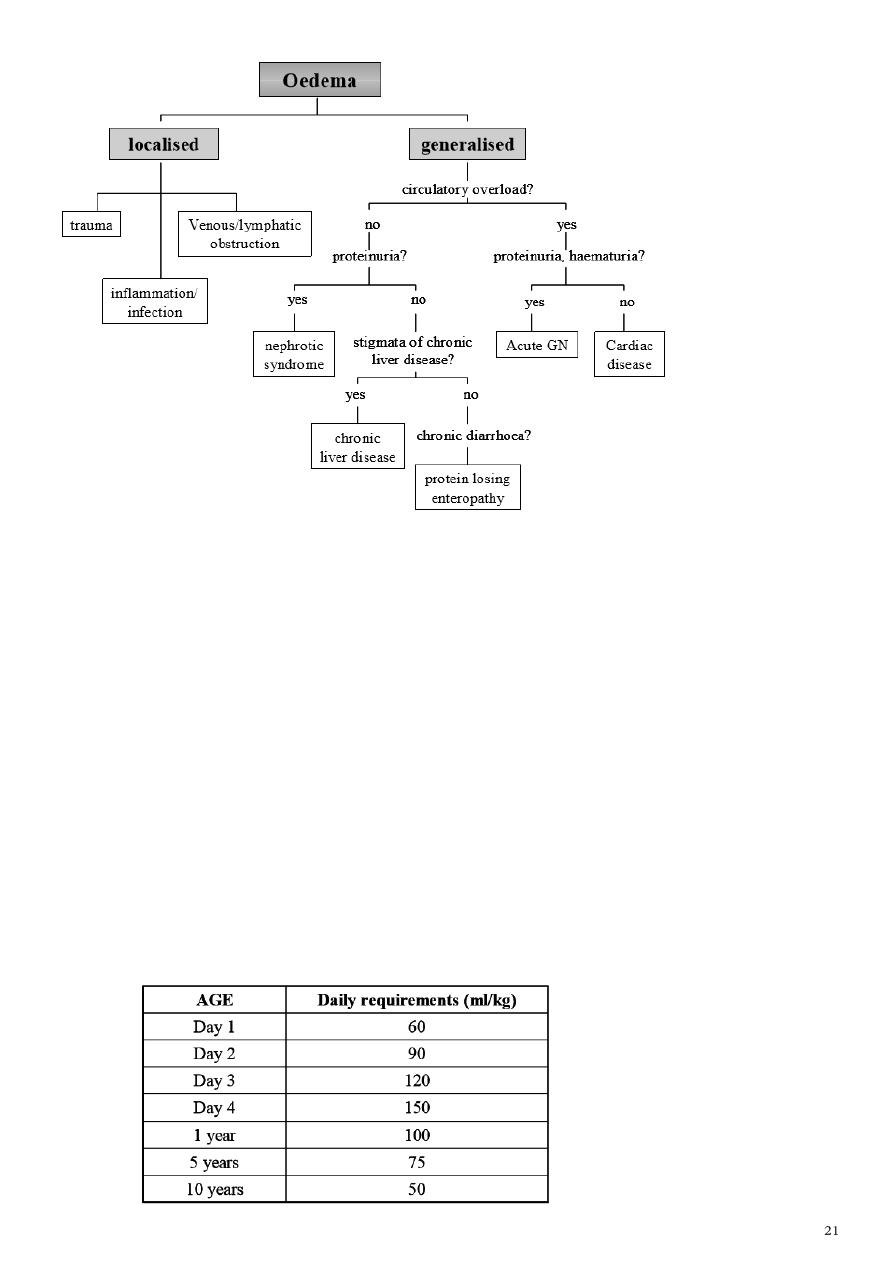
CLINICAL APPROACH TO AN OEDEMATOUS CHILD
1. Confirm oedema.
2. Assess distribution of oedema: Localised versus generalised. In generalised oedema, look
for dependent areas such as pretibial, sacral, scrotal, vulval oedema other than peri-orbital
oedema and ascites.
3. Detailed history and physical examination to assess severity, associated complications and
underlying cause of the oedema (algorithm . A) Localised oedema
i. History of trauma, insect bite or infection ii. Peripheral lymphoedema in newborn –
to exclude Turner’s syndrome iii. Acute oedema of the face and neck – to exclude superior
vena cava obstruction syndrome
B) Generalised oedema
i. Renal disease (most common cause in children) - gross haematuria, oliguria,
hypertension, cardiomegaly, pulmonary oedema to suggest acute glomerulonephritis. Frothy
urine suggests nephrotic syndrome. Absence of circulatory congestion differentiates
nephrotic syndrome from nephritic syndrome. - signs and symptoms of chronic renal
insufficiency such as anaemia, growth retardation and uraemic symptoms such as nausea and
vomiting - exclude secondary causes such as post-infectious glomerulonephritis, systemic
lupus erythematosus, Henoch Schonlein purpura nephritis.

ii. Liver disease - stigmata of chronic liver disease such as jaundice, palmar erythema,
clubbing, pruritic rash - hepatosplenomegaly with gross ascites in the absence of jaundice to
exclude portal vein thrombosis - previous operation scar such as Kasai porto-enterostomy
iii. Allergic reactions - oedema usually mild, commonly periorbital - history of
allergen exposure such as medications, animal dander, food preservatives and colouring -
associated rashes such as urticaria - assess for Stevens-Johnson reaction - if recurrent
oedema, consider C1 esterase deficiency
iv. Cardiac disease - symptoms of congestive cardiac failure such as decreased effort
tolerance, orthopnoea, paroxysmal nocturnal dyspnoea and signs such as cardiomegaly,
gallop rhythm, lung crepitations and turgid liver - assess for underlying cause such as
structural heart disease, cardiomyopathy and myocarditis Note: oedema in cardiac disease
often denotes a late sign in small children
v. Protein losing enteropathy - history of chronic diarrhoea, steatorrhoea and recurrent
abdominal pain - detailed dietary history for possible milk allergy and gluten
hypersensitivity - consider coeliac disease and inflammatory bowel disease in northern
Indians and Caucasians - assess for complications of anaemia, malnutrition and vitamin
deficiency states
4. Investigations
Basic investigations could be conducted at the general practice set-up to delineate the
major causes of oedema and assess its severity. They include:
a) Urine dipstick and microscopy - proteinuria, haematuria and casts are indicative of
renal disease
b) Renal function test - raised serum urea and creatinine are indicative of renal disease
c) Full blood count - normochromic normocytic anaemia suggest chronic disease -
hypochromic microcytic anaemia suggest iron deficiency from occult gastrointestinal
bleeding i.e. cows milk allergy - megaloblastic anaemia suggest vitamin B12 and folate
deficiency from small bowel disease
d) Liver function test - hypoalbuminaemia in the absence of circulatory overload
suggests hypoproteinaemic states - hyperbilirubinaemia and transaminitis suggest liver
disease
e) Chest X-ray and electrocardiogram - cardiomegaly with prominent perihilar
vascular markings/upper lobe diversion and left ventricular hypertrophy confirms
intravascular fluid overload
Further investigations are indicated upon specialist referral depending on the most
likely cause.
Algorithm 1: Clinical approach and assessment of an oedematous child
MANAGEMENT
Subsequent management of oedema is dependent on the primary cause and its
severity. What you can do
A) Localised oedema
Management
1. Insect bite reaction ------->Anti-histamine, anti-inflammatory
(topical steroid)
2. Local infection -------> Incision and drainage, wound dressing, antibiotics
B) Generalised oedema
General measures
a) Dietary management
sodium restriction to 2 grams/m2/day
fluid restriction to 2/3 maintenance depending on severity of oedema
b) Diuretic therapy
i. Loop diuretics
- Frusemide (Lasix)
1-5 mg/kg/dose 6-8 hourly (IV/IM/PO)
- Bumetanide (Burinex)
0.02-0.1 mg/kg/dose 12-24 hourly (PO) 0.1-0.2 mg/kg/dose 8-12 hourly (IV/IM)
Note: higher doses are required in acute glomerulonephritis or chronic renal
impairment
Side effects- hyponatremia, hypokalemia, metabolic alkalosis
ii. Hydrochlorothiazide
- 1 mg/kg/dose 12-24 hourly (PO)
iii. Spironolactone
- used in combination with loop diuretics/ thiazides for potassium sparing effects
0-10 kg: 6.25 mg/dose 12 hourly (PO)
11-20 kg: 12.5 mg/dose 12 hourly (PO)
21-40 kg: 25 mg/dose 12 hourly (PO)
> 40 kg: 25 mg/dose 8 hourly (PO)
SPECIFIC MANAGEMENT UPON SPECIALIST REFERREL

