1
Lecture 02 Pathology D.Lamyaa
Morphologic features of cell and tissue injury
in reversible cell injury the morphological changes are exemplified by:
1. Acute cellular swelling
2. fatty change
In Irreversible cell injury there are two morphologic types of cell death
1. Necrosis
2. Apoptosis
Morphologic examples of reversible injury
Cellular swelling (hydropic change or vacuolar degeneration) is the result of failure of
energy –dependent ion pumps in the plasma membrane, leading to an inability to
maintain ionic gradients across the membranes i.e. there is influx of sodium (with
water) into the cell and departure of the potassium out. It is the first manifestation
of almost all forms of cell injury. When it affects many cells in an organ which appear
also tense. Microscopically: there are small, clear vacuoles within the cytoplasm;
these represent distended segment of the ER.
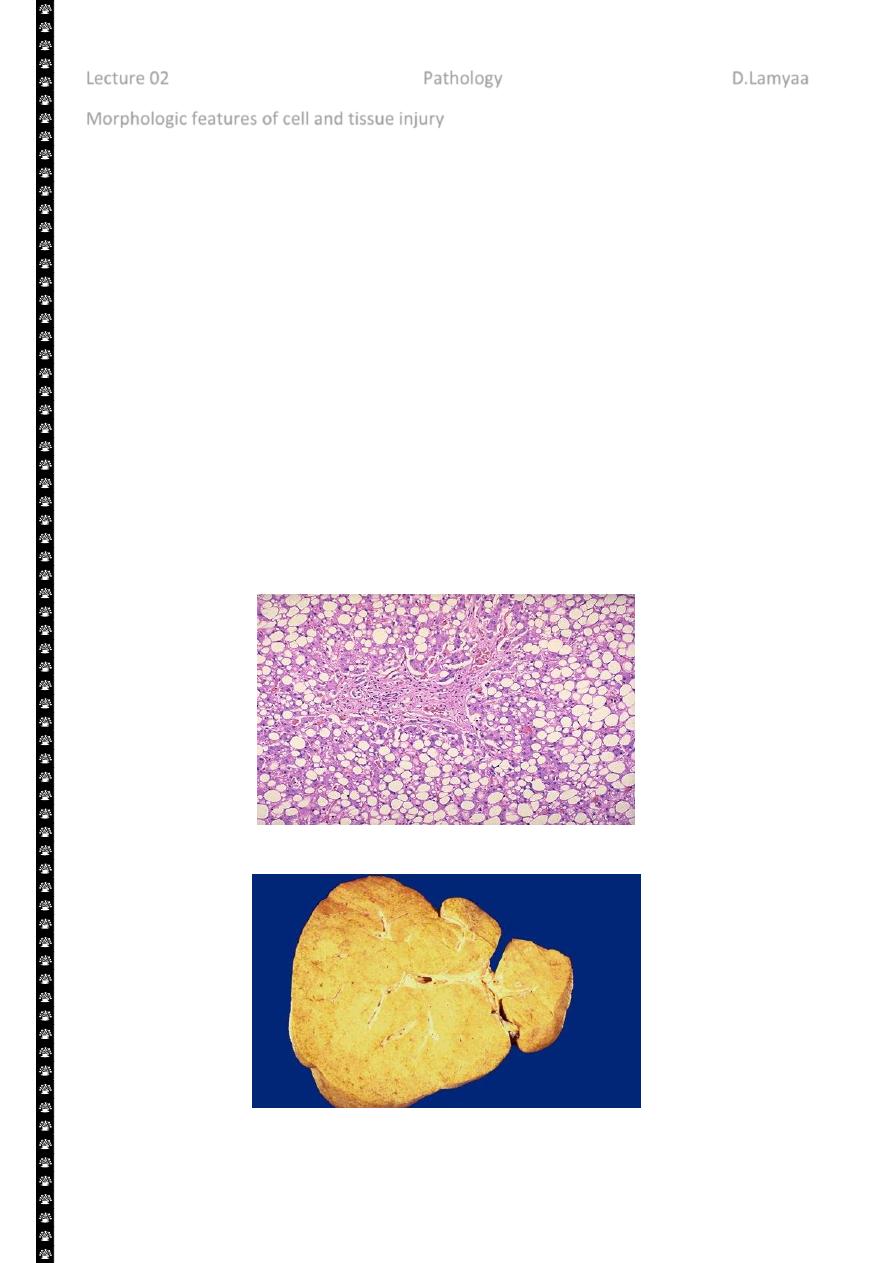
Fatty changes is manifested by the appearance of lipid vacuoles in the cytoplasm. It
is principally encountered in cells participating in fat metabolism (e.g. hepatocytes)
Fatty change liver
Sever fatty change liver

2
Morphologic examples of irreversible injury
Necrosis refers to morphological changes that accompany cell death, largely resulting
from the degradative action of enzymes on lethally injured cells. Necrotic cell are
unable to maintain membrane integrity, and their contents often leak out. The
enzymes responsible for the digestion of the cell are derived either from the
lysosomes of the dying cells themselves or from the lysosomes of leukocytes that are
recruited as part of the inflammatory reaction to the dead cells.
When damage to membrane is severe, enzyme leak out of lysosomes (which are
membrane bound), enter the cytoplasm and digest the cell, resulting in necrosis.
Cellular content also leak out through the damaged plasma membrane and elicit
inflammation.
