HEMODYNAMIC DISORDERSDr.Maha Shakir
Jv = ([Pc − Pi] − σ[πc − πi])Oedema
Thromboembolic DiseaseShock
Definition: Edema is increased fluid in the interstitial tissue spaces or it is a fluid accumulation in the body cavities in excessive amount.
Edema
Depending on the site, fluid accumulation in body cavities can be variously designated as:a) Hydrothorax
b) Hydropericardium
c) Hydroperitoneum (ascites)
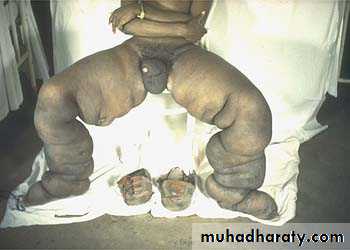
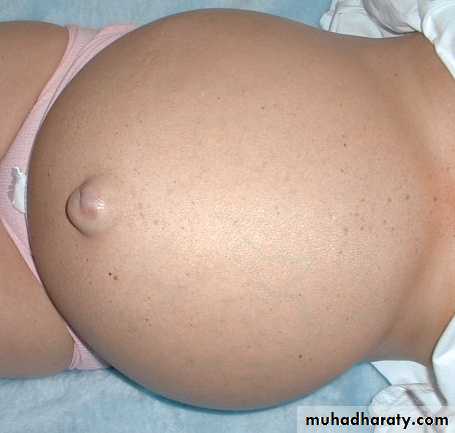
d) Ancsarca – is a severe & generalized edema of the body with profound subcutaneous swelling.
60% of body
2/3 of body water is INTRA-cellular
The rest is INTERSTITIAL
Only 5% is INTRA-vascular
EDEMA is SHIFT to the INTERSTITIAL SPACE
WATERThe capillary endothelium acts as a semi permeable membrane and highly permeable to water & to almost all solutes in plasma with an exception of proteins.
Proteins in plasma and interstial fluid are especially important in controlling plasma & interstitial fluid volume.
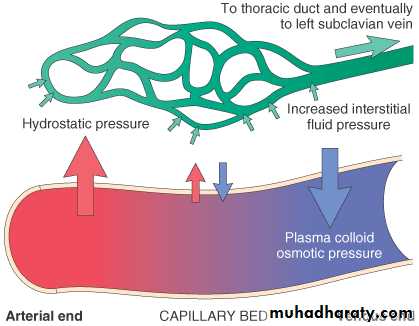
Normally, any outflow of fluid into the interstitium from the arteriolar end of the microcirculation is nearly balanced by inflow at the venular end.
ONLY 5
POSSIBILITIES!!!Increased Hydrostatic Pressure
Reduced Oncotic Pressure
Inreased Vascular permeability
Lymphatic Obstruction
Sodium/Water Retention
Mechanism of development of edema
There are four primary forces that determine fluid movement across the capillary membrane.
I) Hydrostatic and oncotic pressures:
a. The capillary hydrostatic pressureThis pressure tends to force fluid outward from the intravascular space through the capillary membrane to the interstitium.
b. The interstial fluid hydrostatic pressure
This pressure tends to force fluid from the interstitial space to the intravascular space.c. The plasma colloid osmotic (oncotic) pressure
This pressure tends to cause osmosis of fluid inward through the capillary membrane from the interstitium. The plasma oncotic pressure is caused by the presence of plasma proteins.d. The interstial fluid colloid osmotic (oncotic) pressure
This pressure tends to cause osmosis of fluid outward through the capillary membrane to the interstitium.Hence, basically, one can divide pathologic edema into two broad categories:
• Edema due to decreased plasma oncotic pressure.
• 1. Protein loosing glomerulopathies like nephrotic syndrome with leaky glomerulus.
• 2. Liver cirrhosis which leads to decreased protein synthesis by the damaged liver.
