1
Lecture 04 Pathology D.Lamyaa
Apoptosis
This form of cell death is a regulated suicide program in which the relevant cells activated
enzymes capable of degrading the thier own nuclear DNA and nuclear and other cytoplasmic
proteins
Fragments of the apoptotic cells then phagocyte without elicit an inflammatory reaction in
the host. Thus apoptosis differs from necrosis ; the latter is characterized by loss of
membrane integrity, leakage of cellular contents and frequently a host reaction.
Causes of apoptosis:-
Apoptosis in physiologic situations:
1. Death by apoptosis is a normal phenomenon that serves to eliminate cells that
are no longer need it is important in the following physiologic situations.
2. During emberyogenesis (organogenesis).
3. hormone deprivation as in endomaterial cells breakdown during the menstrual
cycle.
4. In proliferating cells such as intestinal crypts epithelia ( to maintain a constant
number).
5. In self-reactive lymphocytes ( to prevent self tissue destruction).
6. Cytotoxic T lymphocytes- induce cell death ( a defense mechanism against viruses
and tumors that serves to kill and eliminate virus infected and neoplastic cells.
Apoptosis in pathologic condition:-
• Apoptosis eliminates cells that are genetically altered or injured beyond repair
without eliciting a host reaction , thus keeping in the damaged as restricted as
possible
Mechanism of apoptosis
The basic process can be understood as four separable but overlapping components:
1. Signaling.
2. Control and integration.
3. Execution.
4. Removal of the dead cells.
1) Signaling:- apoptosis may be triggered by a variety of signals ranging
from intrinsic activation of programmed cell death pathways (e.g during
embryogenesis).
withdrawal of growth factors or hormones.
release of granzymes by cytotoxic T cells.

2
or selected injurious agents radiation, toxins or free radicals (which damage DNA
and activate P53 pathways).
specific receptor –ligand interactions.
The TNF receptor is belong this superfamily of plasma membrane molecules (also FAS
surface molecule is belong this family ). These plasma membrane receptors share an
intracellular (death domain) adapter protein , when this protein is oligomerized lead to
activation of initiator caspases and a cascade of enzyme activation culminating in cell death
2) Control and integration: this is accomplished by specific proteins that connect the
original death signals to the final execution program. There are two broad pathways
in this stage
Direct transmission of death signals by specific adaptor proteins to the execution
mechanism
Regulation of the mitochondrial permeability by members of the BCL-2 family of
proteins. As we know (free radicals, increase of ca,) can result into formation of
mitochondrial transition pore with loss of the mitochondrial potential and more
depletion of ATP and also increase the permeability of the outer mitochondrial
membrane resulting in releasing of the cytochrome c which binds to certain pro-
apoptotic cytosolic proteins triggering the execution caspase culminating in cell
death.
The BCL-2 and BCL-X (found in the mitochondrial membrane) suppress apoptosis by
preventing increased mitochondrial permeability, (act as inhibitors) while BAX and BAD act
as promotors (promote the programmed cell death).
3) Execution :- characterized by specific biochemical events result in synthesis and or
activation of number of cytosolic catabolizing enzymes culminating in morphological
change of apoptosis
4) Removal of the dead cells. The apoptotic cells and their fragments (apoptotic bodies)
express a new ligands on their surfaces that enhancing the phagocytosis and removing
these fragments without releasing of proinflammatory mediators (so inflammation is
abscent).

3
Morphology
In H&E-stained tissue sections, apoptotic cells may appear as round or oval masses with
intensely eosinophilic cytoplasm. Nuclei show various stages of chromatin condensation,
aggregation and karyorrhexis. The cells rapidly shrink, form cytoplasmic buds, and fragment
into apoptotic bodies composed of membrane-bound vesicles of cytosol and organelles.
Because these fragments are quickly extruded and phagocytosed without eliciting an
inflammatory response, even substantial apoptosis may be histologically undetectable.
Apoptosis of a liver cell in viral hepatitis. The cell is reduced in size and contains brightly
eosinophilic cytoplasm and a condensed nucleus.
