1
Lecture 03 Pathology D.Lamyaa
Mechanisms of cell injury
The outcome of the interaction between the injurious agent & the cell depend on:-
1. The type of injury, its duration and its severity.
Thus low doses of toxins or a brief duration of ischemia may lead to reversible cell
injury while large toxin doses or longer ischemic intervals may result in irreversible
injury and cell death.
2. The type, adaptability and genetic makeup of the injured cell The same injury has
vastly outcomes depending on the cell type.
Thus striated skeletal muscle in the leg resist complete ischemia for 2-3- hours
without irreversible injury, whereas cardiac muscle dies after only 20-30 minutes.
The nutritional or hormonal status can also be important; clearly a glycogen filled
hepatocytes will tolerate ischemia much better than one that has just burden its
last glucose molecules.
Genetically determined diversity in metabolic pathways can also be important.
For instance, when exposed for the same dose of a toxin. Individual who inherit
variants in genes encoding cytochrom p-450 may cataboliz the toxin at different
rates leading to different outcomes.
Mechanisms of cell injury
The most important targets of injurious stimuli are-
1. Mitochondria (the site of ATP generation).
2. Cell membrane which influence the ionic and osmotic homeostasis of the cell.
3. Protein synthesis (ribosome).
4. The cytoskeleton (microtubules and various filaments).
5. The genetic apparatus of the cell (nuclear DNA)
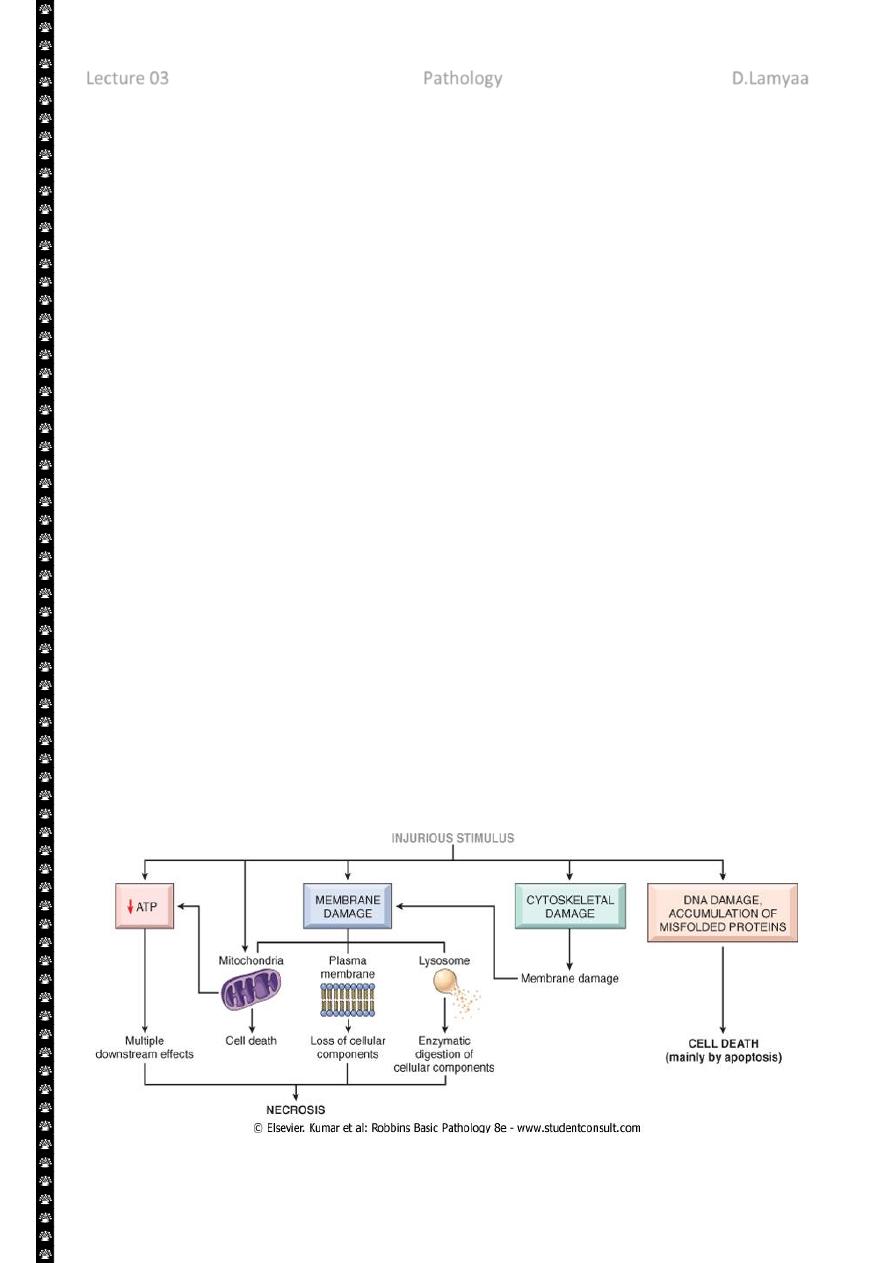
2
The principal cellular and biochemical sites of damage in cell injury. Note that loss of adenosine
triphosphate (ATP) results first in reversible injury (not shown) and culminates in necrosis.
Mitochondrial damage may lead to reversible injury and death necrosis or apoptosis.
ATP Depletion:
ATP the energy fuel of cells, is produced mainly by the oxidative phosphorylation of ATP of
the mitochondria. In addition the glycolytic pathway can generate ATP in the absence of
oxygen using glucose derived either from the circulation or from the hydrolysis of
intracellular glycogen (anerobic glycolysis).
The major causes of ATP depletion:-
1. Reduce supply of oxygen and nutrients.
2. Mitochondrial damage.
3. The action of some toxins (e.g. cyadine)
High energy phosphate in the form of ATP is required for virtually all synthetic and
degrdatative and processes within the cell, include membrane transport, protein synthesis,
phospholipids turnover etc. depletion of ATP to less than 5% -10% of normal levels has