• 3. Malnutrition
• 4. Protein loosing enteropathy
B. Edema resulting from increased capillary hydrostatic pressure as in the following diseases:
1. Deep venous thrombosis resulting in impaired venous return.
2. Pulmonary edema
3. Cerebral edema
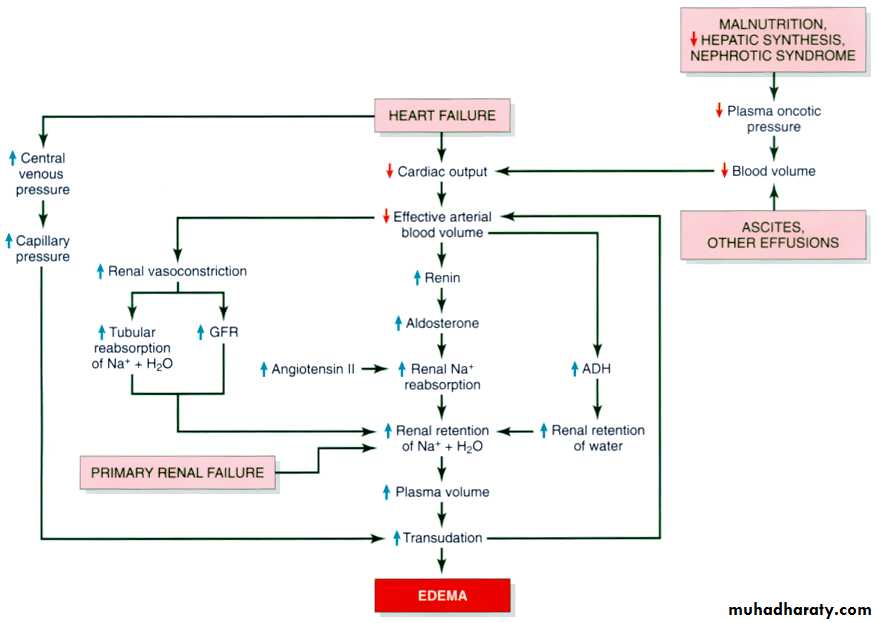
4. Congestive heart failure
Clinical classification of edema:
One can also clinically classify edema into localized & generalized types.1. Localized edema
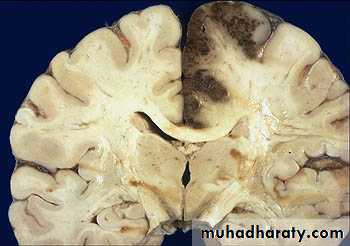
a. Edema of the brain:- May be localized at the site of lesion e.g neoplasm, trauma.
- May be generalized in encephalitis, hypertensive crisis, & trauma
- Narrowed sulci & distended gyri.
- ↑ Edema → compression of medulla towards formen magnum → compression of vital centers lead to →- Hernation of the brain→patient dies
- Usually occurs in left ventricular failure.
- May occur in adult respiratory distress syndrome (ARDS). And infections
- lung ↑ 2.3x its weight.
fluid collect in the alveolar septa around capillaries and impede oxygen diffusion, edema fluid in the alveolar spaces also creates a favorable environment for bacterial infection.
b. Pulmonary edema
a. Reduction of albumin due to excessive loss or reduced synthesis as is caused by:
1) Protein loosing glomerulopathies like nephrotic syndrome2) Liver cirrhosis
3) Malnutrition
4) Protein-losing enteropathy
2. Generalized edema (anasarca) occurs due to:
b. Increased volume of blood secondary to sodium retention caused by congestive heart failure:II) Vascular permeability:
Increased vascular permeability usually occurs due to acute inflammation. chemical mediators are produced. mediators cause increased vascular permeability which leads to loss of fluid & high molecular weight albumin and globulin into the interstitium. Such edema is called inflammatory edema.
Transudate
results from disturbance of Starling forcesspecific gravity < 1.012
protein content < 3 g/dl,
Exudate
results from damage to the capillary wall
specific gravity > 1.012
protein content > 3 g/dl,
Transudate vs Exudate
III) Lymphatic channels:lymphatic system returns to the circulation the small amount of proteinaceous fluid that does leak from the blood into the interstial spaces. Therefore, obstruction of lymphatic channels due to various causes leads to the accumulation of the proteinaceous fluid normally drained by the lymphatic channels. Such kind of edema is called lymphatic edema.
Inflammatory
NeoplasticPostsurgical
Postirradiation
LYMPHATIC OBSTRUCTION(LYMPHEDEMA)
IV)Sodium and water retention:
Sodium & subsequently water retention occurs in various clinical conditions such as congestive heart failure .In these conditions, the retained sodium & water result in increased capillary hydrostatic pressure which leads to the edemaseen in these diseases.
Sodium and water retention:
1- excessive salt intake with renal insufficiency.2- increased tubular reabsorption of sodium
3-Renal hypo perfusion
4-Increased rennin- angiotensin- aldosteron secretion
ASCITES
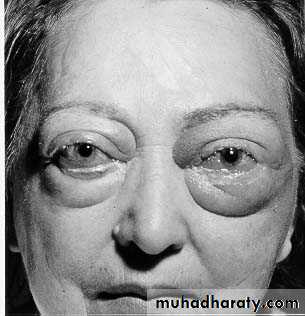
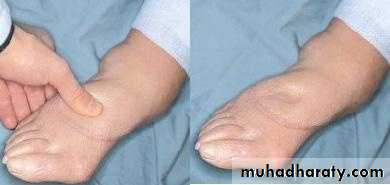
PERIORBITALEDEMA“Pitting” Edema
Thank you
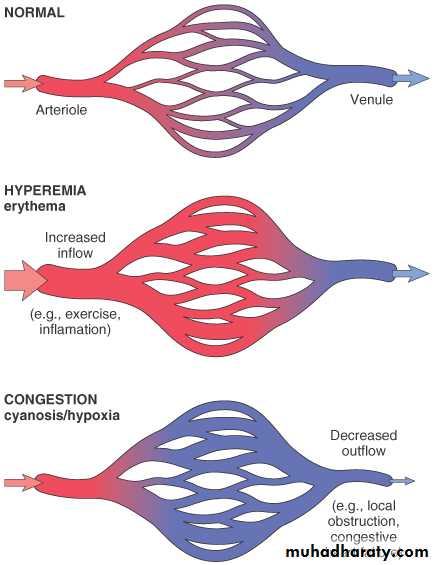
Definition: Both of them can be defined as a local increase in volume of blood in a particular tissue.IV. Hypermia and Congestion
- is an active process resulting from an increased inflow of blood into a tissue because of arteriolar vasodilation.