Example of apoptosis
• Growth Factor Deprivation
Hormone-sensitive cells deprived of the relevant hormone, lymphocytes that are not
stimulated by antigens and cytokines, and neurons deprived of nerve growth factor die by
apoptosis. In all these situations, apoptosis is triggered by the mitochondrial pathway and is
attributable to activation of pro-apoptotic members of the Bcl-2 family and decreased
synthesis of Bcl-2 and Bcl-x
L
.
DNA Damage
Exposure of cells to radiation or chemotherapeutic agents induces DNA damage, and if this
is too severe to be repaired it triggers apoptotic death. When DNA is damaged, the p53
protein accumulates in cells. It first arrests the cell cycle (at the G
1
phase) to allow time for
repair. However, if the damage is too great to be repaired successfully, p53 triggers
apoptosis, mainly by activating sensors that ultimately activate BAX and BAD, and by
stimulating synthesis of pro-apoptotic members of the Bcl-2 family. When p53 is mutated or
absent (as it is in certain cancers), it is incapable of inducing apoptosis, so that cells with
damaged DNA are allowed to survive. In such cells, the DNA damage may result in mutations
or translocations that lead to neoplastic transformation.
4
Cytotoxic T Lymphocyte-Mediated Apoptosis
Cytotoxic T lymphocytes (CTLs) recognize foreign antigens presented on the surface
of infected host cells and tumor cells. Upon activation, CTL granule proteases called
granzymes enter the target cells. Granzymes are able to activate cellular caspases. In this
way, the CTL kills target cells by directly inducing the effector phase of apoptosis, without
engaging mitochondria or death receptors. CTLs also express FasL on their surface and may
kill target cells by ligation of Fas receptors.
Intracellular accumulations
Cells may accumulate abnormal amount of various substances; these may be harmless or
associated with injury. The location of these substances are either cytoplasmic within
organelles ( typically lysosomes) or in the nucleus.
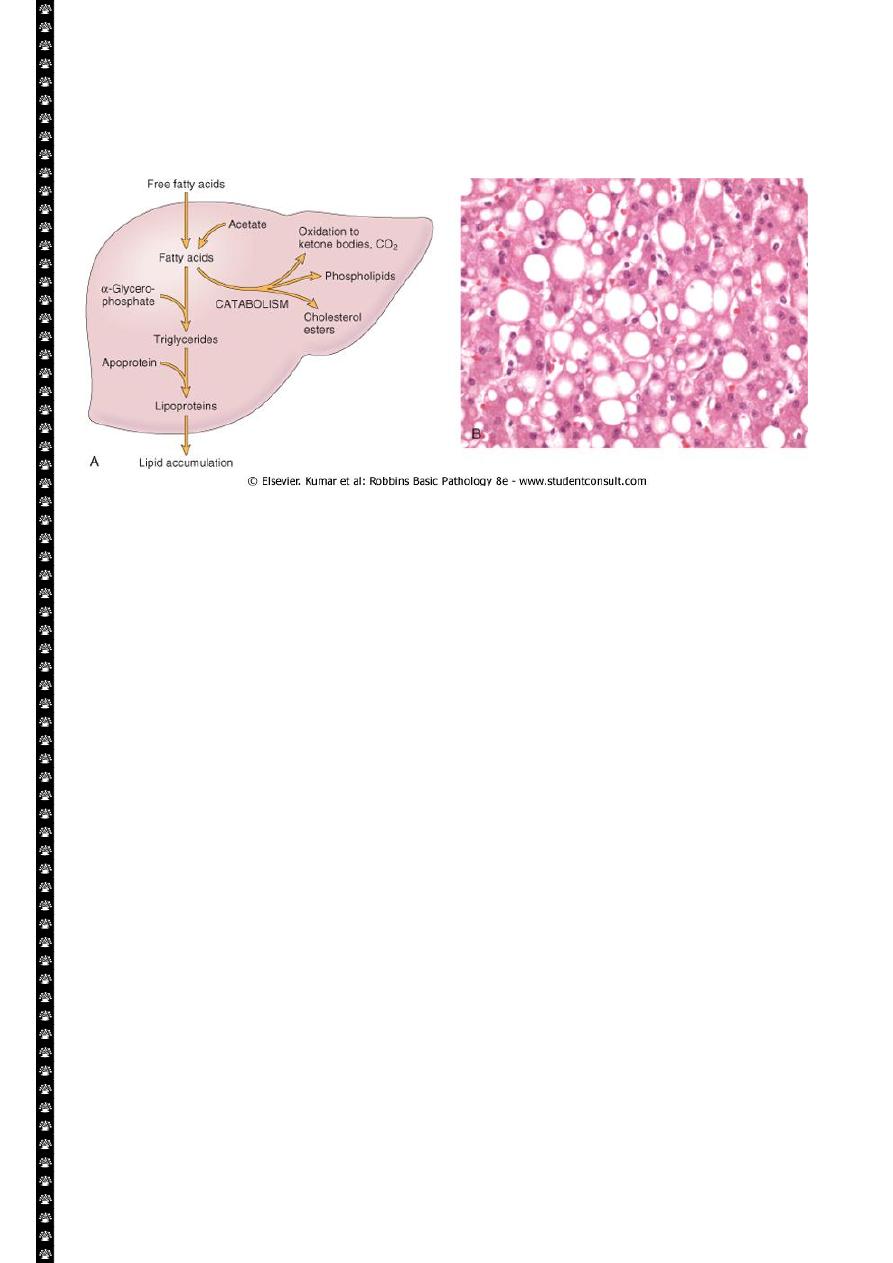
Fatty change (steatosis):-
This refer to an abnormal accumulation of triglycerides within paranchymal cells. It is most
often seen in the liver ,since this is the major organ involved in fat metabolism, but it may
also occur in the heart.
Causes of fatty change include
1. Toxin including alcohol
2. Diabetes mellitus
3. Obesity
4. Protein malnutrition
5. Anoxia
Fatty change (steatosis)
Alcohol abuse and diabetes associated with obesity are the most common cause of fatty