CONCLUSION
Oedema can be due to
increased hydrostatic pressure
decreased plasma oncotic pressure
increased capillary leakage
impaired lymphatic flow
impaired venous flow
This could be a manifestation of a mild or a serious medical conditions such as renal, hepatic
or cardiac diseases. Early recognition of oedema and prompt diagnosis of the underlying
cause is important. Initial assessment and stabilisation of patient should be instituted prior to
specialist referral.
An approach to a child with oedema
Oedema: accumulation excess interstitial fluid
Increased hydrostatic pressure
Acute nephritic syndrome
Congestive cardiac failure
Decreased plasma oncotic pressure
Protein calorie malnutrition, Nephrotic syndrome; protein loosing enteropathy
Increased capillary leakage
Allergy, sepsis, angiooedema.
Impaired venous flow
Vanacaval obstruction, hepatic vein obstruction
Impaired lymphatic flow
Congenital lymphedema, Wuchereria bancrofti infection
Examples for formulation of questions
Localized oedema
Insect bite; trauma; skin infections
Kwashiorkar (bilateral pedal)

Superior vanacaval obstruction
Lymphatic obstruction
Orthostatic
Generalized oedema
Renal: periorbital; hematuria; hypertension; symptoms of collagen disease
(rash, joint pain); frothy urine; symptoms of uraemia (vomiting, nausea, pallor),
convulsion, low urine output.
Cardiac: orthopnoea, joint pain; palpitation; giddiness; fainting episodes; bluish
episodes;
Protein energy malnutrition: low calorie and protein in the diet for long;
precipitating factors (persistent diarrhea, chronic illnesses)
Hepatic: Jaundice; ascites; prominent abdominal veins; neonatal umbilical sepsis;
spleenomegaly; purpura
Collagen diseases: fever, rash, joint pain, pallor
First case:
4 year old girl, who recently recovered from a sore
throat, was brought to the OPD with symptoms of
swelling of both feet. Physical examination reveals
edema around the eyes and the ankle. A routine
urinalysis reveals the following results.
Urine examination
Chemical/Physical Analysis Color:Yellow’ Blood:Moderate;Clarity:Hazy;pH:6.5
Glucose:Negative;Protein:300mg/dL;Ketones:Negative
Specific Gravity:1.015 ;Nitrite:Negative
Microscopic Analysis
20-50 RBC/hpf
10-20 WBC/hpf
2-5 RBC casts/hpf
2-5 Granular casts/hpf
What is the most likely diagnosis?
Second case
5 year male child
Swelling first noticed around eyes.
No history of shortness of breath; fever; cough; jaundice;
umbilical infection; no dark colored urine.
Height: 110cms; Wt: 18kg; liver not enlarged; Ascites
present
The most likely diagnosis is
Third case
12 year male from Pokhara; arrived after traveling by bus
for 12 hours.
History of fever
Upper abdominal pain
Dark colored urine
No past history of sore throat, rash, joint pain diarrhea,
trauma.
Comfortably lying flat in bed
Oral temp: 40C
Respiratory rate: 28.min
Bilateral pedal edema, non tender
Absence of Jaundice
Weight: 38 Kg.
Chest: normal
Abdomen: Tender R hypo. No free fluid
Normal blood count
Urine: routine normal
Liver function: normal
X-ray chest: normal
What causes we have excluded?
Increased hydrostatic pressure?
Decreased plasma oncotic pressure?
Increased capillary leakage?
Impaired venous flow?
Impaired lymphatic flow?
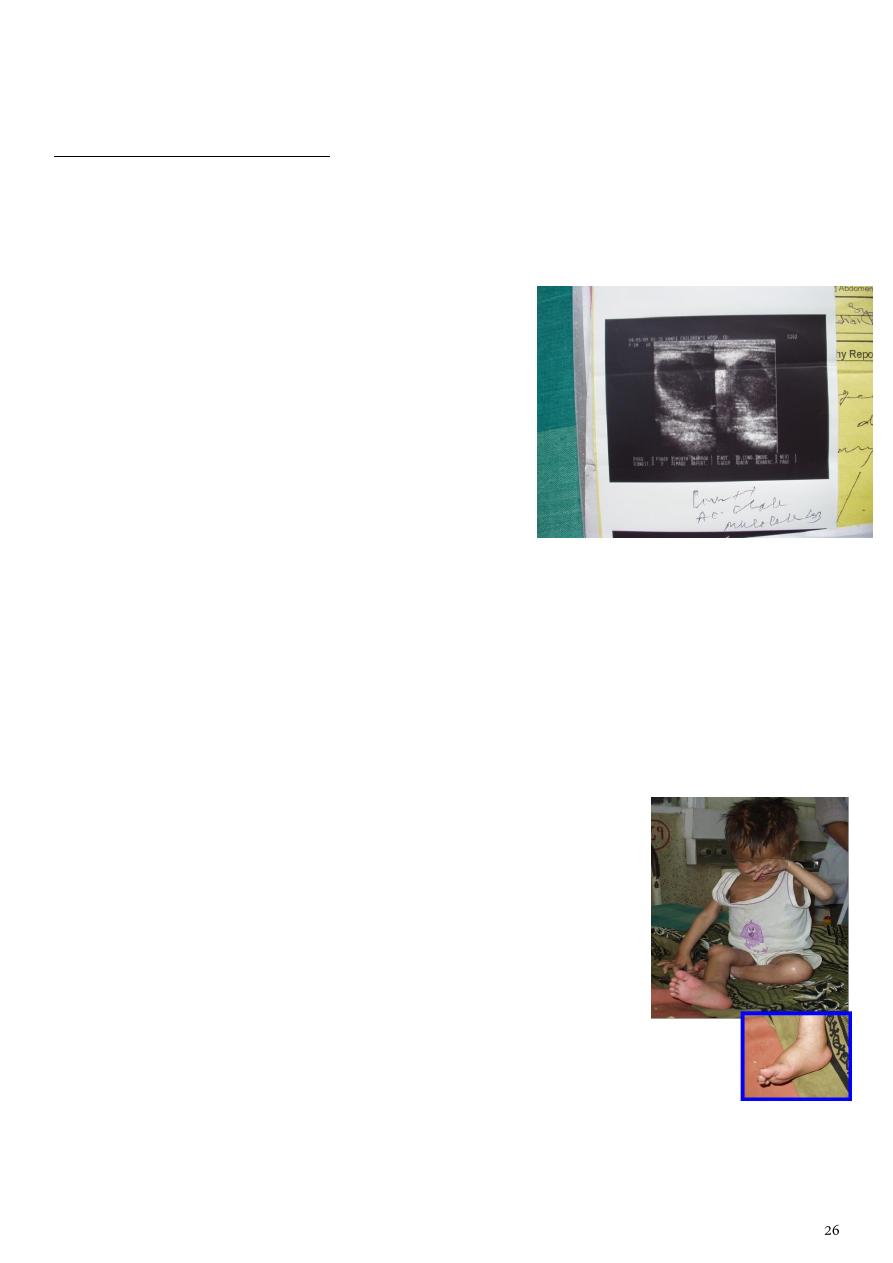
Bilateral edema and tender R hypochondrium.
Ultrasound of the abdomen:
Thickened Gall Bladder wall
Mucocoele
Third case :Final diagnosis and pathophysiology
Edema: increased hydrostatic pressure due to gravitational effect from prolonged leg
hanging.
R. Hypochondrium pain and fever: cholecystitis and mucocele of gall bladder
(ultrasound supported)
Edema subsided on the next day after admission.
Fourth case
5 year male child
Swelling started from limb : one month
No history of cough, shortness of breath, cyanosis, jaundice,
dark colored urine, umbilical infection.
Persistent diarrhea +.
Irritable; wt: 12 kg; Ht: 100cms. Serum protein: 1.5G/dL;
Urine normal
What is the diagnosis?