widespread effect on many cellular systems
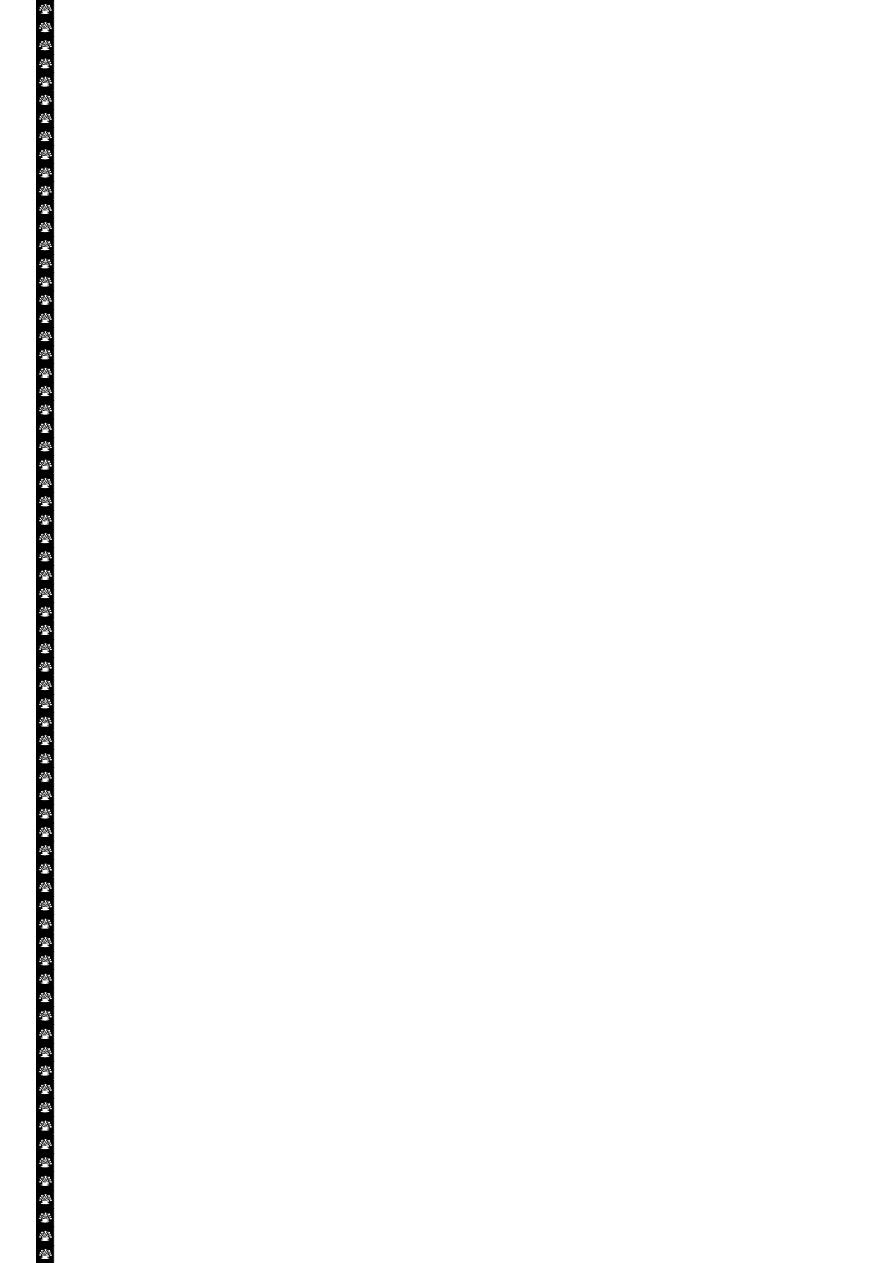
ATP depletion
The activity of plasma membrane energy dependent sodium pump is reduce,
resulting in intracellular accumulation of sodium and efflux of potassium. The net gain
of solute is accompanied by iso- osmotic gain of water, causing cell swelling.
There is a compensatory increase in anaerobic glycolysis in an attempt to maintain
the cell energy sources. As a consequence, intracellular glycogen stores are rapidly
depleted and lactic acid accumulates, leading to decrease activity of many cellular
enzymes (due to decrease pH level).
Failure of the Ca pump lead to influx of Ca with damaging effects on numerous cellular
components, describe below.
Structural disruption of the protein synthetic apparatus manifested as detachment of
ribosomes from the rough endoplasmic reticulum (RER) and polysomes into
monosomes, with a consequent reduction in protein synthesis
Ultimately, there is irreversible damage to the mitochonerial and lysosomal
membranes and the cells undergoes necrosis.
3
The initial functional and morphologic consequences of decreased intracellular adenosine
triphosphate (ATP) during cell injury. ER, Endoplasmic reticulum.