The leakage of intracellular proteins through the damaged cell membrane and
ultimately into the circulation provides a means of detecting tissue specific necrosis
using blood or serum samples. Cardiac muscle for example contains a unique enzyme
creatin kinase and of the contractile protein troponin. Hepatocytes contain
transaminases. Irreversible injury and cell death in these tissue are reflected in
increase levels of such proteins and measurement of serum levels is used clinically to
assess damage to these tissues.
Morphologic examples of irreversible injury:
Apoptosis is the mode of cell death when
The cell is deprived of growth factors or
The cell's DNA or proteins are damaged beyond repair
3
This is an active, energy dependent regulated type of cell death., apoptosis servers many
normal functions and is not necessarily pathological. Necrosis involve a large number of
contagious cells (so part of tissue may involved) & it is always a pathologic process
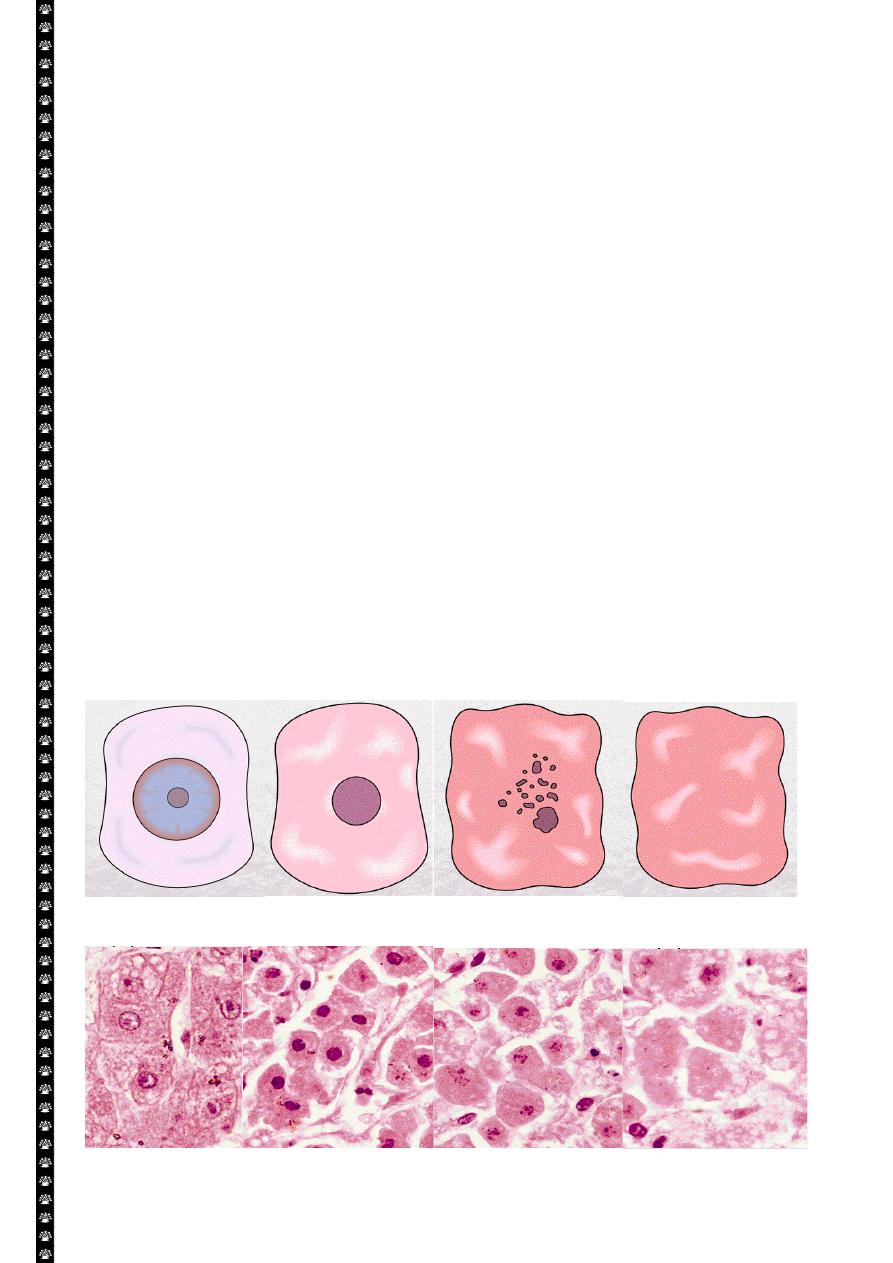
Morphologic features of the necrotic cells
Cytoplasmic changes
1. The necrotic cells (e.g. as a result of oxygen deprivation) show increased
eosinophilia i.e. appear deep pink in color than normal cells. This is attributable in
part to increased binding of eosin to denaturated cytoplasmic proteins and in part
to loss of the basophilia that is normally imparted by the RNA in the cytoplasm
(basophilia is the blue staining from the hematoxilin dye)
2. The cells may have more homogenous appearance than the viable cells, mostly
because of the loss of glycogen particles
3. When enzymes have digested the cytoplasmic organelles, the cytoplasm becomes
vacuolated and appears mouth eaten.
Nuclear changes:-
Assume one of three patterns, all due to breakdown of DNA and chromatin
1. Pyknosis characterized by the nuclear shrinkage and increased basophilia, the
DNA condenses into a solid shrunken mass
2. Karyorrhexis the pyknotic nucleus undergoes fragmentation onto 1 to 2 part, the
nucleus in a dead cell completely disappears.