- commonly occurs in:
exercising skeletal muscle
or acute inflammation.
- Affected tissue becomes red as there is engorgement with oxygenated blood.
Hypermia
- is a passive process resulting from impaired outflow of blood from a tissue.
occurs systemically as in:cardiac failure
or locally as in isolated venous obstruction.
- Affected tissue appears blue-red due to accumulation of deoxygenated blood.
Congestion
In long-standing congestion (also called chronic passive congestion states),poorly oxygenated blood causes hypoxia → results in parenchyma cell degeneration or cell death.
HYPEREMIA/(CONGESTION)
Active Process
HYPEREMIACONGESTION
Passive Process
Acute or Chronic
LUNG
ACUTE
CHRONIC
LIVER
ACUTE
CHRONIC
CEREBRAL
CONGESTION
Cut surface:
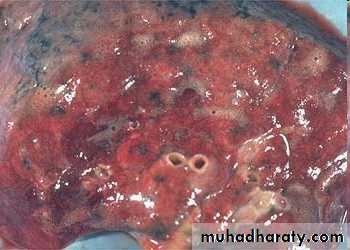
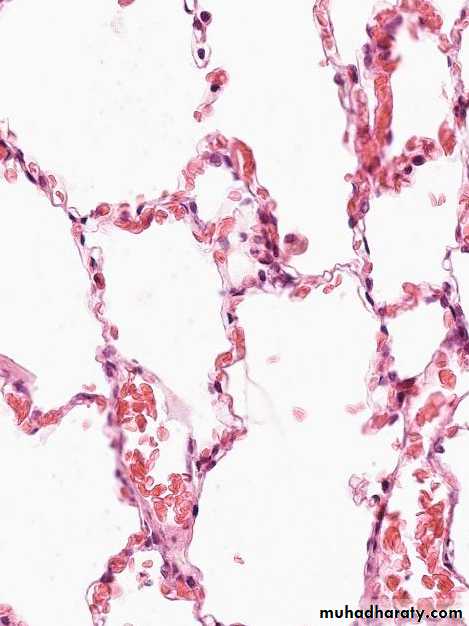
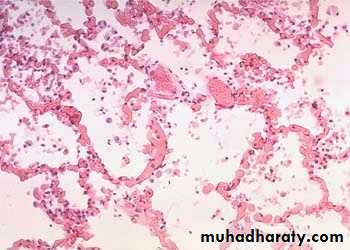
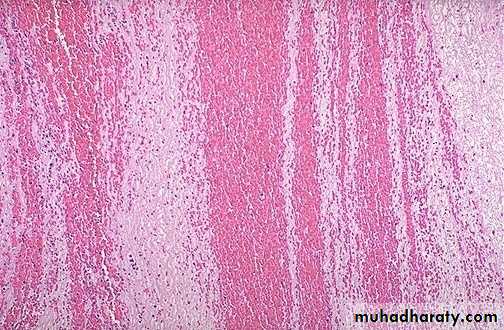
hemorrhagic & wet.1. Acute pulmonary congestion:
Alveolar capillaries engorged with blood
Septal edema
a) Pulmonary congestion
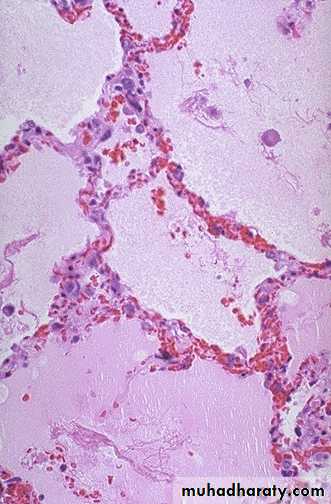
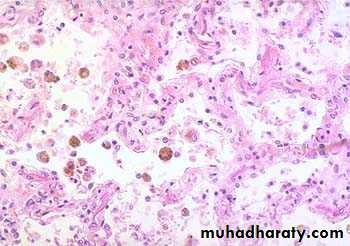
Thickened & fibrotic septaAlveolar spaces contain hemosiderin-laden macrophages resulting in an appearance termed brown indurations.
Can result in pulmonary hypertension.
2. Chronic pulmonary congestion:
Lung, acute pulmonary congestion and edema
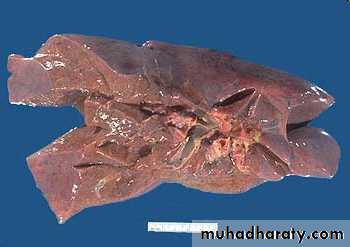
The lung has a red, hyperemic cut surface, reflecting passive congestion, due to increased hydrostatic pressure, as seen in cases of left heart failure. The transudate, mixed with air in the alveoli, gives the cut surface a frothy appearance
Lung, chronic passive congestion
surface The lung has a red-brown color due to accumulation of hemosiderin from extravasated erythrocytes. Fibrosis causes the cut edges to stand up rather than collapse.