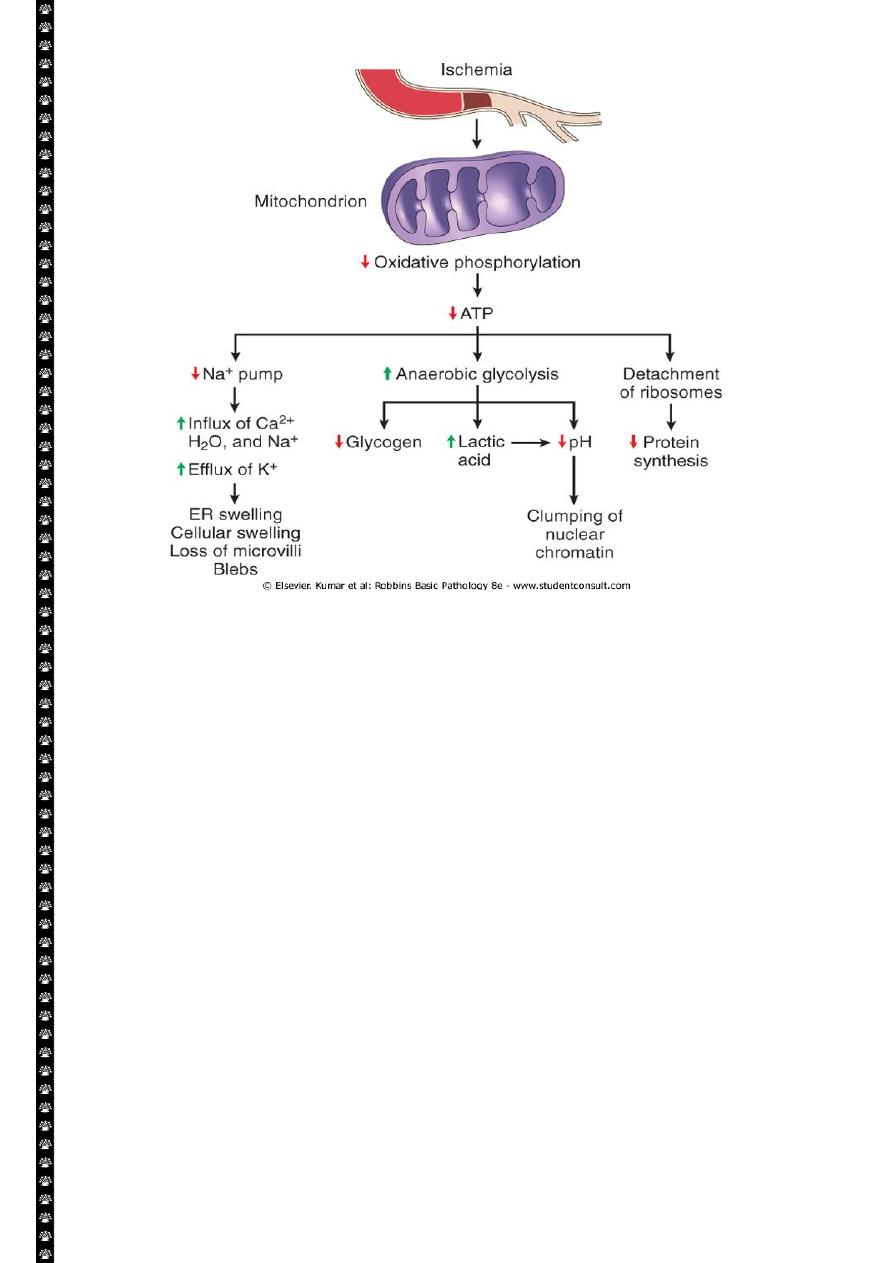
Mitochondrial Damage:
Mitochondria are supplies ATP, but they are also critical players in the cell injury and
death. Mitochondria can be damaged by increase of cytosolic Ca, reactive oxygen species,
and oxygen deprivation, and so they are sensitive to virtually all types of injurious stimuli,
including hypoxia
1. The formation of a channel in the mitochondrial membrane, called the
permeability transition pore. The opening of this channel leads to the loss of
mitochondrial membrane potential and PH changes, resulting in failure of
oxidative phosphorylation and progressive depletion of ATP, culminating in
necrosis of the cells.
2. Increase permeability of the mitochondrial membrane may result in leakage of
cytochrome c ( the major protein involved in electron transport) that are capable
for activating apoptotic pathways. Thus cytochrome c play a key dual role in cell
survival and death in its normal location inside the mitochondria, it's essential for
energy generation and the life of the cell, but when mitochoneria are damaged so
severely that cytochrome c leaks out; its signal to die by apoptosis.