3. Karyolysis i.e. the basophilia of the chromatin may fade, presumably secondary to
deoxyribonuclease (DNase) activity.
Cell necrosis / Nuclear changes
Liver cell necrosis / Nuclear changes
4
Morphological patterns of tissue necrosis:
Six types of Necrosis
1. Liquefactive
2. Coagulative
3. Gangrenous
4. Fibrinoid
5. Fat
6. Caseous
There are several morphological patterns of tissue necrosis, which may provide clues about
the underlying cause
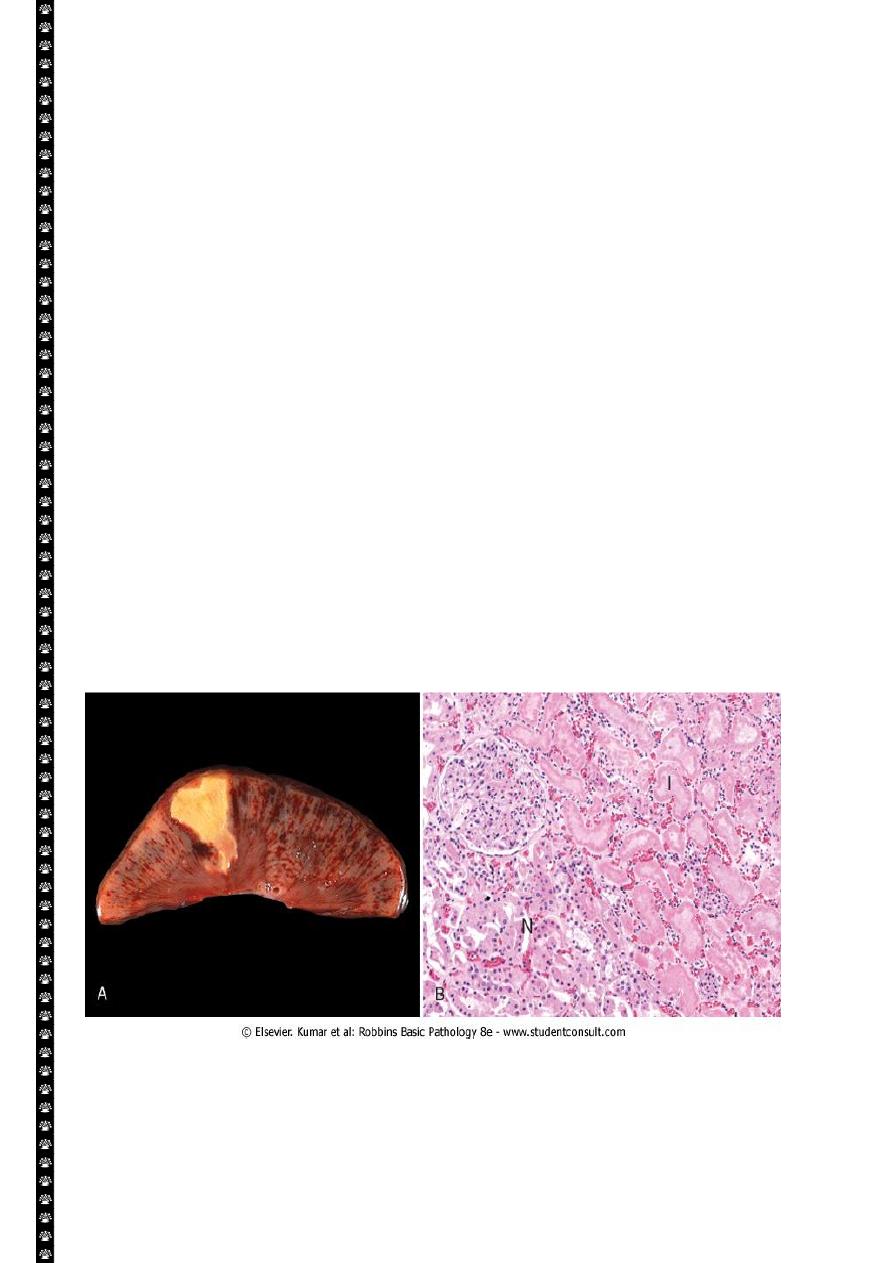
Coagulative necrosis is:-
A form of tissue necrosis. Coagulative necrosis is characteristic of infarcts (area
of ischemic necrosis) in all solid organ except brain. Grossly the affected tissue
have a firm texture
In which the final structural details are lost but the basic tissue architecture is
preserved. This appears to be due to denaturation not only of structural
proteins but also of enzymes, which blocks proteolysis of the dead cells. These
changes are reflected microscopically as homogeneously eosinophilic cells
that are devoid of nuclei.
Ultimately, the necrotic cells are removed by phagocytosis of the cellular
debris by infiltrating leukocytes and by digestion by the action of the lysosomal
enzymes of the leukocytes.
Coagulative necrosis. A, A wedge-shaped kidney infarct (yellow) with preservation of the outlines.
B, Microscopic view of the edge of the infarct, with normal kidney (N) and necrotic cells in the infarct
(I). The necrotic cells show preserved outlines with loss of nuclei, and an inflammatory infiltrate is
present (difficult to discern at this magnification).