ACUTE PASSIVE HYPEREMIA/CONGESTION, LUNG
CHRONIC PASSIVE HYPEREMIA/CONGESTION, LUNG
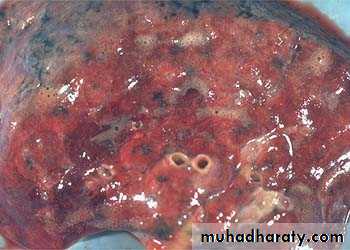
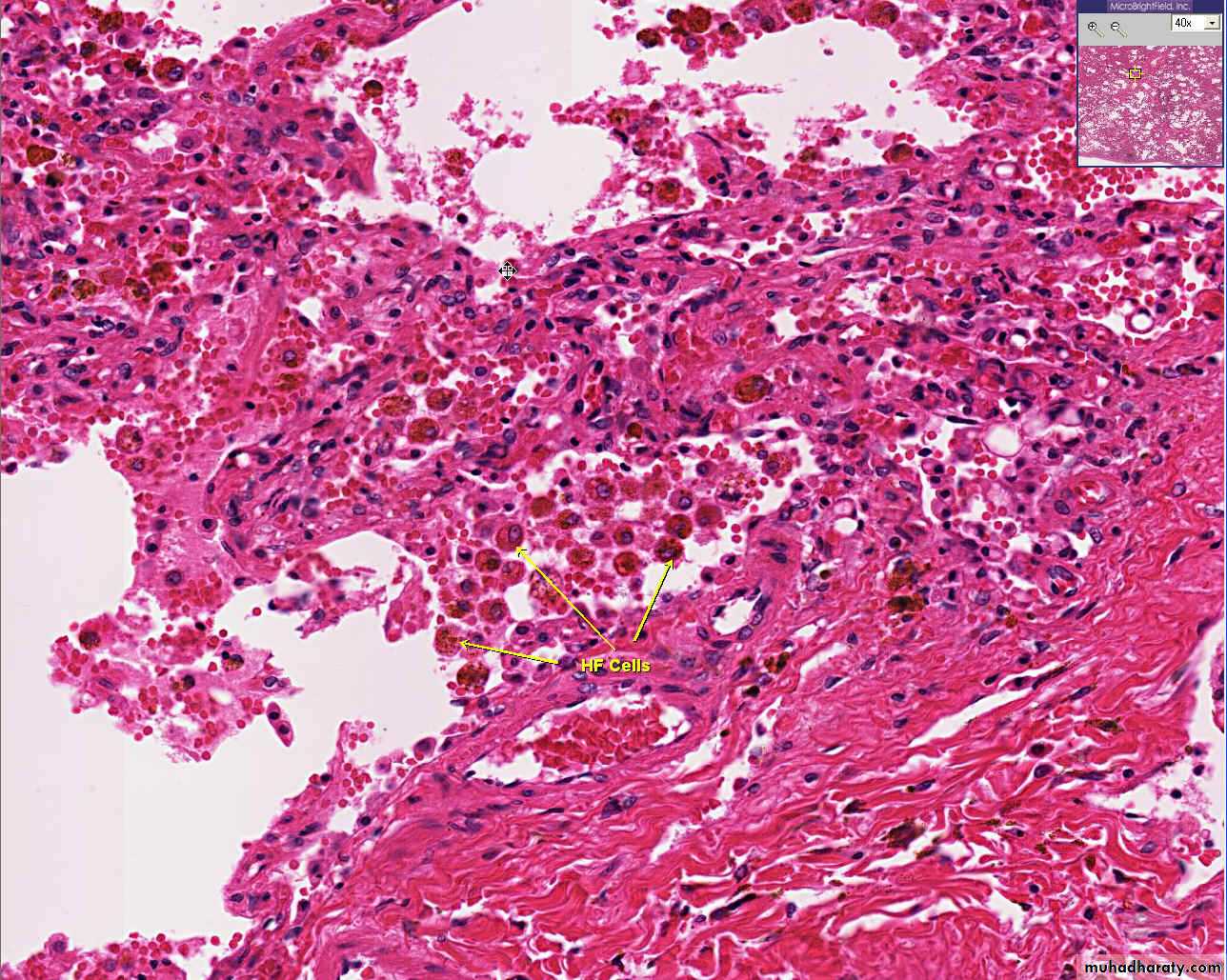
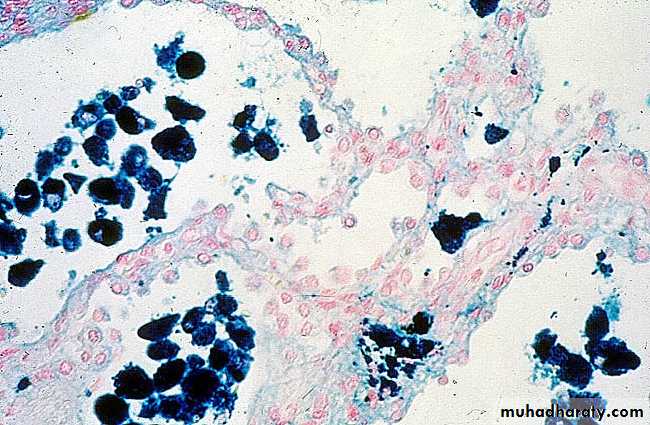
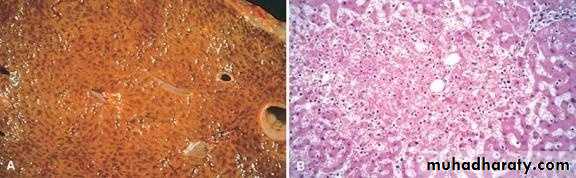