Seminar3
: Pyrexia of unknown origin
Children having fever >38.3 C prolonged of at least 8 days duration and initial 3-7 days of
hospital or outpatient; history and physical examination and basic investigations as
CBC,ESR, x ray,urine test,stool,test, within this 8 days, without reaching to the diagnosis for
the source or the cause of fever, are considered as having PUO. About 5-15% of cases with
fever considered as having PUO. PUO is usually caused by common problems but with
unusual presentations.
Aetiology;
The most common causes are
1.infections 30-40%
2. malignancy 20-30%,As Leukemia, Lymphoma, solid tumors, Willm and neuroblastoma,
bone tumors
3.connective tissue disorders 10-15% AS juvenile rheumatoid arthritis, SLE.
Miscellaneous causes;
l. drug fever
2.Kawasaki disease
3.HIV
4.Infamatory bowel disease
5. Factitious fever (MAUNCHAUSEN OF PROXY).
INFECTIONS INCLUDES
BACTEREMIA IN CHILDREN UNDER 2 YEAR
UTI, rare to be the cause as easy to diagnose it
INFECTIVE ENDOCARDITIS
CAT SCRACH DISEASE
TUBERCULOSIS
EB-VIRUS INFECTION

TYPHOID FEVER
BRUCELLOSIS
SARCOIDOSIS
TULEREMIA
TOXOPLASMOSIS
-------------------------------------------------------------------------------------------------------------common
suggestions of PUO or prolonged fever in children if not resolve within 3-5 days ,is to think
of EB VIRUS ,KAWASAKI DISEASE, OR UTI.
Approach for diagnosis of FUO OR PUO
Good history and physical examinations are critical components of evaluation of febrile
child and frequently result in detection of the underlying cause .
History
Fever characteristics as duration pattern, rigor, sweating, timing as at night in TB, and in
lymphoma . its response to antipyretics and after response; how is the child appears;
usually viral will be normal but in bacterial pt looks toxic and ill.
Rash
Rash may be present with fever and the study of the type of the rash as petechial or
maculopapular. Bacterial or viral infection may cause maculopapular or petechial or
urticarial rash or and its distribution may help us in diagnosis. Palpable purpura may be
seen in vasculitis. Vesicles in viral infections.
Accompanying symptomatology;
Inflammatory or malignancy or vasculitis may result in multisystemic involvement such as
cough and shortness of breath due to to carditis, pleuritis.GI symptoms CNS symptoms and
joint features .
Arthralgia in joint septic infection and in leukemia and in rheumatoid arthritis, lyme disease,
Bone pain in leukemia and SLE .
GI symptoms as in Crohn disease and ulcerative colitis.
CNS features as confusion and seizures as in bacterial endocarditis, cerebral abscess,
tuberculous meningitis.
Urinary symptoms as frequency and dysuria.

Poor growth and anorexia as in chronic disease opposing bacterial infection, as acute state.
Immunization status .
Drug history
Past history; as sickle cell disease increase possibility of infection.
Exposure to causative agent as travel to malarial area or contact typhoid fever
or TB patient. Or exposed to a cat scrach.
Psychological history ;incase of factitious fever mother may create the fever as by heating
the thermometer, or give some drugs as she want to stay in the hospital.
Physical examination
Confirm the temperature
Toxic child is more likely to have bacterial infection. Most well appearing children do not
have bacterial infection
BP,PULSE RATE ,RR,ARE CRUCIAL FOR THE STASTUS OF THE PATIENT.
COMPLETE PHYSICAL EXAMINATION FOR LYMPHNODES AND APPEARANCE AND PALLOR
AND SKIN AND NAIL AND ORGANOMEGALY AND ABDOMEN ,CVS,RESPIRATORY
S,CNS,LOCOMOTOR SYSTEM., WILL NARROW THE LIMIT OF POSSIBLE CAUSES FOR THE
FEVER.
INVESTIGATIONS
More extensive lab investigations are needed like blood culture,liver function ,kidney
function test,thyroid function as in hyperthyroidism, ,serology for viral infections or
bacterial,CTscan of abdomen or brain or the chest or pelvis, Us STUDY AND CXR. Etc…
------------------------------------------------------------------------------------
Most of the cases finally can be diagnosed for the cause of fever, but still few cases may not
find the cause and the child remits with time called ;( Fever without source ) FWS.
------------------------------------------------------------------------------------

Seminar4
: Vaccination
The immune system is a complex network of specialized organs and cells protects the body
from destruction by foreign agents and microbial pathogens , degrades and removes
damaged or dead cells, and exerts a surveillance function to prevent the development and
growth of malignant cells. The immune system is composed of immune cells and central and
peripheral lymphoid structures. The immune cells move throughout the body, searching for
and destroying foreign substances but avoiding cells regarded as self.
Natural immunity:
It is not produced by the immune response. This type of immunity is present at birth and
appears to be present in all members of a species.
Acquired immunity:
It develops after birth as a result of exposure to an antigen, thereby activating the immune
response. Acquired immunity can be either active or passive, depending on whether the
immune response took place in the host or a donor.
Differences of immune system of children and adult
The normal human no fully active immune system at birth because of immaturity. It relies
instead on passively transferred antibodies from the mother. This maternal antibody slowly
decreases in concentration and for all practical purposes, has waned by 1 year.
The infant own production of antibody begins to be meaningful at 7 or 8 months of age when
the total of maternal and infant antibody is low. One has waned and the other is not up to
full strength. This is age when many of the infectious disease processes of infancy begin /e.g.
otitis media, pneumonia.
Vaccination
Administration of a substance to a person with the purpose of preventing a disease
Traditionally composed of a killed or weakened microorganism
Vaccination works by creating a type of immune response that enables the memory cells
to later respond to a similar organism before it can cause disease

Early History of Vaccination
Pioneered India and China in the 17th century
The tradition of vaccination may have originated in India in AD 1000
Powdered scabs from people infected with smallpox was used to protect against the
disease
Smallpox was responsible for 8 to 20% of all deaths in several European countries in the
18th century
In 1721 Lady Mary Wortley Montagu brought the knowledge of these techniques from
Constantinople (now Istanbul) to England
Two to three percent of the smallpox vaccinees, however, died from the vaccination
itself
Benjamin Jesty and, later, Edward Jenner could show that vaccination with the less
dangerous cowpox could protect against infection with smallpox
The word vaccination, which is derived from vacca, the Latin word for cow.
Era of Vaccination
English physician Edward Jenner
observed that milkmaids stricken with a viral disease called cowpox were rarely victims
of a similar disease, smallpox
Jenner took a few drops of fluid from a pustule of a woman who had cowpox and
injected the fluid into a healthy young boy who had never had cowpox or smallpox
Six weeks later, Jenner injected the boy with fluid from a smallpox pustule, but the boy
remained free of the dreaded smallpox.
In those days, a million people died from smallpox each year in Europe alone, most of
them children.
Those who survived were often left with blindness, deep scars, and deformities
In 1796, Jenner started on a course that would ease the suffering of people around the
world for centuries to come.
By 1980, an updated version of Jenner vaccine lead to the total eradication of smallpox.
Early History of Vaccination
In 1879 Louis Pasteur showed that chicken cholera weakened by growing it in the
laboratory could protect against infection with more virulent strains
1881 he showed in a public experiment at Pouilly-Le-Fort that his anthrax vaccine was
efficient in protecting sheep, a goat, and cows.