4
Consequences of mitochondrial dysfunction, culminating in cell death by necrosis or
apoptosis. ATP, Adenosine triphosphate.
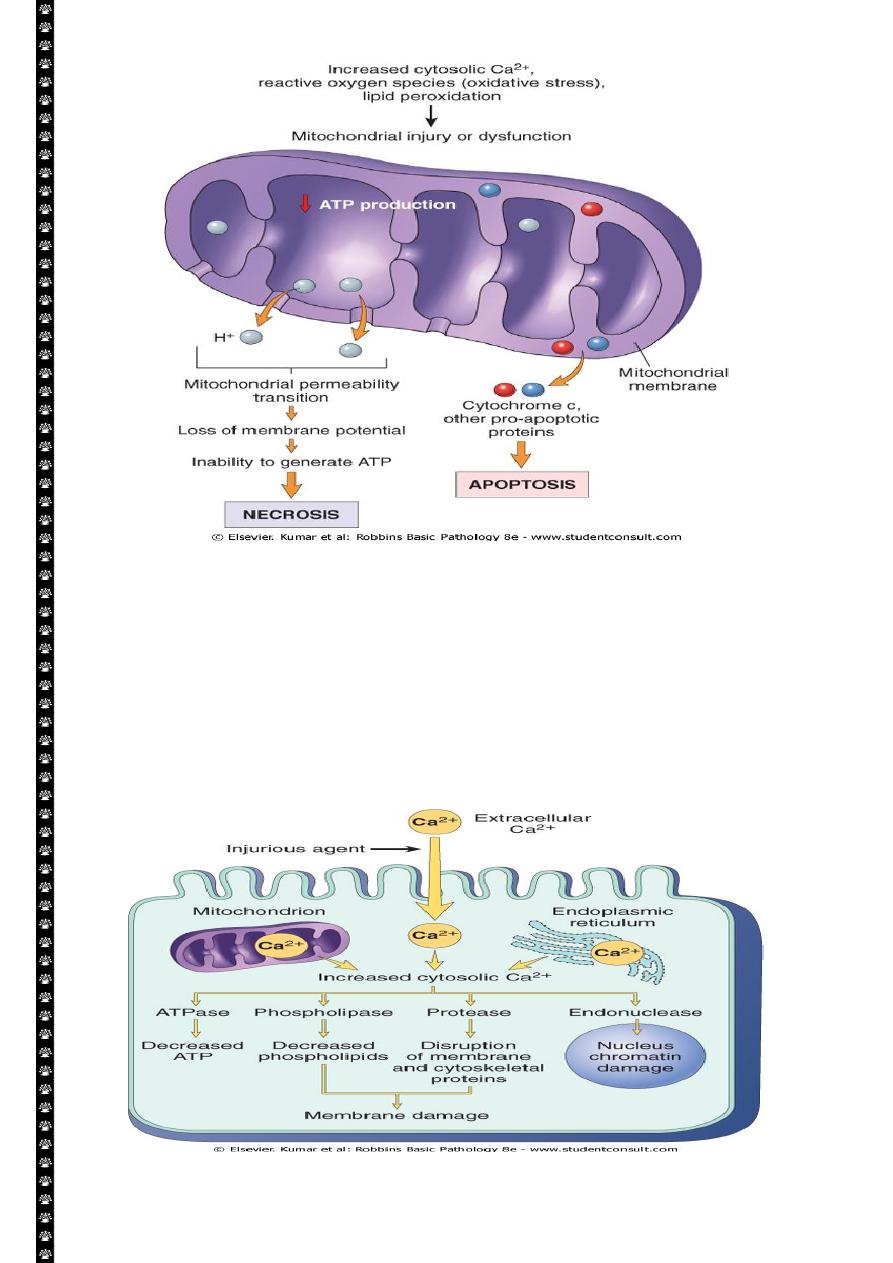
Influx of calcium:
Cytoplasmic free calcium is normally maintained by ATP –dependent calcium pump(
transporter) at concentration that are 10,000 times lower than the concentration of
extracellular calcium or intracellular mitochondrial and ER calcium. Ischemia and certain
toxins causes an increase in cytoplasmic calcium concentration , initially because of release
of Ca from intracellular stores, and later resulting from increased influx across the plasma
membrane.