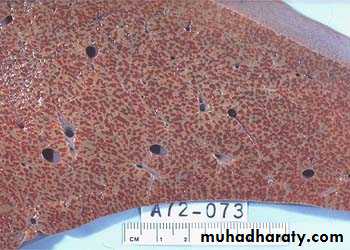
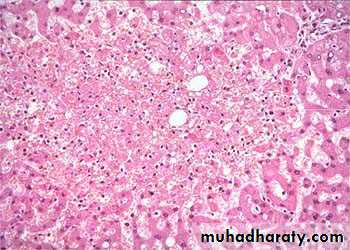
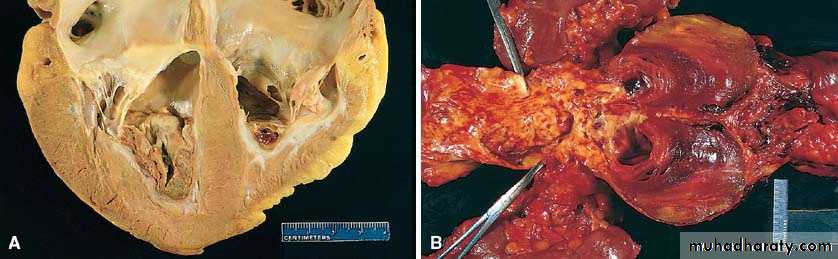
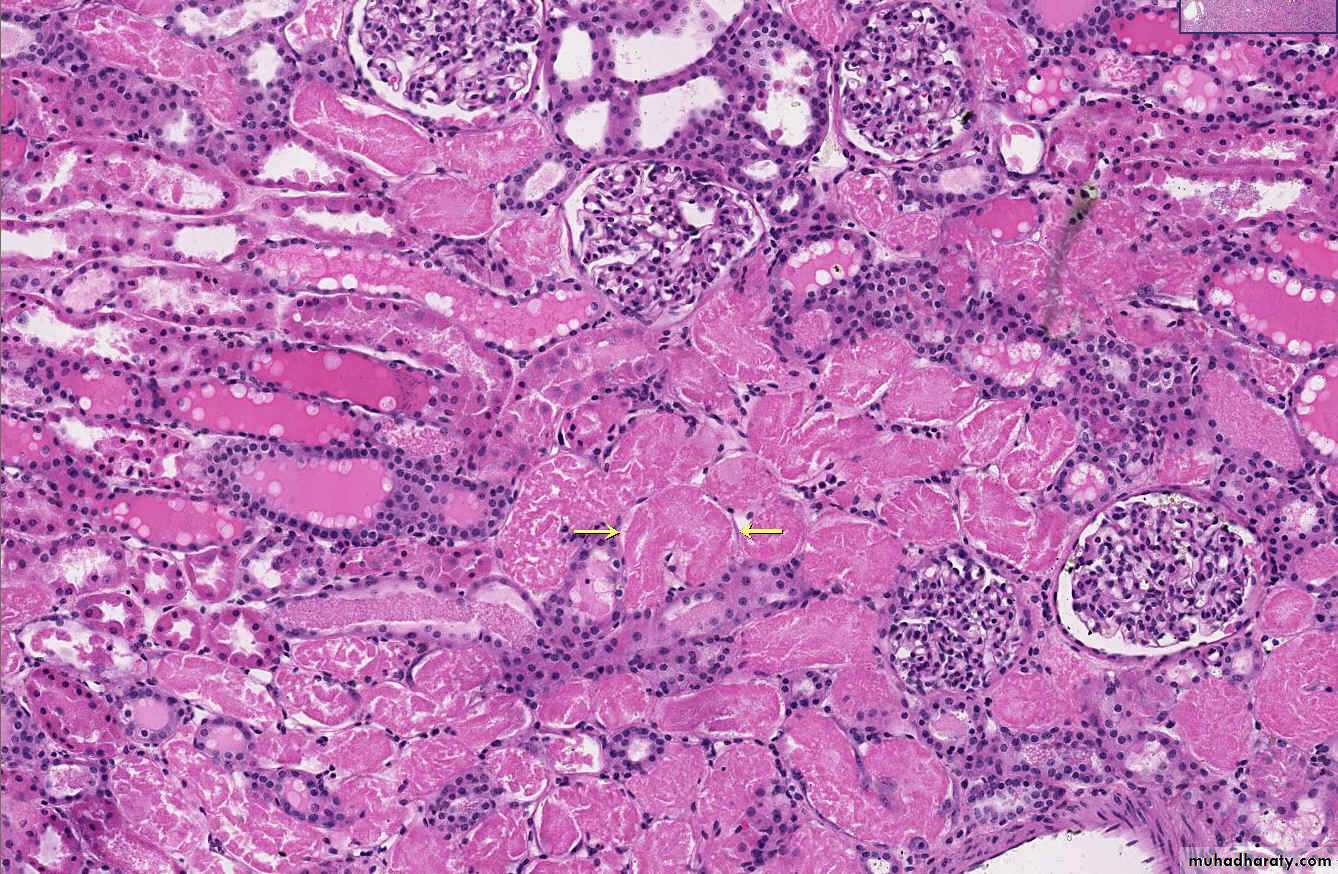
1) Acute hepatic congestion:
Central vein & sinusoids are distendedThere may be even central hepatocyte degeneration.