5
Coagulative necrosis / myocardial cells
This myocardial infarction is about 3 to 4 days old. There is an extensive acute inflammatory cell
infiltrate and the myocardial fibers are so necrotic that the outlines of them are only barely visible.
The cytoplasm is rather homogeneous, deeply eosinophilic, devoid of cross striation and there are
no nuclei.
Liquefactive necrosis :-
in this type of necrosis there is complete digestion of the dead cells resulting in
transformation of the affected tissue into a liquid viscous mass enclosed within a cystic
cavity. Liquefaction necrosis is seen in two situations
1. Focal bacterial infection( or occasionally fungal) infections. This is because
microbes stimulate the accumulation of inflammatory cells and the enzymes of
the leukocytes digest (liquefy) the tissue. The process is usually due to pyogenic
bacterial –associates acute suppurative inflammation ( abscess); the liquefied
material is frequently creamy yellow and is called pus
2. Ischemic destruction of the brain tissue : for obscure reasons hypoxic death of
cells within the central nervous system often evokes liquefactive necrosis.
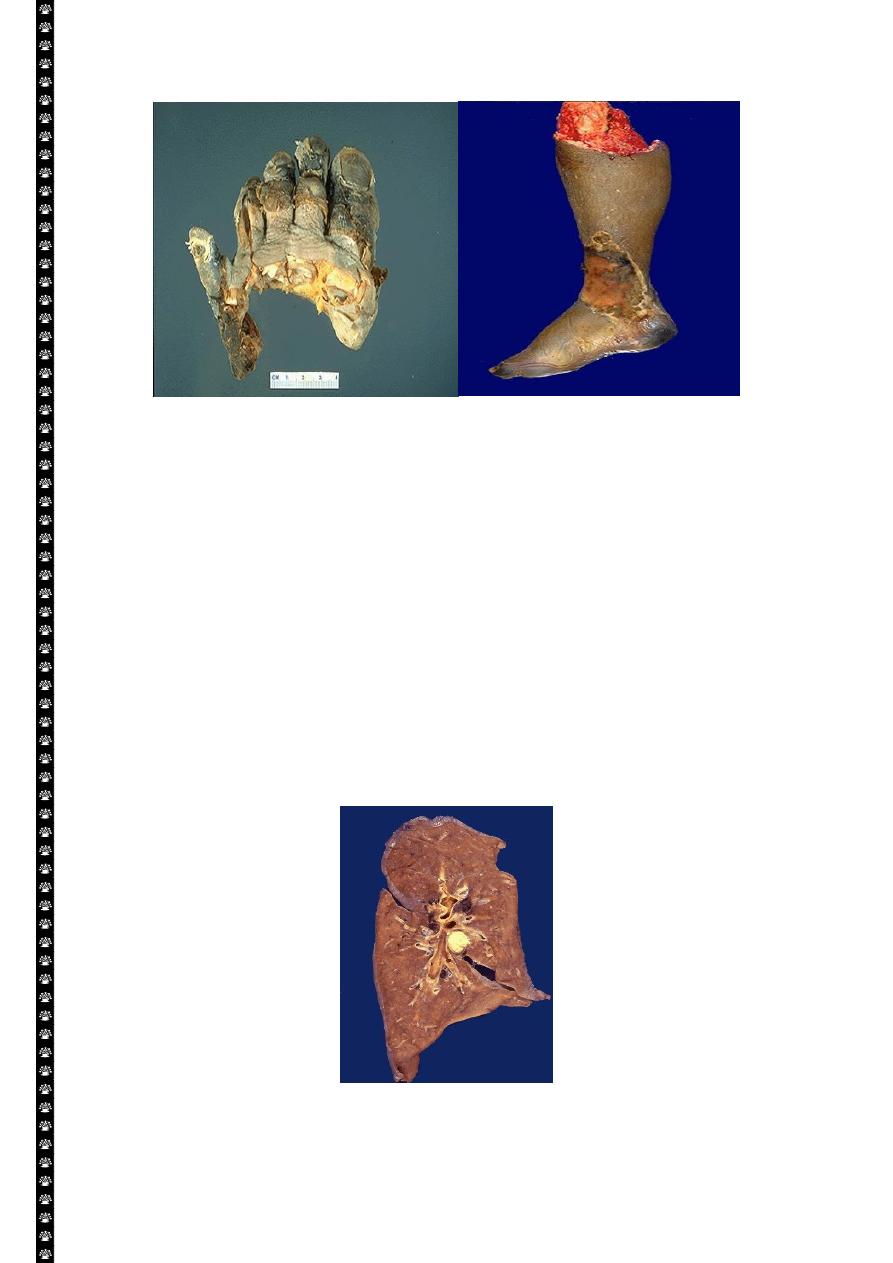
Gangrenous necrosis:-
It is not a distinctive pattern of cell death, however the term is still commonly used in clinical
practice. It is usually applied to a limb, generally the lower leg that has lost its blood supply
and has undergone coagulative necrosis involving multiple tissue layers (dry gangrene) when
bacterial infection is superimposed, coagulative necrosis is modified by the liquefactive
action of the bacteria and the attracted leukocytes (wet gangrene).
Liquefactive necrosis brain
6
Gangrene-lower limb
Left: this is gangrene, or necrosis of the toes that were involved in a frostbite injury. This is an
example of "dry" gangrene in which there is mainly coagulative necrosis from the anoxic injury.