change in the liver ( fatty liver) in industrialized nations.
Free fatty acids from adipose tissue or ingested food are normally transported into
hepatocytes, where they are esterified to triglycerides converted into cholesterol or
phospholipids or oxidized to ketone bodies. Triglycerides from the hepatocytes required the
formation of complexes with apoprotiens to form lipoproteins which are able to enter the
circulation.
Excess accumulation of triglycerides may result from defect at any step from fatty acid entry
to lipoprotein exit.
Hepatotoxins e.g.(alcohol) alter mitochondrial and SER function and thus inhibit
fatty acid oxidation;
CCL4 and protein malnutrition decreases the synthesis of apoprotiens
anoxia inhibits fatty acid oxidation
starvation increase fatty acid mobilization from peripheral stores.
5
The significance of fatty change depends on the cause and severity of the accumulation.
When mild it may have no effect. More severe fatty change may transiently impair cellular
function, but the change is reversible. In the severe form, fatty change may precede cell
death.
Gross features:-
Fatty changes is most commonly seen in the liver and heart.
In the liver mild fatty change may not affect the gross appearance
With increasing the accumulation, the organ enlarged and become progressively
yellow, until in extreme cases it may weight 5 Kg (3 times the normal weight) and
appear bright yellow, soft and greasy
Microscopic features:-
Early fatty changes is seen by light microscopy as small fat vacuoles in the
cytoplasm around the nucleus
In later stages, the vacuoles coalesce to create cleared spaces that displace the
nucleus to the cell periphery.
Cholesterol and cholesterol esters accumulations:-
Cellular cholesterol metabolism is tightly regulated to ensure normal cell membrane
synthesis without significant intracellular accumulation. However, phagocytic cell may
become overloaded with lipid ( triglycerides, cholesterol and cholesteryl esters) in several
different pathologic processes
Macrophage in contact with lipid debris of necrotic cells or abnormal (e.g.
oxidized) form of lipoproteins may become stuffed with phagocytosed lipid. These
macrophages may be filled with minutes, membrane bound vacuoles of lipid,
imparting a foamy appearance to their cytoplasm (foam cells).
In atherosclerosis, smooth muscle cells and macrophages are filled with lipid
vacuoles composed of cholesterol and cholesterol esters; these give
6
atherosclerotic plaques their characteristic yellow color and contribute to the
pathogenesis of lesion.