Peripheral hepatocytes better oxygenated & develop only fatty changes.
b) Hepatic congestion
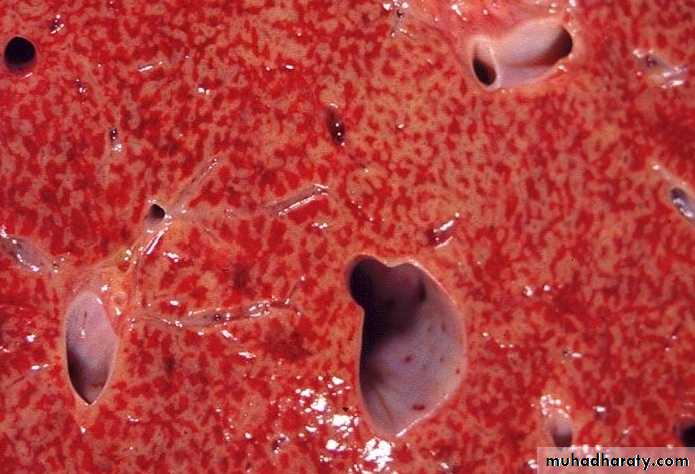
- Central lobules grossly depressed because of loss of cells & appear red brown the surrounding zones uncongested tan, sometimes fatty liver, (nutmeg liver).- Hemosiderin laden macrophages
- In longstanding hepatic congestion, commonly associated with cardiac failure, there is a grossly evident hepatic fibrosis called cardiac cirrhosis.
2) Chronic passive congestion of liver:
Acute Passive Congestion, Liver
Acute Passive Congestion, Liver
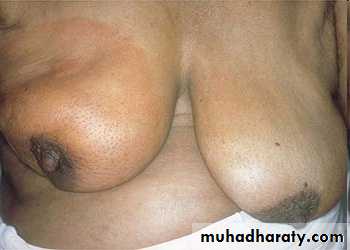
CHRONIC PASSIVE HYPEREMIA/CONGESTION, LIVERBreast, lymphedema secondary to breast carcinoma
– Clinical presentation Breast cancer cells have blocked the lymphatic channels draining fluid from the skin of the right breast, leading to passive congestion and resulting in a peau d'orange (orange skin) appearance. Compare with the normal left breast.
V. Haemorrhage
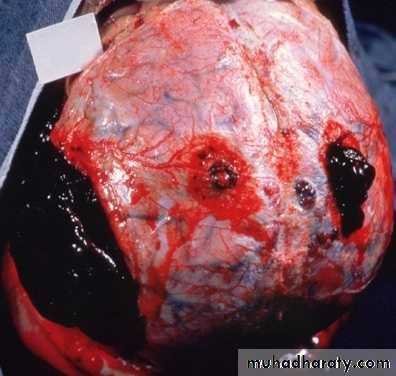
Definition:Hemorrhage is extravasation of blood outside the blood vessel.
Causes:
- Physical trauma – Stabbing
- Stick injury
- Gunshot
- Motor vehicle accident
- Inadequacies in blood clotting which can be due to:
A. Too few or poorly functioning platelets (i.e. qualitative & quantitative defect of platelets)
B. Missing or low amount of clotting factors
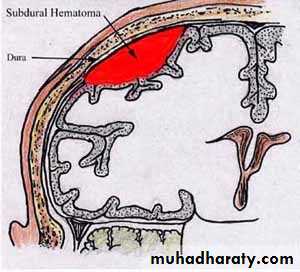
E.g. Low levels of prothrombin, fibrinogen & other precursors.HEMATOMA (implies MASS effect)
PETECHIAE (1-2mm) (PLATELETS)PURPURA <1cm
ECCHYMOSES >1cm (BRUISE)
HEMO-: -thorax, -pericardium, -peritoneum, HEMARTHROSIS
TERMS
ACUTE CHRONICPURPLE GREEN BROWN
HGB BILIRUBIN HEMOSIDERIN
EVOLUTION of HEMORRHAGE
Effects of haemorrhage: depend on the:-rate
-amount of blood loss:
- If > 20% the total blood volume is rapidly lost from the body, it may lead to hypovolemic shock & death.