Right: this is gangrene of the lower extremity. In this case the term "wet" gangrene is more
applicable because of the liquefactive component from superimposed infection in addition to the
coagulative necrosis from loss of blood supply. This patient had diabetes mellitus.
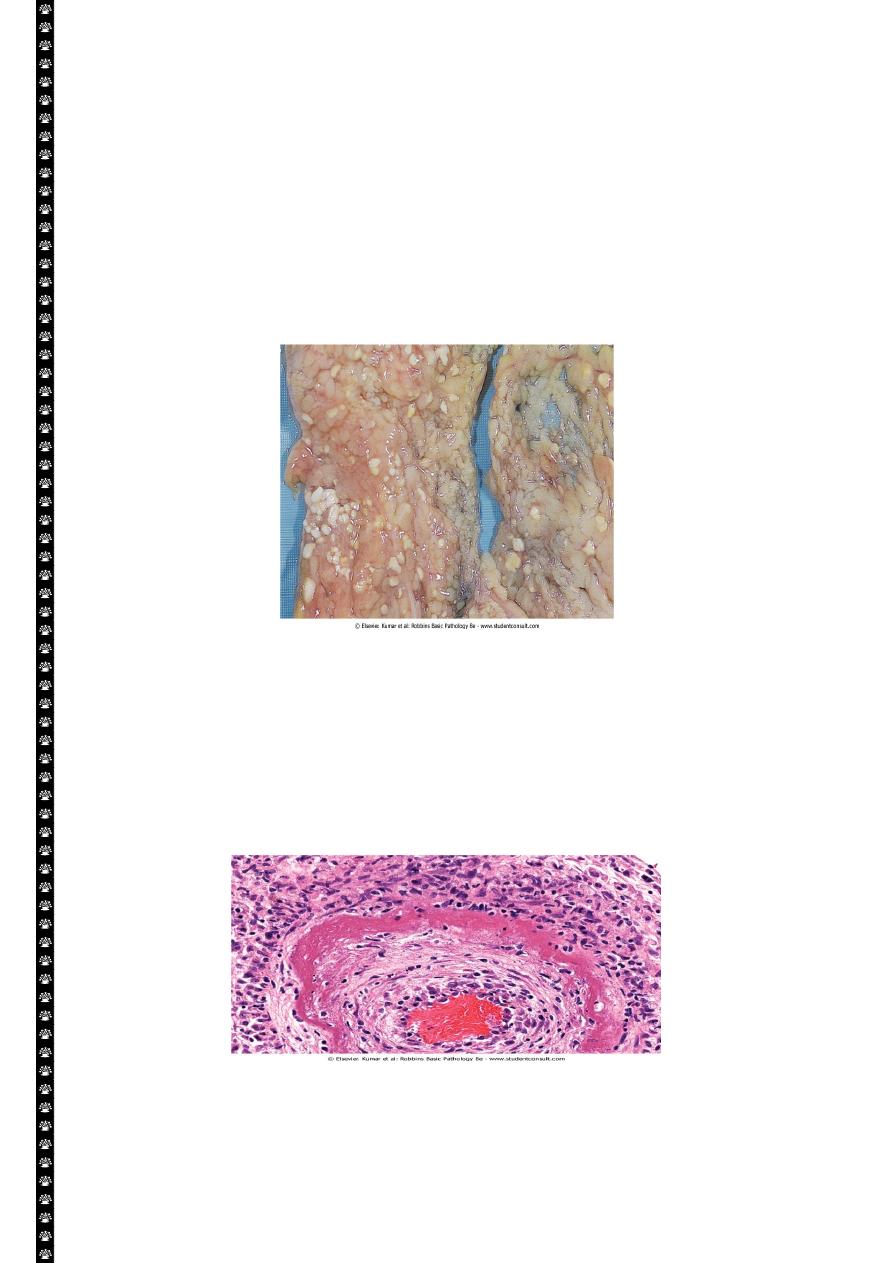
Caseous necrosis :-
Is encountered most often in the foci of the tuberculous infection. The term caseous is ( a
cheese like) is derived from friable yellow white appearance of the area of the necrosis. The
tissue architecture is completely lost and cellular outlines cannot be discerned. Caseous
necrosis is often enclosed within a distinctive inflammatory border; this appearance is
characteristics of granulomatous inflammation.
Caseous necrosis-pulmonary hilar LN
This is the gross appearance of caseous necrosis in a hilar lymph node infected with tuberculosis.
The node has a cheesy tan to white appearance.
7
Fat necrosis
Typically seen in acute pancreatitis and results from release of activated pancreatic lipases
into the substance of the pancreas and the peritoneal cavity. Pancreatic enzymes that have
leaked out of acinar cells liquefy the membranes of fat cells releasing fatty acids that
combined with calcium to produce grossly visible chalky white areas (fat saponification).this
typical appearance enables the surgeon to identify the lesions. Microscopically the foci of
necrosis contain shadowy outlines of necrotic fat cells with bluish calcium deposits
surrounded by an inflammatory reaction. Another example of fat necrosis is seen in female
breasts; at least some of these cases are preceded by a history of trauma (traumatic fat
necrosis)
Fat necrosis in acute pancreatitis. The areas of white chalky deposits represent foci of fat necrosis
with calcium soap formation (saponification) at sites of lipid breakdown in the mesentery
.