Protein accumulations:-
Morphologically visible protein accumulations may occur because excesses are
presented to the cells or because the cells synthesize excessive amounts. In the
kidney for example; trace amount of albumin filtered through the glomerulus are
normally reabsorbed by pinocytosis in the proximal convoluted tubules. However
in disorders with heavy protein leakage across the glomerular filter (e.g. nephrotic
syndrome), there is a much larger reabsorption of protein. Pinocytic vesicles
containing this protein fuse with lysosomes, resulting in the histologic appearance
of pink, hyaline cytoplasmic droplets.
Another example is the accumulation of intracellular proteins are also seen in
certain type of cell injury. For example, the Mallory body (or alcoholic hyaline) is
an eosinophilic cytoplasmic inclusion in liver cells that is highly characteristic of
alcohol liver disease. Such inclusions are composed predominantly of aggregated
intermediate filaments that presumably resist degradation.
Pigments:-
are colored substances that exogenous, coming from outside the body, or endogenous
synthesized within the body itself.
Exogenous pigments; the most common of these is carbon (an example is coal
dust), a ubiquitous air pollutant of urban life. When inhaled, it is phagocytosed by
alveolar macrophages and transported through lymphatic channels to the
regional tracheobronchial lymph nodes. Aggregates of the pigment blacken the
draining lymph nodes and pulmonary parenchyma (anthracosis) heavy
accumulations may induce fibroblastic reaction that can result in a serious lung
diseases called coal workers pneumoconiosis
Endogenous pigments; include lipofuscin, melanin and certain derivatives of
hemoglobin material that accumulates in a variety of tissues (particularly the
heart, liver and the brain) as a function of age or atrophy.
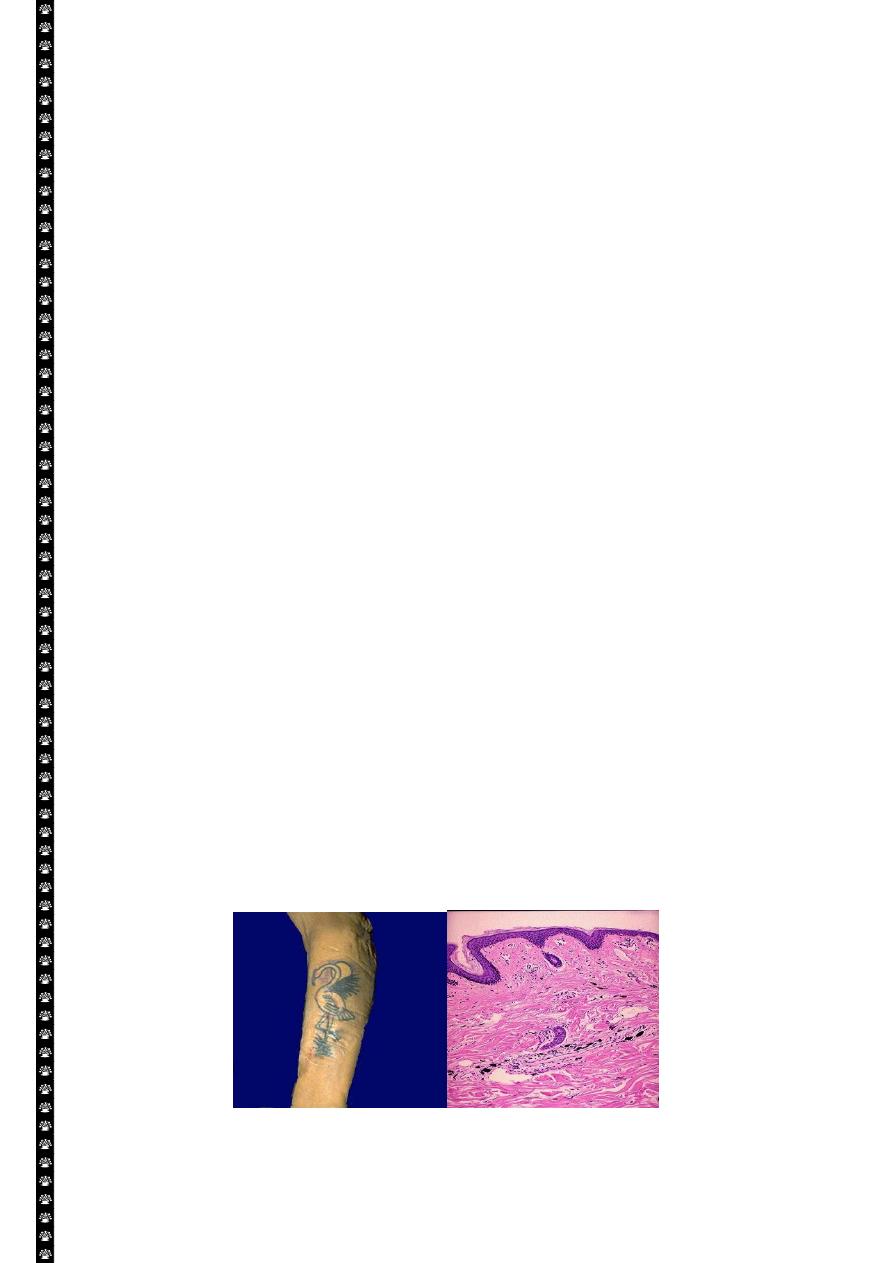
Skin tattoo
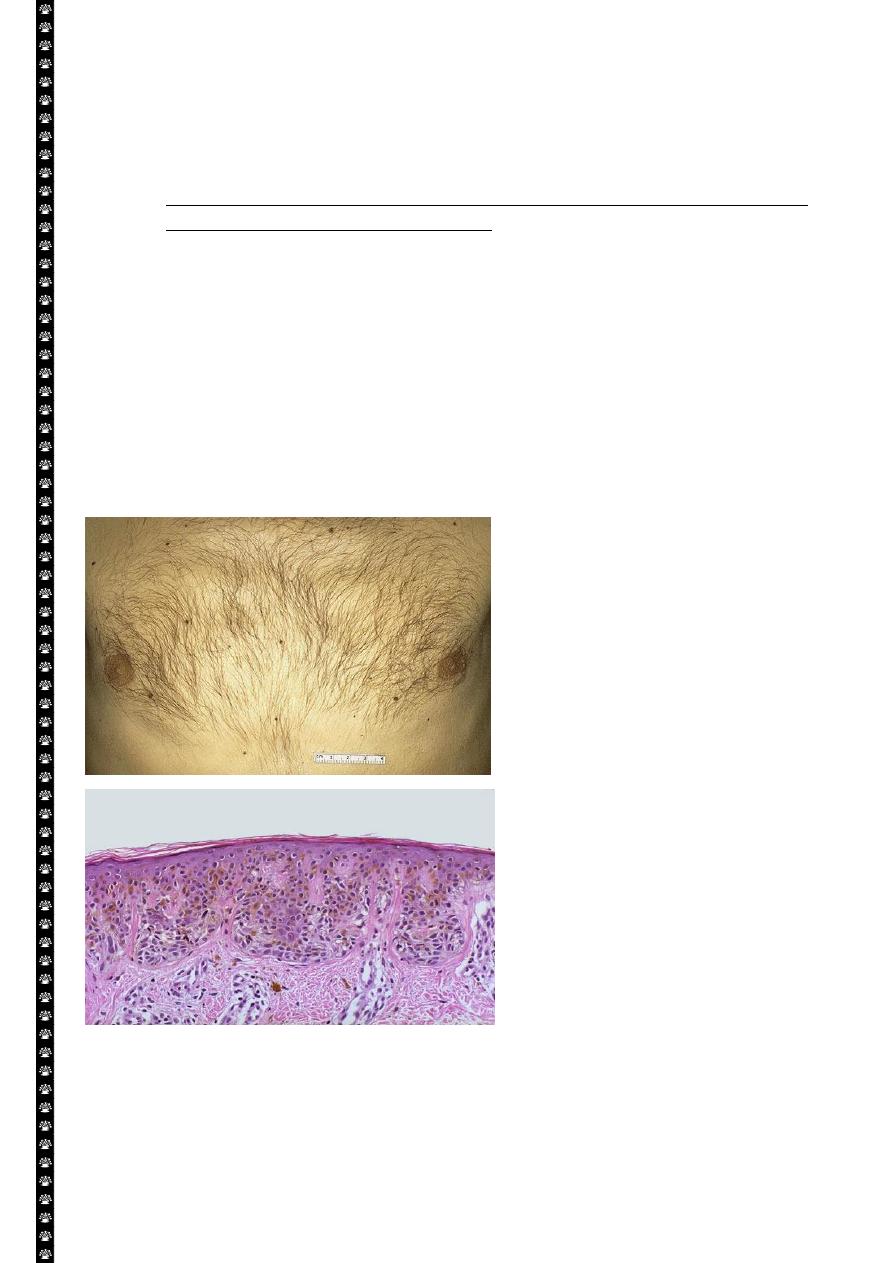
7
Lt. Here is a tattoo. Tattooing is a practice that is thousands of years old. In many cultures, tattoos
have great significance. The pigment in tattoos is transferred to the dermis with a needle.