- Chronic loss of blood leads to anaemia.
Definition: Thrombosis is defined as the formation of a solid or semisolid mass from the constituents of the blood within the vascular system during life.
VII. Thrombosis
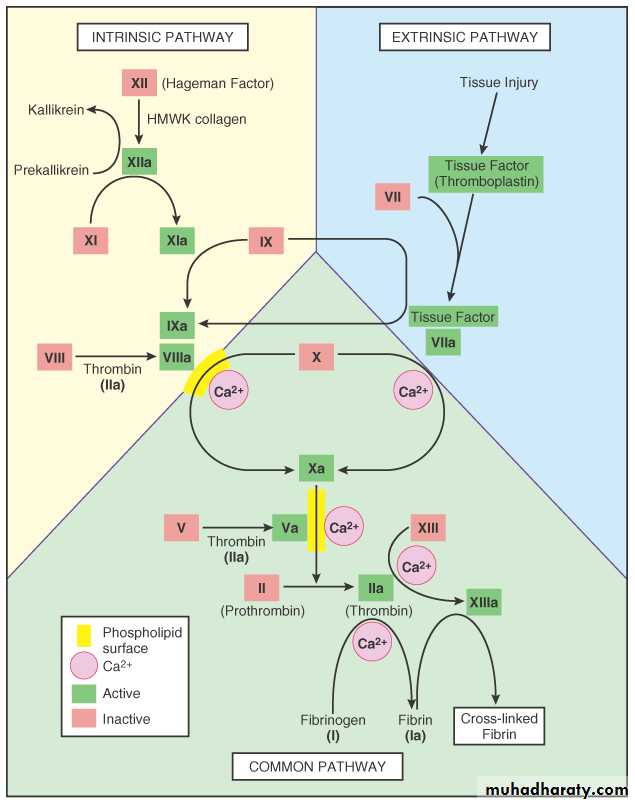
Definition: Hemostasis is the maintainence of the clot-free state of blood & the prevention of blood loss via the formation of hemostatic plug.
Hemostasis
ENDOTHELIUM
PLATELETS
COAGULATION “CASCADE”
PLAYERS
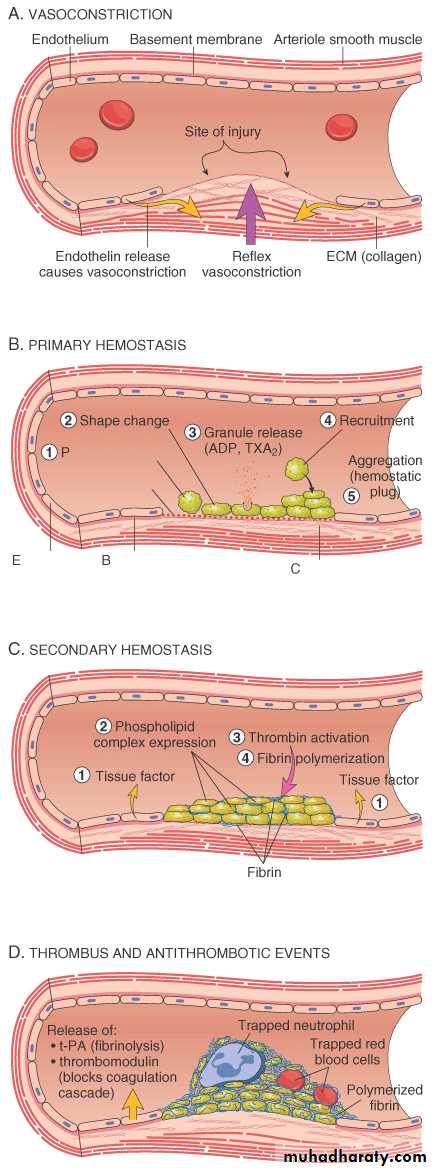
ARTERIOLAR VASOCONSTRICTION
Reflex NeurogenicEndothelin, from endothelial cells
THROMBOGENIC ECM at injury site
Adhere and activate platelets
Platelet aggregation (1˚ HEMOSTASIS)
TISSUE FACTOR released by endothelium, plats.
Activates coagulation cascadethrombinfibrin (2˚ HEMOSTASIS)
FIBRIN polymerizes, TPA limits plug
SEQUENCE of EVENTSfollowing VASCULAR INJURY
Virchow’s TRIANGLE
THROMBOSIS
ENDOTHELIAL INJURY
ABNORMAL FLOW(NON-LAMINAR)
HYPER-
COAGULATION
* It is the most important factor in thrombus formation and by itself can lead to thrombosis.
* Endothelial injury is particularly important in thrombus formation in the heart & arterial circulation.