Fibrinoid necrosis.
Typically seen immune reactions involving blood vessels. Deposits of immune complexes,
together with fibrin that has leaked out of vessels result in a bright pink and amorphous
appearance in H&E stains. This type is exemplified by the necrosis seen in polyarteritis
nodosa.
Fibrinoid necrosis in an artery in a patient with polyarteritis nodosa. The wall of the artery shows a
circumferential bright pink area of necrosis with protein deposition and inflammation (dark nuclei
of neutrophils).
8
Mechanisms of cell injury
The outcome of the interaction between the injurious agent & the cell depend on:-
1. The type of injury, its duration and its severity.
Thus low doses of toxins or a brief duration of ischemia may lead to reversible cell
injury while large toxin doses or longer ischemic intervals may result in irreversible
injury and cell death.
2. The type, adaptability and genetic makeup of the injured cell The same injury has
vastly outcomes depending on the cell type.
Thus striated skeletal muscle in the leg resist complete ischemia for 2-3- hours
without irreversible injury, whereas cardiac muscle dies after only 20-30 minutes.
The nutritional or hormonal status can also be important; clearly a glycogen filled
hepatocytes will tolerate ischemia much better than one that has just burden its
last glucose molecules.
Genetically determined diversity in metabolic pathways can also be important.
For instance, when exposed for the same dose of a toxin. Individual who inherit
variants in genes encoding cytochrom p-450 may cataboliz the toxin at different
rates leading to different outcomes.
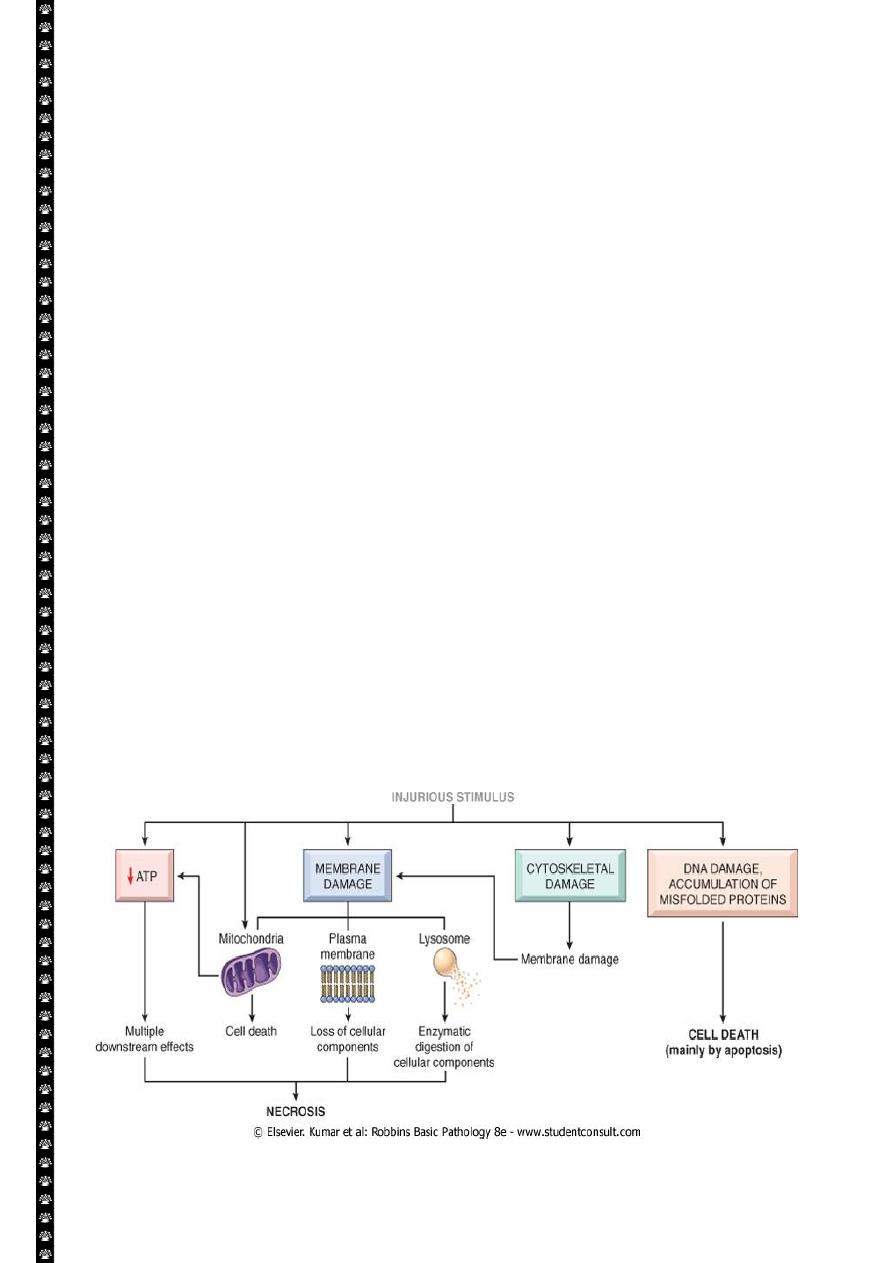
Mechanisms of cell injury
The most important targets of injurious stimuli are-
1. Mitochondria (the site of ATP generation).
2. Cell membrane which influence the ionic and osmotic homeostasis of the cell.
3. Protein synthesis (ribosome).
4. The cytoskeleton (microtubules and various filaments).
5. The genetic apparatus of the cell (nuclear DNA)
9
The principal cellular and biochemical sites of damage in cell injury. Note that loss of adenosine
triphosphate (ATP) results first in reversible injury (not shown) and culminates in necrosis.