5
Sources and consequences of increased cytosolic calcium in cell injury. ATP, Adenosine
triphosphate; ATPase, adenosine triphosphatase
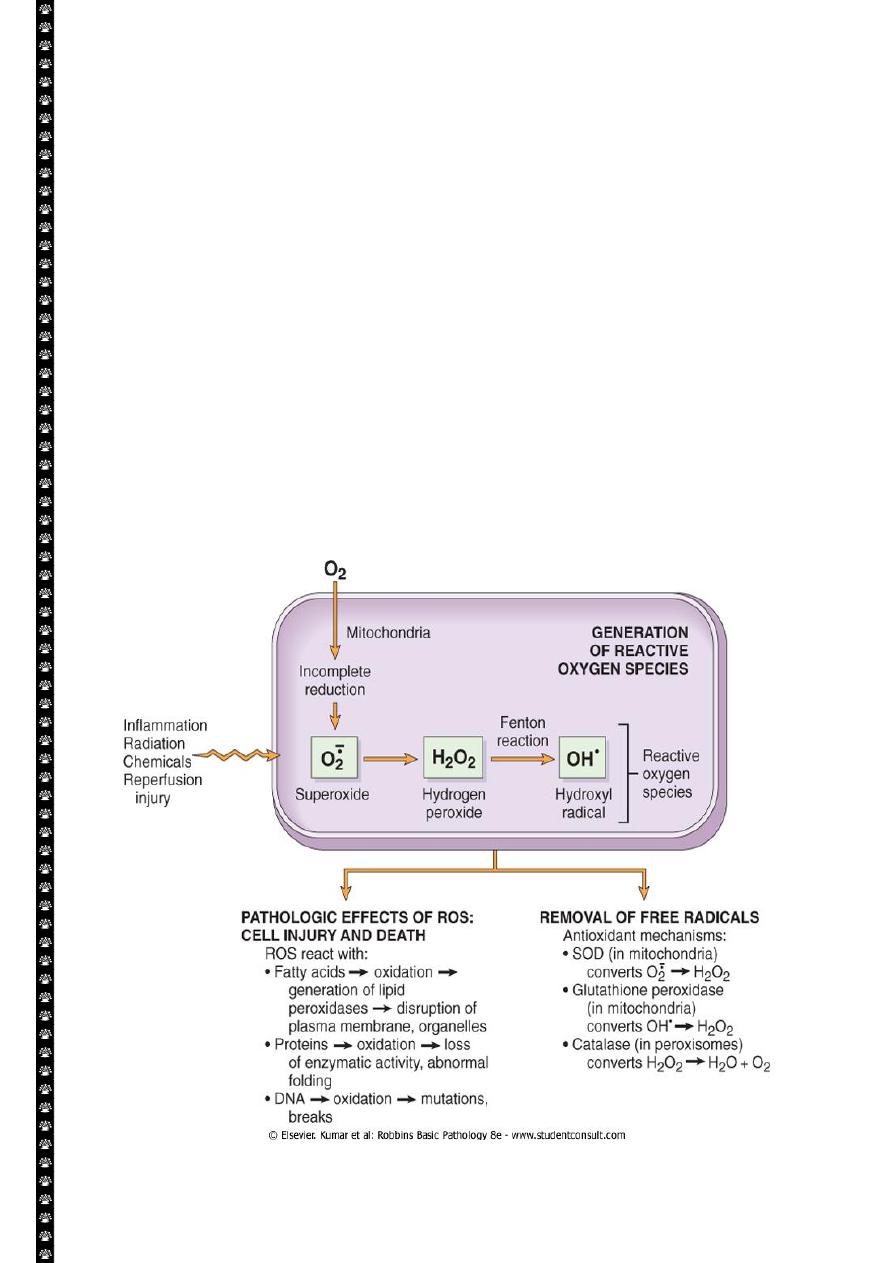
Accumulation of oxygen derived free radicals (oxidative stress)
These are designated as reactive oxygen species (ROS) & these are units with a single
unpaired electron in their outer orbit. When generated in cells they avidly attack the nucleic
acids, cellular protein and lipids. The molecules that react with free radicals are in turn
converted into free radicals, thus propagating the chain of damage.ROS are produced
normally in cells during mitochoderial respiration and energy generation, but they are
degraded and removed by cellular defense systems. When their production increase or the
defense systems are ineffective, the result is excess of these free radicals, leading to a
condition called oxidative stress.