In 1885 Pasteur developed a vaccine against rabies based on a live attenuated virus
A year later Edmund Salmon and Theobald Smith developed a (heat) killed cholera
vaccine.
Over the next 20 years killed typhoid and plague vaccines were developed
In 1927 the bacille Calmette-Guérin (BCG vaccine) against tuberculosis vere developed
Since Jenner's time, vaccines have been developed against more than 20 infectious diseases
•
The date of introduction of first generation of vaccines for use in humans*
–
1798 Smallpox
–
1885 Rabies
–
1897 Plague
–
1923 Diphtheria
–
1926 Pertussis
–
1927 Tuberculosis (BCG)
–
1927 Tetanus
–
1935 Yellow Fever
•
After World War II
–
1955 Injectable Polio Vaccine (IPV)
–
1962 Oral Polio Vaccine (OPV)
–
1964 Measles
–
1967 Mumps
–
1970 Rubella
–
1981 Hepatitis B
Vaccination Today
Vaccines have been made for only 34 of the more than 400 known pathogens that are
harmful to man.
Immunization saves the lives of 3 million children each year, but that 2 million more lives
could be saved if existing vaccines were applied on a full-scale worldwide
Human Vaccines against pathogens


Type of Vaccination
Live Vaccines
• Characteristics
• Able to replicate in the host
• Attenuated (weakened) so they do not cause disease
• Advantages
• Induce a broad immune response (cellular and humoral)
• Low doses of vaccine are normally sufficient
• Long-lasting protection are often induced
• Disadvantages
• May cause adverse reactions
• May be transmitted from person to person
Subunit Vaccines
• Relatively easy to produce (not live)
• Classically produced by inactivating a whole virus or bacterium
• Heat
• Chemicals
• The vaccine may be purified
• Selecting one or a few proteins which confer protection
• Bordetella pertussis (whooping cough)
• Create a better-tolerated vaccine that is free from whole microorganism cells
Subunit Vaccines: Polysaccharides
• Polysaccharides
• Many bacteria have polysaccharides in their outer membrane

• Polysaccharide based vaccines
• Neisseria meningitidis
• Streptococcus pneumoniae
• Generate a T cell-independent response
• Inefficient in children younger than 2 years old
• Overcome by conjugating the polysaccharides to peptides
• Used in vaccines against Streptococcus pneumoniae and
Haemophilus influenzae.
Subunit Vaccines: Toxoids
• Toxins
• Responsible for the pathogenesis of many bacteria
• Toxoids
• Inactivated toxins
• Toxoid based vaccines
• Bordetella pertussis
• Clostridium tetani
• Corynebacterium diphtheriae
• Inactivation
• Traditionally done by chemical means
• Altering the DNA sequences important to toxicity
Subunit Vaccines: Recombinant
• The hepatitis B virus (HBV) vaccine
• Originally based on the surface antigen purified from the blood of chronically
infected individuals.

• Due to safety concerns, the HBV vaccine became the first to be produced using
recombinant DNA technology (1986)
• Produced in bakers’ yeast (Saccharomyces cerevisiae)
• Virus-like particles (VLPs)
• Viral proteins that self-assemble to particles with the same size as the native
virus.
• VLP is the basis of a promising new vaccine against human papilloma virus (HPV)
• Merck
• In phase III
Genetic Vaccines
• Introduce DNA or RNA into the host
• Injected (Naked)
• Coated on gold particles
• Carried by viruses
• vaccinia, adenovirus, or alphaviruses
• bacteria such as
• Salmonella typhi, Mycobacterium tuberculosis
• Advantages
• Easy to produce
• Induce cellular response
• Disadvantages
• Low response in 1st generation
Type of Vaccination
Live attenuated Vaccine
OPV

Measles
Rubella
Mumps
BCG
Varicella Vaccine
Inactivated organism or their products
Diphtheria
Tetanus
Pertussis( whole cell/acellular)
Hepatits Avaccine
Hepatitis B
Pneumococcal Polysaccharide vaccine
Influenza
IPV
Hib
Passive Immunity
Transfer of antibody produced by one human or other animal to another
Transplacental most important source in infancy
Temporary protection
Sources of Passive Immunity
Almost all blood or blood products
Homologous pooled human antibody (immune globulin)
Homologous human hyperimmune globulin
Heterologous hyperimmune serum (antitoxin)
IMMUNOGLOBULIN PREPARETION
Normal human Ig.

Normal human Ig is an antibody-rich fraction, obtained from a pool of at least 1000 donors.
The preparation should contain at least 90% intact IgG; it should be as free as possible from
IgG aggregates; all IgG sub-classes should be present; there should be a low IgA
concentration; the level of antibody against at least two bacterial species and two viruses
should be ascertained
Normal human Ig used to prevent measles in highly susceptible individuals and to provide
temporary protection /up to 12 weeks/ against hepatitis A infection.
Live vaccines should not normally be given for 12 weeks after an injection of normal human
Ig.
· Specific human Ig.
These preparations are made from the plasma of patient who have recently recovered from
an infection or are obtained from individuals who have been immunized against a specific
infection.
The advantages of Ig-s are:
1. freedom from hepatitis B
2. concentration of the antibodies into a small volume for intramuscular use.
3. stable antibody content, if properly stored.
Vaccine Preventable Diseases
An infectious disease for which an effective preventive vaccine exists.
If a person dies from it, the death is considered a vaccine-preventable death.
FULLY IMMUNIZED CHILD
A child who received
One dose of BCG,
Three doses of DPT and OPV,
One dose of measles
before one year of age.

This gives a child the best chance for survival.
Control
Reduction of prevalence or incidence of disease to lower acceptable level.
Elimination
Eradication of disease from a large geographic region or political jurisdiction
Either reduction of infectious disease’s prevalence in regional population to zero or
reduction of global prevalence to a negligible amount.
Eradication
Termination of all transmissions of infection by extermination of infectious agent
through surveillance and containment.
Reduction of infectious disease’s prevalence in global host population to zero.
EXPANDED PROGRAMME ON IMMUNISATION (EPI)
EPI launched in 1974
Build on smallpox infrastructure
Targeted 6 diseases
EPI progressively adopted by all countries
Universal by early 1098s
Addition to EPI
Yellow fever in 1988
• For endemic countries only : 33 in Africa, 11 in S. America.
• Given with measles vaccine
Hepatitis B in 1992
• In high seroprevalence countries by 1995
• In all countries by 1997
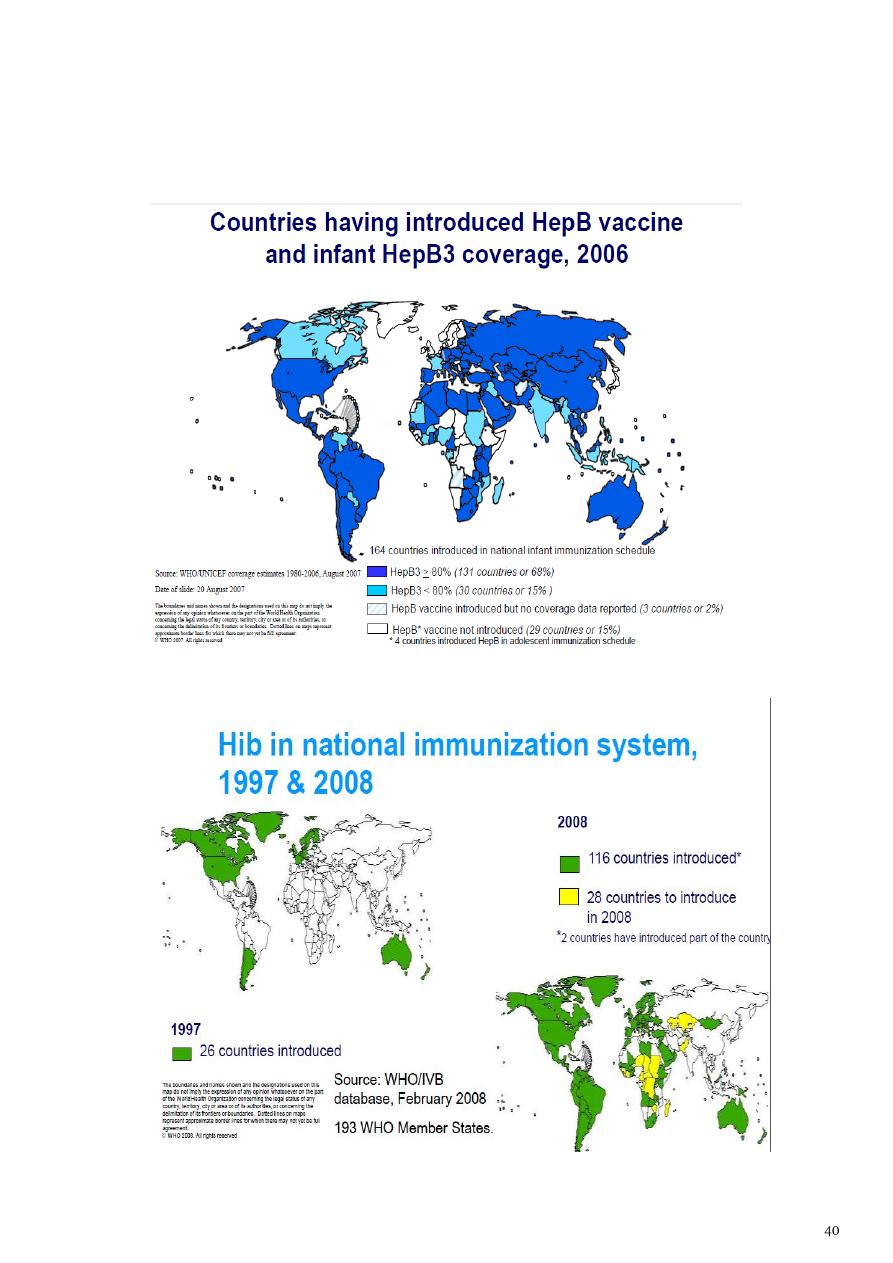
Haemophilus influenzae type b (Hib)
• 1998 : based on disease burden and capacity
• 2006 : all countries. ( lack of data should not be obstacle)