Mitochondrial damage may lead to reversible injury and death necrosis or apoptosis.
ATP Depletion:
ATP the energy fuel of cells, is produced mainly by the oxidative phosphorylation of ATP of
the mitochondria. In addition the glycolytic pathway can generate ATP in the absence of
oxygen using glucose derived either from the circulation or from the hydrolysis of
intracellular glycogen (anerobic glycolysis).
The major causes of ATP depletion:-
1. Reduce supply of oxygen and nutrients.
2. Mitochondrial damage.
3. The action of some toxins (e.g. cyadine)
High energy phosphate in the form of ATP is required for virtually all synthetic and
degrdatative and processes within the cell, include membrane transport, protein synthesis,
phospholipids turnover etc. depletion of ATP to less than 5% -10% of normal levels has
widespread effect on many cellular systems
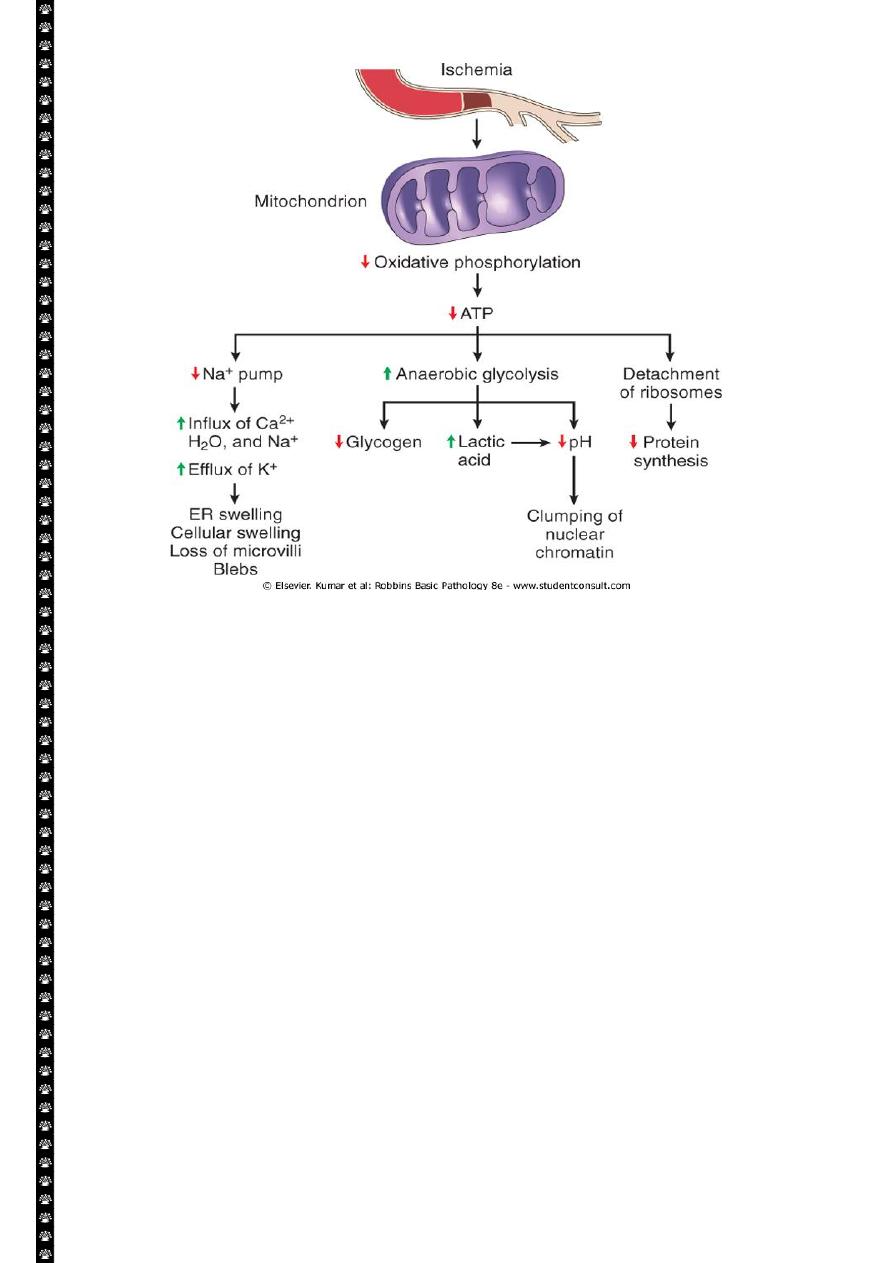
ATP depletion
The activity of plasma membrane energy dependent sodium pump is reduce,
resulting in intracellular accumulation of sodium and efflux of potassium. The net gain
of solute is accompanied by iso- osmotic gain of water, causing cell swelling.
There is a compensatory increase in anaerobic glycolysis in an attempt to maintain
the cell energy sources. As a consequence, intracellular glycogen stores are rapidly
depleted and lactic acid accumulates, leading to decrease activity of many cellular
enzymes (due to decrease pH level).