Rt. This is the microscopic appearance of tattoo pigment (black) in the dermis. Note that this
pigment is well within the dermis and, therefore, difficult to remove.
Lipofuscin represents complexes of the lipid and protein that derived from lipids
peroxidation of subcellular membranes. It is not injurious to the cell but is
important as a marker of past free radical injury. The brown pigment, when
present in large amounts, imparts an appearance to the tissue that is called brown
atrophy
Melanin:- is an endogenous, brown-black pigment produced in melanocytes
following
the
tyrosinase-
catalyzed
oxidation
of
tyrosine
to
dihydroxyphenylalanine. It is synthesized exclusively by melanocytes located in
the epidermis and acts as a screen against harmful ultraviolet radiation. Although
melanocytes are the only source of melanin, adjacent basal keratinocytes in the
skin can accumulate the pigment (e.g. in freckles), as can dermal macrophages.
Pigmented nevi
• Above: these are benign nevi. Small brown flat to slightly raised nevi are quite common. They
are usually less than a centimeter in diameter.
• Below: in this nevus, there are nevus cells in nests in the lower epidermis. They show brownish
melanin pigment.
8
Hemosiderin: is a hemoglobin-derived granular pigment that is golden yellow to brown and
accumulates in tissues when there is a local or systemic excess of iron. Hemosiderin pigment
represent large aggregates of iron, readily visualized by light microscopy; the iron can be
identified by the Prussian blue histochemical reaction. Local excesses of iron and
consequently of hemosiderin, result from hemorrahage. The best example is the common