COMPONENTS of program
1. Immunization of pregnant women against tetanus.
2. Immunization of children in their first year of life against 6 VPDs.
3) Immunisation in preterm infants
All vaccines except Hepatitis B
If BW < 2Kg & mother HBsAg negative :- postpone till baby attaines 2kg wt or 2 mths
of age.
If BW < 2Kg & mother HBsAg positive :- give vaccine + immunoglobulin.
4) Children receiving corticosteroids
Children receiving corticosteroids at the dose of 2 mg/kg/day for more than 14 days
should not receive live virus vaccines until steroid has been discontinued for at least
1 month.
Tetanus toxoid
Intramuscular – upper arm – 0.5 ml
Pregnancy – 2 doses - 1
st
dose as early as possible and second dose after 4 weeks of
first dose and before 36 weeks of pregnancy

Pregnancy – booster dose (before 36 weeks of pregnancy) – If received 2 TT doses in
a pregnancy within last three years. Give TT to woman in labour, if she has not
received TT previously
TT booster for both boys and girls at 10 years and 16 years
No TT required between two doses in case of injury
BCG
At birth or as early as possible till one year of age
0.1 ml (0.05ml until one month of age)
Intra-dermal
Left upper arm
Hepatitis B
Birth dose – within 24 hours of birth
0.5 ml
Intramuscular
Antero-lateral side of mid-thigh
Rest three doses at 6 weeks, 10 weeks and 14 weeks
OPV
Zero dose – within first 15 days of birth
2 drops
Oral
First, second and third doses at 6, 10 and 14 weeks with DPT-1, 2 and 3
OPV booster with DPT booster at 16-24 months
DPT
Three primary doses at 6, 10 and 14 weeks with OPV-1, 2 and 3
0.5 ml
Intra-muscular

Antero-lateral side of mid-thigh
One booster at 16-24 m with OPV booster (antero-lateral side of mid-thigh) and
second booster at 5-6 years (upper arm)
Measles
At 9 completed months to 12 months
Give up to 5 years if not received at 9-12 months age
Second dose at 16-24 months (select states after catch-up campaign) – Measles
Containing Vaccine
0.5 ml
Sub-cutaneous
Right upper arm
Along with Vitamin A (1
st
dose) – 1ml (1 lakh IU) - oral
Constraints
Illiteracy
Non uniform coverage
Poor implementation
Poor monitoring
High drop outs
Declining coverage in some major states
Over reporting
Poor injection safety
Reorientation of staff being not carried out
Vacany of staff at field level not filled
Poor surveillance of vaccine preventable diseases
Poor vaccine logistics
Poor maintainance of equipments
Extra ordinary emphasis on polio vaccine

Route of Administration
Site of Administration
Who should not be vaccinated?
Allergy
Fever
HIV infection
Immunodeficiency
IG administration
Neurological disorder
Prematurity
Reactions to Previous vaccine
Simultaneous administration of Vaccines
Thrombocytopenia

Allergy
A. Allergic Reactions to Egg-related antigens
1. Yellow fever and influenza vaccines do contain egg proteins and rarely induce
immediate allergic reactions. Skin testing is recommended before
administration with an history of allergic to egg
2. MMR- Even those with severe hypersensitivity are at low risk of anaphylaxis.
B. Antibiotic-induced allergic reaction
Delayed type local reaction 48-96 hours afterwards and is usually minor
IPV and OPV – streptomycin, neomycin and polymyxin B
MMR and varicella vaccine-neomycin
C. Gelatin- MMR, Varicella vaccine
Fever
Low-grade fever or mild illness is not a contraindication for vaccination
Children with moderate or severe febrile illnesses can be vaccinated as soon as they
are recovering and no longer acutely ill
Vaccination in Pregnancy
Risk to a developing fetus from vaccination of the mother during pregnancy is mostly
theoretical
Only smallpox vaccine has ever been shown to injure a fetus
The benefits of vaccinating usually outweigh potential risks
Inactivated vaccines
– Routine (influenza)
– Vaccinate if indicated (hep B, Td, mening, rabies)
– Vaccinate if benefit outweighs risk (all other)
– Live vaccine – do not administer
– Exception is yellow fever vaccine
HIV Infection
No BCG
OPV is Contraindicated
– in household contact, in recipient ( asymptomatic or symptomatic)

– IPV for these children and household contacts
MMR vaccination should be considered for all asymptomatic and to all symptomatic
HIV-infected persons who do not have evidence of severe immunosuprresion or
measles immunity
Pneumococcal vaccine, Hib, DTP (or DTaP), Hepatitis B vaccine, Influenza vaccines are
all indicated
Immunosupression
No live viral vaccines and BCG. IPV for these patients, their siblings and their
household contacts
No live vaccine (except varicella) until six months after immunosuppressive therapy
Neurological disorder
Progressive developmental delay or changing neurological findings (e.g. infantile
spasm) - defer pertussis immunization
Personal history of convulsions
Recent seizures - defer pertussis immunization
Conditions predisposing to seizures or neurological deterioration (e.g. tuberous
sclerosis) - defer pertussis immunization
Reactions
Severe Reactions to DTP
– Insonable cry lasting more than 3 hrs with 48 hrs of dose
– Seizure with 3 days
– Severe local reactions
– Family hx of adverse event
Not a contraindication, but consider carefully the benefits and risks, if need to
vaccinate can use acellular DTP for less reactions
GBS with 6 weeks after a dose of DTP
– Again based on risks and benefits for further dose of DTP and risk of GBS
recurrence.
Contraindication for further dose of DTP
– encephalopathy within 7 days of a dose of DTP