Cell injury in many circumstances involves damage by free radicals; these include:
1. Reperfusion injury.
2. Chemical and radiation injury.
3. Toxicity from oxygen and other gases.
4. Cellular aging.
5. Inflammatory cells mediated tissue injury
6
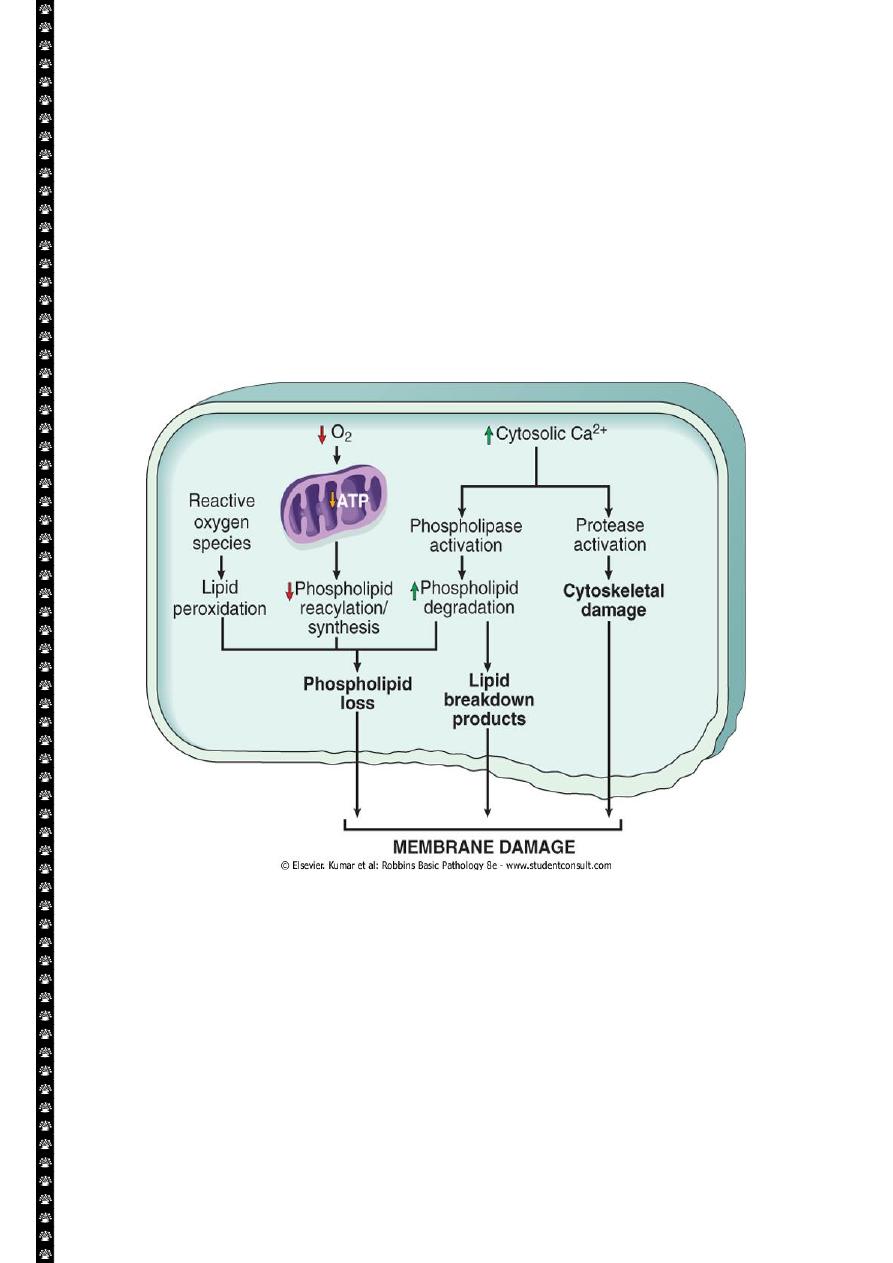
Defects in membrane permeability:-
Biochemical mechanisms contribute to membrane damage include:-
1. Decrease phopholipid synthesis due to fall in ATP levels. This affect all cellular
membrane including mitochondrial, which worsen the loss of ATP.
2. Degredation of membrane phospholipids due to activation of intracellular
phospholipase through increased levels of intracellular Ca.
3. Injury to cell membranes by oxygen free radicals (ROS) by lipid peroxidation.
4. Damaged to the cytoskeleton through activation of proteases by increased
cytoplasmic Ca.
5. They detergent effect of free fatty acids on membranes. These products result
from phospholipid degradation
Mechanisms of membrane damage in cell injury. Decreased O
2
and increased cytosolic Ca
2+
are
typically seen in ischemia but may accompany other forms of cell injury. Reactive oxygen species,
which are often produced on reperfusion of ischemic tissues, also cause membrane damage (not
shown).
The most important site of membrane damage during cell injury are:
1. Mitochondrial membrane damage: damage to mitochondrial membranes result
in decreased production of ATP , culminating in necrosis, alternatively release of
proteins triggers apoptotic death.

7
2. Plasma membrane damage:- lead to loss osmotic balance and influx of fluid and
ions as well as loss of cellular contents.
3. Injury to lysosomal membranes results in leakage of their enzymes into the
cytoplasm and activation of acid hydrolases in the acidic intracellular PH of the