bruise.
Whenever there is systemic overload of iron, hemosiderin is deposited in many organs and
tissues including their macrophages. This condition is called hemosiderosis . with progressive
accumulation, paranchymal cells throughout the body (but principally the liver, pancreas,
heart, and endocrine organs) become (bronzed) with accumulating pigments.
Hemosiderosis occurs in the setting of
Increased absorption of dietary iron
Impaired utilization of iron
Hemolytic anemia and
Transfusion (the transfused red cells constitute an exogenous load of iron)
In most of instances of systemic hemosidrosis, the iron pigment does not damage he
parenchymal cells or impair organ function despite an impressive accumulation. However
more extensive accumulations of iron are seen in hereditary hemochromatosis, with tissue
injury including liver fibrosis, heart failure, and diabetes mellitus.
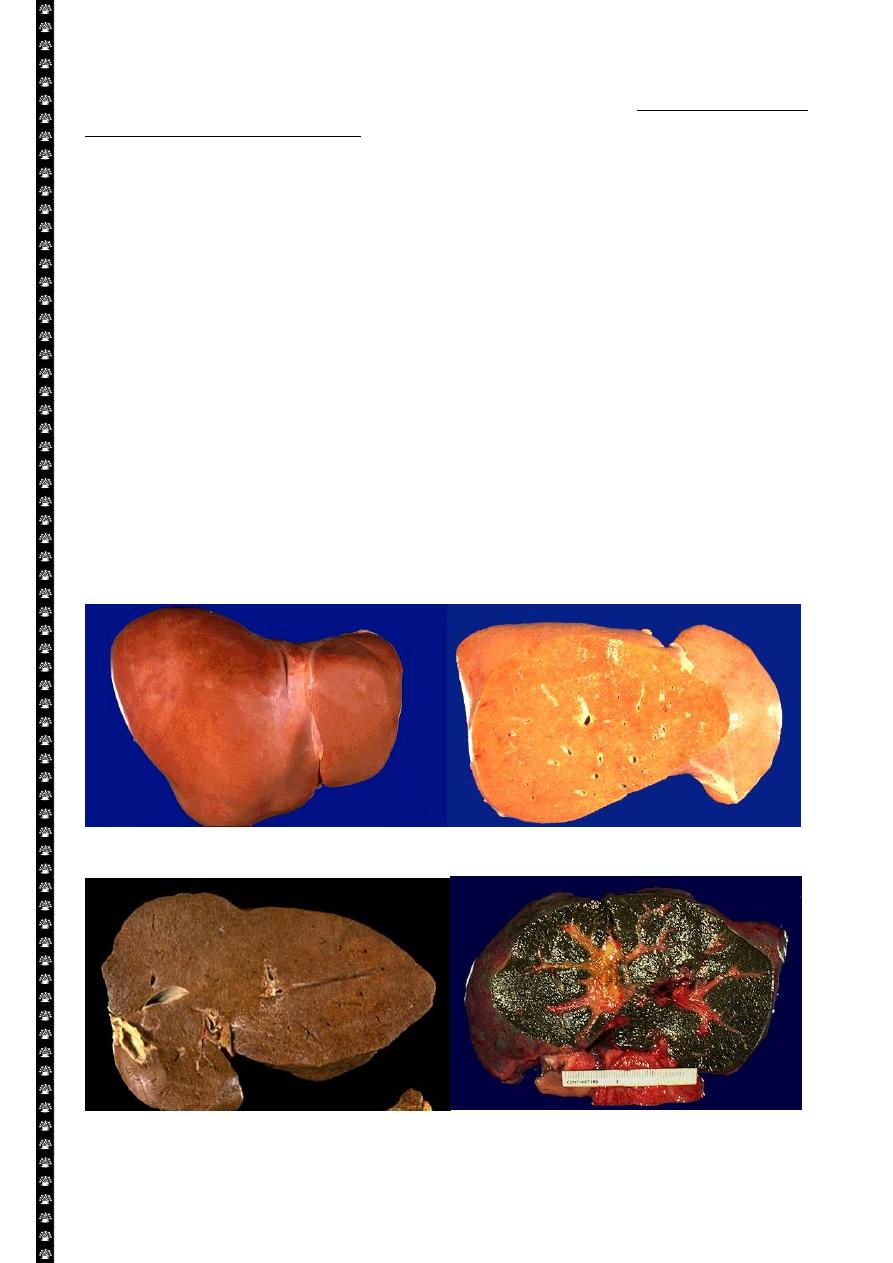
Normal Fatty change
Hemochromatosis Biliary obstruction
9
Pathological calcification:-
Pathologic calcification is a common process in a wide variety of diseases states;
it implies the abnormal deposition of calcium salts.
When the deposition occurs in dead or dying tissues, it is called dystrophic
calcification; it occur in the absence of calcium metabolic derangements (i.e. with
normal serum levels of calcium).
In contrast, the deposition of calcium salts in normal tissues is known as
metastatic calcification and almost always reflects some derangement in calcium
metabolism (hypercalcemia). It should be noted that while hypercalcemia is not a
prerequisting for dystrophic calcification, it can exacerbate it.