VACCINE REACTIONS
Common, minor reactions
– vaccine stimulates immune system
– settle on their own
– warn parents and advise how to manage
Rare, more serious reactions
– anaphylaxis (serious allergic reaction)
–
vaccine specific reactions
RARE, MORE SERIOUS REACTIONS
Simutaneous administration of Vaccine
A theoretical risk that administration of multiple live virus vaccine: OPV, MMR, and
varicella ) within 28 days of one another if not given on the same day will result in a
sub optimal immune response
No data to substantiate this
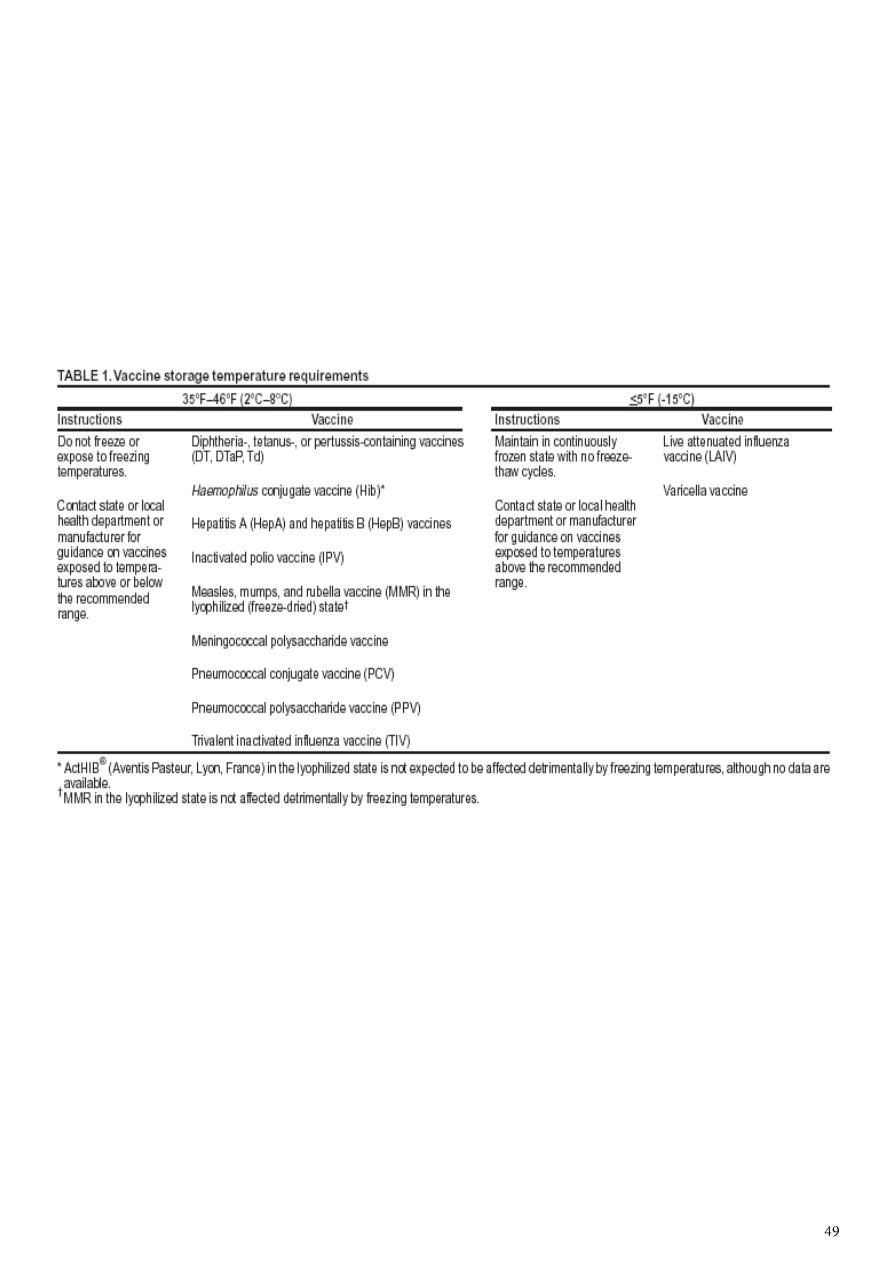
Vaccine Cold Chain
Maintaining proper vaccine temperatures during storage and handling to preserve
potency
The success of efforts against vaccine-preventable diseases is attributable in part to
proper storage and handling of vaccines.
Exposure of vaccines to temperatures outside the recommended ranges can affect
potency adversely, thereby reducing protection from vaccine-preventable diseases
Recommended Storage Temperatures
Table 5: Vaccination Schedule for Infants and Children 2012
Age Type of vaccine
0
-1 Week OPV0 dose , HepB1 , BCG
2 Months OPV1 , PENTA1,ROTA1
4 Months OPV2 , TETRA1,ROTA2
6 Months OPV3 , PENTA2,ROTA3
9 Months Measles + VIT A
15 Months MMR (Measles , Mumps , Rubella)
18 Months TETRA2, OPV First Booster dose + VIT A
4-6 Years DPT , OPV Second Booster dose + MMR2
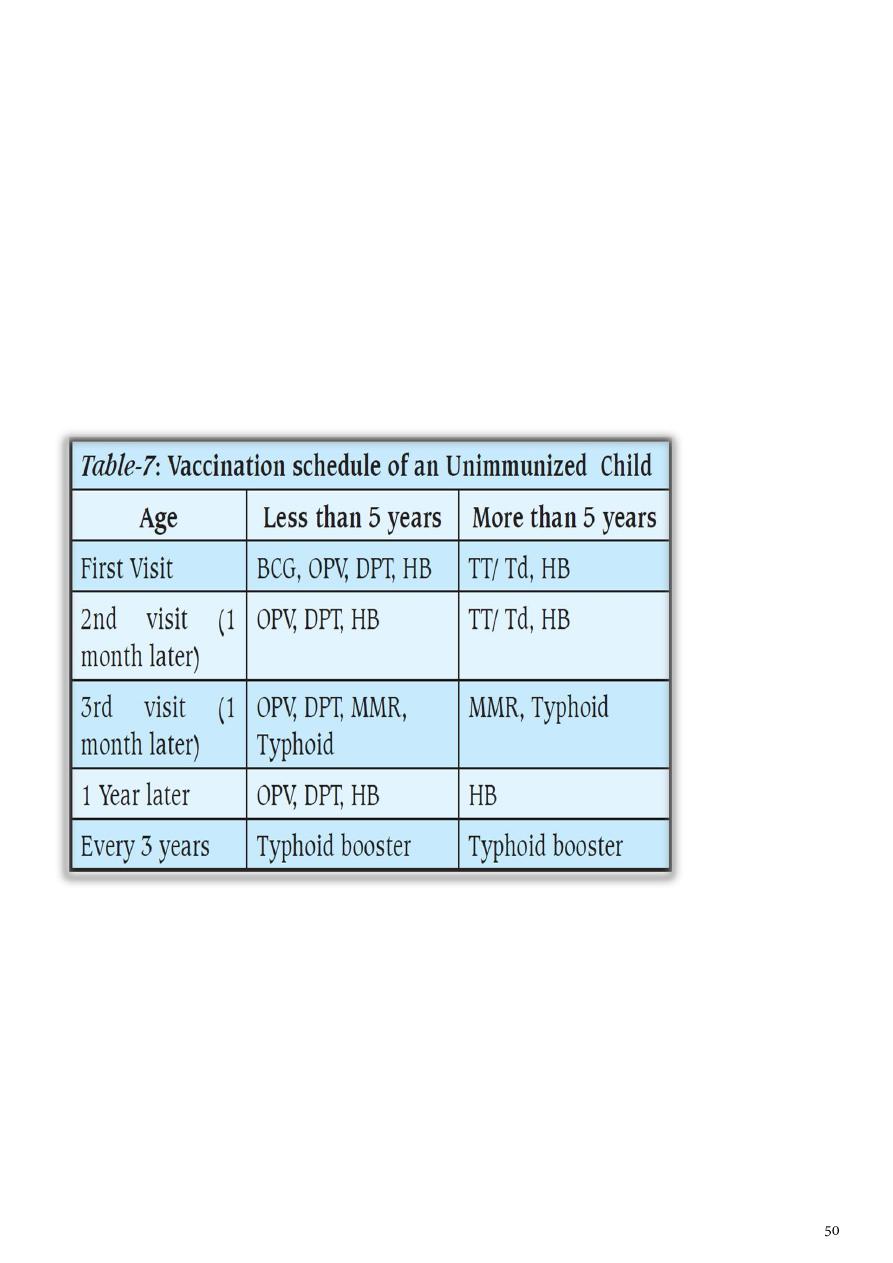
Table 6: National Immunization Schedule for Infants and Children 2015
Age Type of vaccine
0-1 Week HepB1 , BCG + OPV0dose
2 Months HEXA 1,ROTA1 ,PREV13-1+OPV1
4 Months HEXA2,ROTA2,PREV13-2 + OPV2
6 Months HEXA3,ROTA3,PREV13-3 + OPV3
9 Months Measles + VIT A
15 Months MMR(Measles , Mumps , Rubella)
18 Months PENTA (DTP+IPV+Hib ) OPV + VIT A
4-6 Years TETRA (DTaP +IVP ) + OPV + MMR