Failure of the Ca pump lead to influx of Ca with damaging effects on numerous cellular
components, describe below.
Structural disruption of the protein synthetic apparatus manifested as detachment of
ribosomes from the rough endoplasmic reticulum (RER) and polysomes into
monosomes, with a consequent reduction in protein synthesis
Ultimately, there is irreversible damage to the mitochonerial and lysosomal
membranes and the cells undergoes necrosis.
10
The initial functional and morphologic consequences of decreased intracellular adenosine
triphosphate (ATP) during cell injury. ER, Endoplasmic reticulum.
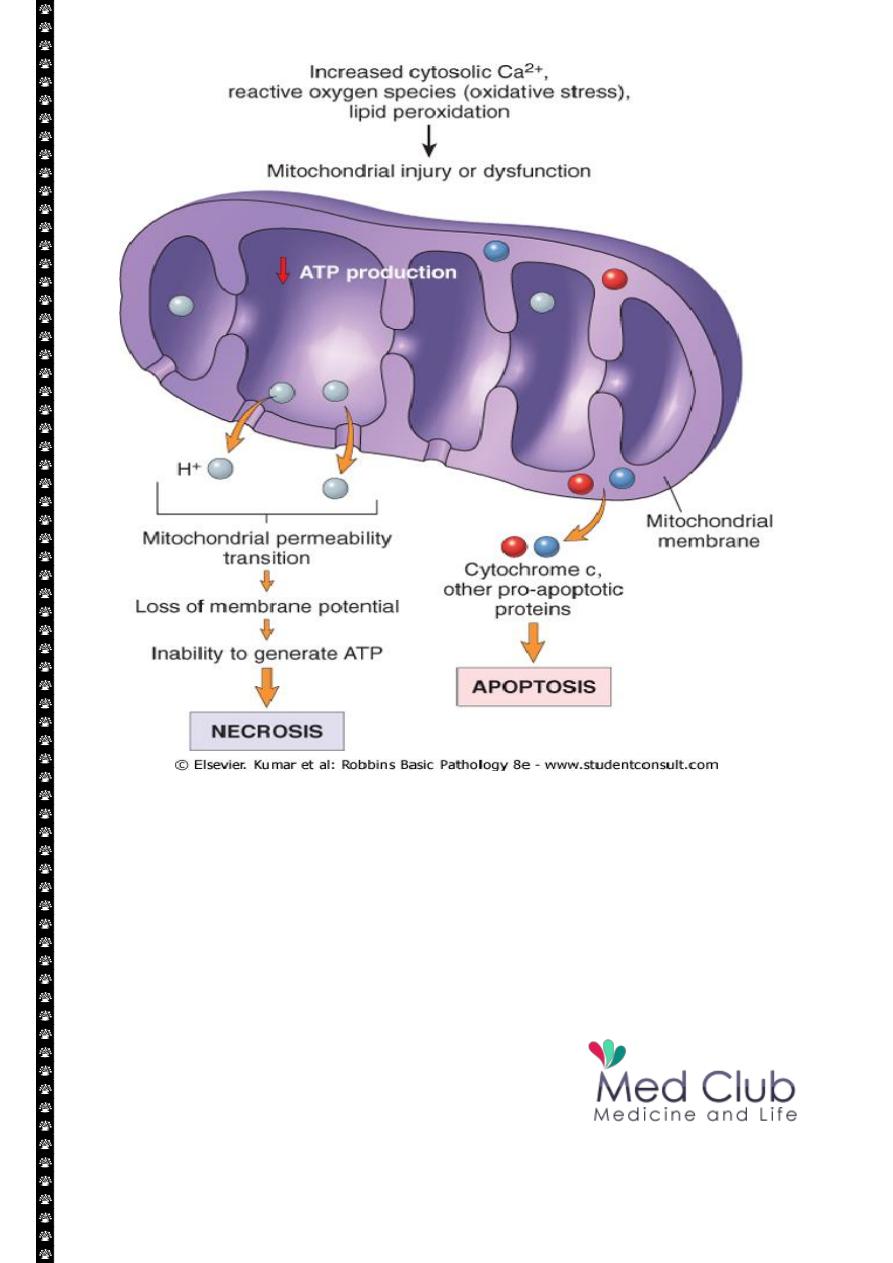
Mitochondrial Damage:
Mitochondria are supplies ATP, but they are also critical players in the cell injury and
death. Mitochondria can be damaged by increase of cytosolic Ca, reactive oxygen species,
and oxygen deprivation, and so they are sensitive to virtually all types of injurious stimuli,
including hypoxia
1. The formation of a channel in the mitochondrial membrane, called the
permeability transition pore. The opening of this channel leads to the loss of
mitochondrial membrane potential and PH changes, resulting in failure of
oxidative phosphorylation and progressive depletion of ATP, culminating in
necrosis of the cells.
2. Increase permeability of the mitochondrial membrane may result in leakage of
cytochrome c ( the major protein involved in electron transport) that are capable
for activating apoptotic pathways. Thus cytochrome c play a key dual role in cell
survival and death in its normal location inside the mitochondria, it's essential for
energy generation and the life of the cell, but when mitochoneria are damaged so
severely that cytochrome c leaks out; its signal to die by apoptosis.
11
Consequences of mitochondrial dysfunction, culminating in cell death by necrosis or
apoptosis. ATP, Adenosine triphosphate.