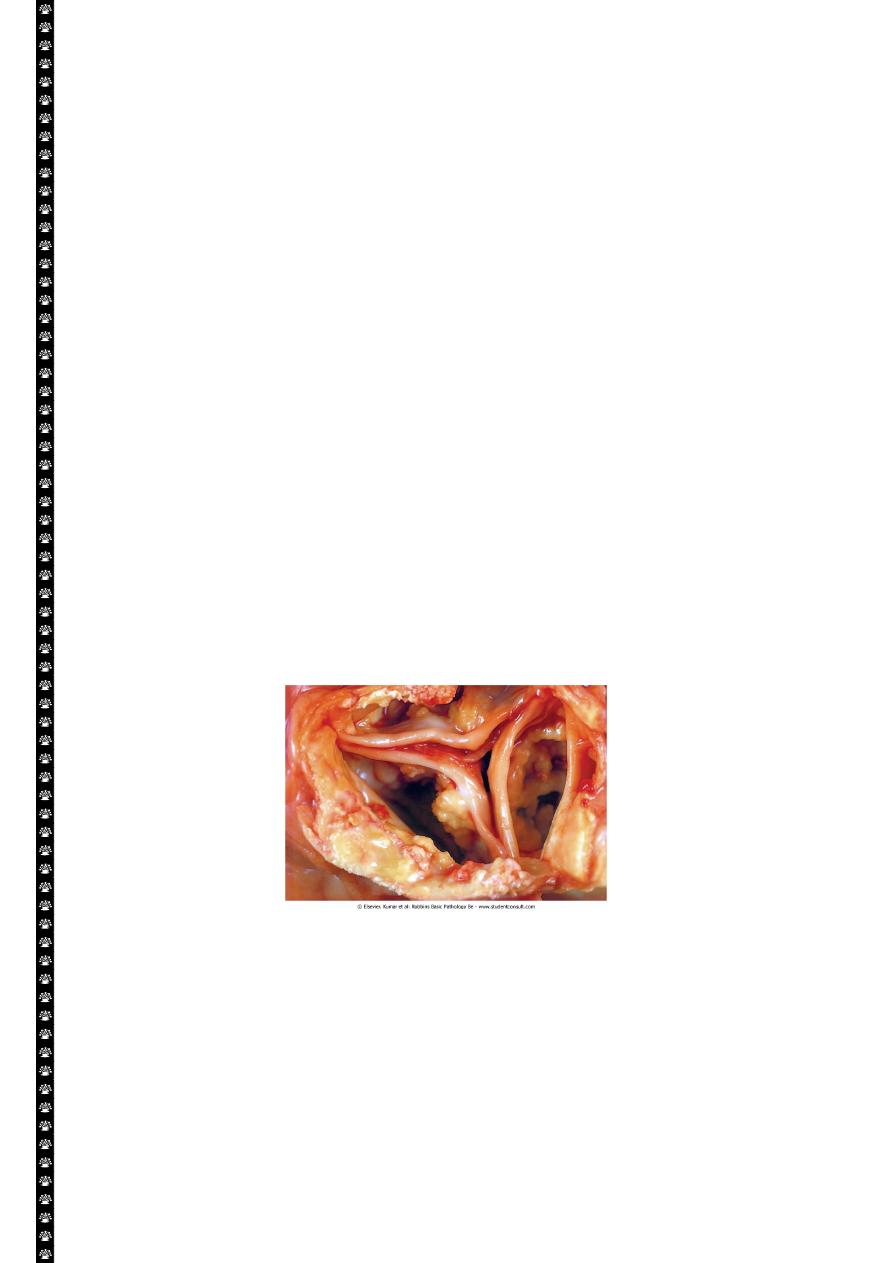
Dystrophic calcification:- is encountered in
Areas of necrosis ( of any type as coagulative, caseous, etc.)
Advanced atherosclerosis ( as in the aorta and coronaries)
Aging or damaged heart valves resulting in severely impaired valve motion.
Dystrophic calcification of the aortic valves is an important cause of aortic stenosis
in the elderly.
Regardless of the site, calcium salts are grossly seen as fine white granules or
clumps, often felt as gritty deposits.
Microscopically:- calcification appear as intracellular and/or extracellular
basophilic (bluish) deposits. In time, metaplastic bone may be formed in the focus
of calcification.
Dystrophic calcification

10
Metastatic calcification:-
This is seen in cases of hypercalcemia of any cause. The four major causes of hypercalcemia
are:-
Increased secretion of parathyroid hormone (primary parathyroid tumors or
production of parathyroid hormone-related protein by other malignant tumors)
Destruction of bone (effect of accelerated turnover as in Paget disease,
immobilization or tumors due to increase bone catabolism associated with
multiple myeloma, leukemia or diffuse skeletal metastases
Vitamin D related disorders including vitamin D intoxication and sarcoidosis (in
which macrophages activate a vitamin D precursor)
Renal failure, in which phosphate retention leads to secondary
hyperparathyroidism.
Metastatic calcification can occur widely throughout the body but principally
affects the interstitial tissues of the vessels, kidneys, lungs and gastric mucosa.
The calcium deposits morphologically resemble those described in dystrophic
calcification. Although they do not generally cause clinical dysfunction, extensive
calcifications in the lungs may produce remarkable radiographs and respiratory
deficits and massive deposits in the kidney (nephrocalcinosis) can cause renal
damage.