Seminar5
: Acute respiratory infection
CLASSIFICATION
CHILD AGED 2 MONTHS TO 5 YEARS
Classifying the illness means making decisions about the type and severity of disease. The
sick child should be put into one of the four classifications:
VERY SEVERE DISEASE
SEVERE PNEUMONIA
PNEUMONIA(not severe)
NO PNEUMONIA
1. VERY SEVERE DISEASE
The danger signs and possible causes are:
a) Not able to drink: A child who is not able to drink could have severe pneumonia or
bronchiolotis, septicaemia, throat abscess, meningitis or cerebral malaria.

b) Convulsions, abnormally sleepy or difficult to wake: A child with these signs may have
severe pneumonia resulting in hypoxia, sepsis, cerebral malaria or meningitis. Meningitis
can develop as a complication of pneumonia or it can occur on its own.
c) Stridor in calm child: If a child has stridor when calm, the child may be in danger of life
threatening obstruction of the air-way from swelling of larynx, trachea or epiglottis.
d) Severe malnutrition: A severely malnourished child is at high risk of developing and dying
from pneumonia. In addition, the child may not show typical signs of the illness.
2. SEVERE PNEUMONIA
The most important signs to consider when deciding if the child has pneumonia are the
child’s respiratory rate, and whether or not there is chest indrawing may not have fast
breathing if the child becomes exhausted, and if the efforts needed to expand the lungs is
too great.
In such cases , chest indrawing maybe the only sign in a child with severe pneumonia. A
child with chest indrawing is at higher risk of pneumonia than a child with fast breathing
alone.
A child classified as having severe pneumonia also has other signs such as;
. Nasal flaring, when the nose widens as the child breaths in
. Grunting, the short sounds made with the voice when the child has difficulty in breathing
and
. Cyanosis, a dark bluish or purplish coloration of the skin caused by hypoxia
Children who have chest indrawing and a first episode of wheezing often have severe
pneumonia, however with recurrent wheezing do not have severe pnuemonia.
3. Pneumonia (not severe)
A child who has fast breathing and no chest indrawing is classified as having pneumonia(not
severe). Most children are classified in this category if they are brought early for treatment.
Fever , cough, tachpnoea, crackles , signs of consolidation , and constitutional symptoms
are the general clinical features seen in a patient suffering with pneumonia.
4. No pneumonia: cough or cold
Most children with a cough or difficult breathing do not have any danger signs
or signs of pneumonia ( chest indrawing or fast breathing). These children have
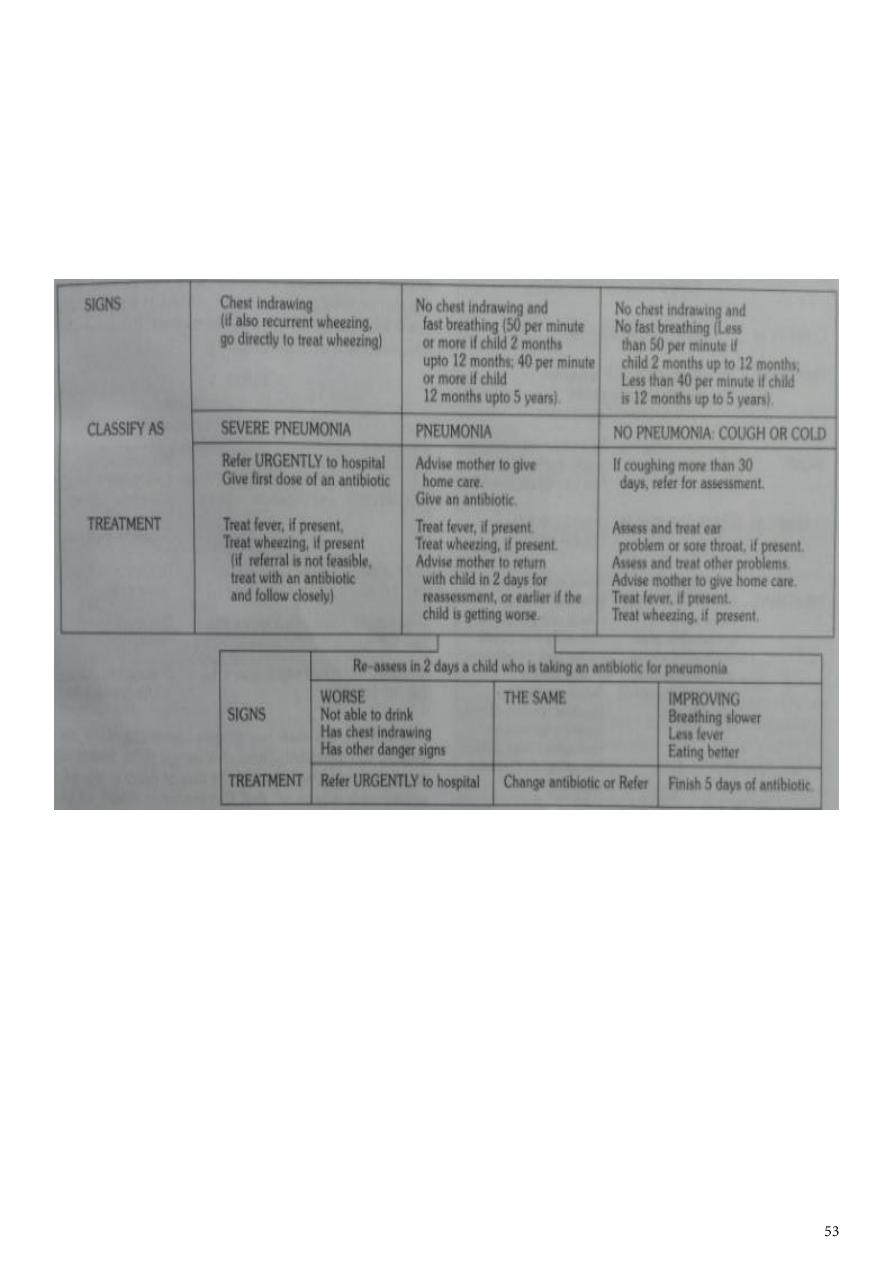
a simple cough or cold. They are classified as having ‘no pneumonia: cough or
cold’. They do not need any antibiotic. Majority of such cases are viral infections
where antibiotics are not effective. Normally a child with cold will get better
within 1-2 weeks.
MANAGEMENT OF PNEUMONIA IN A CHILD AGED 2 MONTHS UPTO 5 YEARS
Classifying illness of young infant
Infants less than 2 months of age are referred to as young infants. They have special
characteristics that must be considered when their illness is classified. They can become
sick and die very quickly from bacterial infections, are much less likely to cough with
pneumonia, and frequently have only non specific signs such as poor feeding, fever or
hypothermia.
1) Further mild chest indrawing is normal in young infants because their chest wall bones
are soft. The presence of these characteristics means that they will be classified and treated
differently from older children.

2) Many of the cases may have added risk factor of low birth weight. Such children are very
susceptible to temperature changes and even in tropical climates, death due to cold stress
or hypothermia are common.
3) In young infants the cut off point for fast breathing is 60 breaths per minute. Any
pneumonia in young infants is considered to be severe.
Some of the danger signs of very severe disease are:
a. convulsions, abnormally sleepy or difficult to wake: a young infant with
these signs may have hypoxia from pneumonia, sepsis or meningitis.
Malaria infection is unusual in children of this age, so antimalarial
treatment is not advised.
b. Stridor when calm: infections causing stridor viz diphtheria, bacterial
tracheitis, measles or epiglottitis are rare in young infants. A young infant
who has stridor should be classified as having very severe disease.
c. Stopped feeding well : a young infant who stops feeding well(i.e takes less
than half of the usual amount of milk) may have a serious infection and
should be classified as having very severe disease.
d. wheezing: it is uncommon in infants and is usually associated with
hypoxia.
e. Fever or low body temperature: fever(38 degree or more) is uncommon in
young infants and more often means a serious bacterial infection. It may
be the only sign of serious bacterial infection. Sometimes infection can
cause hypothermia.