injured (e.g. ischemic) cell. Lysosomes contain RNases, DNases, proteases and
components and the cells die b necrosis.
Damage to DNA& proteins
Cells have mechanisms that repair damage to DNA, but if this damage is too severe to be
corrected (e.g. after radiation injury or oxidative stress), the cell initiates its suicidal program
and die by apoptosis , a similar reaction is triggered by improperly folded (configured)
protein (see unfolded protein response) which may be the result of inherited mutations or
through free radicals. These mechanisms of cell injury typically cause apoptosis.
Examples of cell injury and necrosis:-
1. Ischemic and hypoxic injury.
2. Reperfusion injury
Subcellular response to cell injury
Certain agents and stresses induce distinctive alterations involving only subcellular
organelles. Although some of these alterations occur in acute lethal injury, other are seen in
chronic forms of cell injury and still others are adaptive responses
E.g. Abnormalities of the cytoskeleton may be manifested as an abnormal
appearance and function of cells (e.g. Mallory bodies, which represent
intracellular accumulations of fibrillar material in alcoholic liver disease).
Autophagy :-refers to lysosomal digestion of the cells own components it is a
survival mechanism whenever there is nutrient deprivation ; the starved cell lived
by eating its own components. In this process, intracellular organelles are first
sequestrated from the cytoplasm in an autophagic vacuoles.
Induction (hypertrophy of smooth ER):- the smooth ER is involve in the
metabolism of various chemicals and cells exposed to these chemicals show
hypertrophy of the ER as an adaptive response that may have important
functional consequences.