CLASSIFICATION AND MANAGEMENT OF ILLNESS IN YOUNG INFANTS
Treatment
A. TREATMENT FOR CHILDREN AGED 2 MONTHS UPTO 5 YEARS
.PNEUMONIA (child with cough and fast breathing)
cotrimoxazole is the drug of choice for the treatment of pneumonia. Cure rates are 95%. It
is less expensive with few side effects and can be used safely by health workers at the
peripheral health facilities and at home by the mothers.
The condition of the child should be assessed after 48 hours.
Cotrimoxazole should be continued for another 3 days in children who show
improvement in clinical condition.
If there is no improvement in the condition then it should be continued for 48 hours
and then reassesed.
If at 48 hours or earlier the condition worsens, the child should be hospitalised
immediately.

Severe pneumonia (chest indrawing)
Children with severe pneumonia should be treated as inpatients with intramuscular
injections of benzylpenicilline(after test dose) ampicillin or chloramphenicol.
The condition of the child must be monitored everyday and reviewed after 48hrs for
antibiotic therapy.
Antibiotic therapy must be given for a minimum of 5 days and continued for atleast 3 days
after the child gets well.
Very severe disease
-Children with signs of very severe disease are in imminent danger of death and should be
treated in a health facility, with provision of oxygen therapy and intensive monitoring.
-Chloramphenicol IM is the doc in all such cases. Treat for 48 hrs ,if conditons improve
switch over to oral chloramphenicol. It should be given for a total of 10 days.
-If condition worsens or does not improve after 48hrs switch to IM injections of cloxacillin
and gentamicin.
B. PNEUMONIA IN YOUNG INFANTS UNDER 2 MONTHS OF AGE
The treatment in these condition is basically the same.
1.The child must be hospitaised.
2.Treatment with cotrimoxazole maybe started by the health worker before referring the
child.
3. If pneumonia is suspected in the child should be treated with IM injection of
bezylpenicilline or ampicillin, along with injection gentamycin.
Besides antibiotics, therapy for the associated conditions if any, must be instituted
immediately. The child must be kept warm and dry. Breast feeding must be promoted
strongly as the child who is not breast fed is at a much higher risk of diarrhoea.
Management of AURI (no pneumonia)
-> Many children with presenting symptoms of cough, cold and fever do not have
pnenumonia and do not require treatment with antibiotics.
->They are not recommended as majority of cases are caused by viruses and antibiotics are
not effective, they increase resistant strains and cause side effects while providing no
clinical benefit, and are wasteful expenditure.
->Symptomatic treatment and care at home is generally enough for such cases.

Seminar6
: ARI control and prevention
ARI control
Improving the primary medical care services and developing better methods for early
detection , treatment and prevention of acute respiratory infection is the best way to
control ARI
mortality rate due to pneumonia is reduced if treated correctly
Education of mothers about pneumonia because compliance with treatment and
seeking proper care when child suffers determine outcome of the disease
WHO recommendation for management of ARI
Clinical assesment
History taking and management are very important
1)age
2)feeding habits
3)fever
4)convulsions
5)irregular breathing
6)history of treatment during the illness
7)activity
Physical examination
1:count the breaths in one minute
Breathing count depends on the age of the child
Count respiratory rate for a minute
Fast breathing is present when RR is
-60 breaths /min or more in a child less than two months of age
-50/min or more in child aged 2months upto 12 months
-40 breaths/min or more in a child aged 12 months upto 5 years

Chest indrawing
Look for chest indrawing when child breaths IN
Child has indrawing if the lower chest wall goes in when the child breaths IN
Occurs when the effort required to breath in ,is much greater than normal
Stridor
Harsh noise while breathing IN is stridor
Occurs due to narrowing of trachea ,larynx or epiglottis
These conditions often called croup
Wheeze
A child with wheeze makes a soft whistling noise
OR
shows signs that breathing OUT is difficult
This is due to narrowing of the air passages
Fever
Check for body temperature
Cyanosis
Sign of hypoxia
Malnutrition
If malnutrition is present its high risk and case fatality rates are higher
In severely malnourished:
1) children with pneumonia, fast breathing and chest indrawing may not be evident
2)Impaired or absent response to hypoxia and a weak or absent cough reflex
3)Careful evaluation and mangement
ARI control programmes
ARI control in children
• ARI is an episode of acute symptoms & signs resulting from infection of
any part of respiratory tract & related structures
• Constitutes 22-66% of outpatients & 12-45% of inpatients

• The aim of the program is to identify children with ARI at the community
level by training the field workers to recognize easily & reliably identifiable
clinical signs of ARI & early reference
WHO protocol comprises 3 steps:
1. Case finding & Assessment
2. Case Classification
3. Institution of appropriate therapy
Step 1: Case finding & Assessment
• Cough & difficult breathing in children < 5 years age
• Fever is not an efficient criteria
Step 2: Case Classification
• Children grouped into 2:
Infants < 2months & Older children
• Specific signs to be looked: In younger children like feeding difficulty,
lethargy, hypothermia, convulsions
In infants < 2 months
• Pneumonia is diagnosed if RR 60/min with other clinical signs
• All should be hospitalized
• All should receive IV medications
• Minimum duration of 10 days
• Combination of Ampicillin & Gentamicin
Step 3:Institution of appropriate therapy
Antibiotics

Prevention of ARI
Breastfeeding infants exclusively (no other food or drinks, not even water)
for the first six months breast milk has excellent nutritional value and it
contains the mother’s antibodies which help to protect the infant from
infection.
Avoiding irritation of the respiratory tract by indoor air pollution, such as
smoke from cooking fires; avoid the use of dried cow dung as fuel for
indoor fires.
Immunization of all children with the routine Expanded Programme on
Immunization
Feeding children with adequate amounts of varied and nutritious food to
keep their immune system strong.
control the spread of respiratory bacteria by educating parents to avoid
contact as much as possible between their children and patients who have
ARIs.
people with ARIs should cough or sneeze away from others, hold a cloth
to the nose and mouth to catch the airborne droplets when coughing or
sneezing
Immunization also increases control, by reducing the reservoir of
infection in the community and increasing the level of herd immunity
Immunization
Measles vaccine
Pneumonia is a serious complication of measles
Reducing the incidence of measles helps reduce death from pneumonia
Live attenuated vaccine
Freeze dried product
0.5ml dose subcutaneously also effective intramuscularly
Schedule :9 th month

HIB vaccine
Haemophilus influenza B most important cause of death due to meningitis and
pneumonia in developing countries
Available for more than a decade
Expensive
Included in the immunization schedule
combined preparation with DPT and poliomyelitis
Three or four doses are given dependin on type of vaccine
Schedule : 6 ,10, 14 weeks booster dose 12-18 months
Vaccine is not offered to children more than 24 months
Pneumococcal vaccine
A)ppv23: polysaccharide non conjugate vaccine containing capsular antigen of 23
serotypes against this infection
Children under two years and immunocompromised do not respond well to this
vaccine
Select groups –sickle cell disease ,chronic heart disease , DM, organ transplants etc
Dose -0.5ml
Administration – intramuscular in the deltoid
Pcv-7: pneumococcal conjugate vaccine
New vaccine suitable for infants and toddlers
It is included in the immunization schedule
Induces a t- cell dependent immune response
Prevents pneumococcal pneumonia and meningitis moderately effective against
otitis media
dose- 1)6,10,14 weeks ,booster after 12 months
OR
2)2, 4,6 months and booster after 12 months
administration-intramuscular

Seminar7
: Neonatal emergencies