* Some Examples:
- Endocardial injury during myocardial infarction
- Injury over ulcerated plaque in severely atherosclerotic arteries.
A: Endothelial injury
- In hemodynamic stress like severe hypertension & turbulence of flow over scarred valves directly damaging the endothelium.- Bacterial endtoxin & hyperchloestrolemia, radiation & cigarette smoking may be sources of endothelial injury.
the final event is exposure of the highly thrombogenic subendothelial extracellular matrix, mainly collagen & tissue factors up on which platelets undergo adherence & contact activation.
Under physiologic conditions normal blood flow is laminar, that is, the cellular elements flow centrally in the vessel lumen separated from endothelium by slowing moving clear zone of plasma.
B: Turbulence or Stasis (Alterations in normal blood flow)
a. Disrupt the laminar flow and bring platelets in to contact with the endothelium
b. Prevent dilution of activated clotting factors by freshly flowing bloodc. Retard the inflow of clotting factor inhibitors and permit the build up of thrombi.
Stasis & turbulence therefore:
Stasis is a major factor in the development of venous thrombi
while turbulence contributes to arterial & cardiac thrombosis by causing direct endothelial injury or by forming countercurrents & local pockets of stasis.a) Ulcerated atherosclerotic plaque
b) Aneurysms are favored sites of stasisc) Myocardial infarction has a region of noncontractile myocardium
d) Mitral valve stenosis after chronic rheumatic fever may result in left atrial dilation,
e) Hypervisicosity syndrome
Examples:
Definition: Hypercoagulability is any alteration of the coagulation pathway that predisposes to thrombosis.Hypercoagulability is a less common cause of thrombosis .
C: Hypercoagulablity
1. Primary (Genetic)
- Mutations in factor V [Lieden factor]- Anti thrombin III deficiency
- Protein C or S deficiency
Causes
A: High-risk for hypercoagulablity
- prolonged bed rest or immobilization- Myocardial infarction
- Tissue damage (surgery, fracture, burns)
- cancers
- Prosthetic cardiac valves
- Disseminated intra vascular coagulation.
2. Secondary (Acquired) which, in turn, can be categorized into:
B: Low risk factor for hypercoagulablity
- Atrial fibrillation- Cardiomyopathy
- Nephrotic syndrome
- Smoking
- Oral contraceptives
- Hyperestrogenic state eg. Pregnancy.
Thrombi may develop anywhere in the cardiovascular system.
Variable size and shape.
Arterial or cardiac thrombi usually begin at the site of endothelial injury, e.g. atherosclerotic plaque, or turbulence.
Venous thrombi characteristically occur in site of stasis.
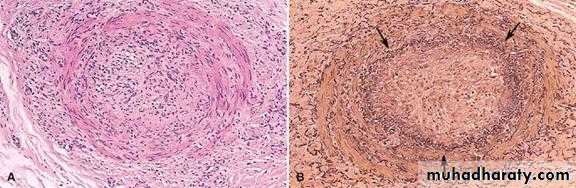
Morphology of Thrombi
-An area of attachment of the underlying vessel or heart wall is characteristically to all thrombi. Tail may not attach and is prone to fragment creating an embolus.Arterial thrombi tend to grow in a retrograde direction from the point of attachment,
whereas venous thrombi extend in the direction of blood flow (i.e., toward the heart).When formed in the heart or aorta, thrombi may have grossly and microscopically apperant laminations called Lines of Zahn , these are produced by pale layers of platelets and fibrin that alternate with darker layers of more red cells. In veins and smaller arteries the lamination typically not apparent, and the thrombi resembles statically coagulated blood.
Such laminations signify that a thrombus has formed in flowing blood; their presence can therefore distinguish antemortem thrombosis from the bland nonlaminated clots that occur postmortem
-When arterial thromi arise in heart chambers or aorta, they are usually applied well to the wall of the underlying structure and are termed as mural thrombi.
-ARTERIAL THROMBI are usually occlusive, the most common site in descending order, are
coronary,cerebral and
femoral arteries.
-The thrombus are usually superimposed on an atherosclerotic plaque.
Abnormal myocardial contraction (arrhythmias), dilated cardiomyopathy, or myocardial infarction leads to cardiac mural thrombi
while ulcerated atherosclerotic plaque and aneurysmal dilation are the precursors of aortic thrombus formation
CARDIAC AND ARTERIAL THROMBOSIS
VENOUS THROMBI or phlebothrombosis, is almost invariably occlusive,most often create a long cast off the vein lumen
known as red thrombi.WHY???
Phlebothrombus most commonly 90% of cases affects the veins of lower extremities.
Postmortem clots are gelatinous with a dark red dependant portion where red cells have settled by gravity, and a yellow chicken fat supernatant,they are not attached to the underlying wall.
In contrast red thrombi are firmer,
almost always have a point of attachment.
Difference from postmortem clots
Thrombi are significant clinically because:
- They cause obstruction of arteries and veins- They are possible source of emboli.
Clinical significance of thrombi- Usually occurs in saphenous venous system, particularly when there are varicosities.
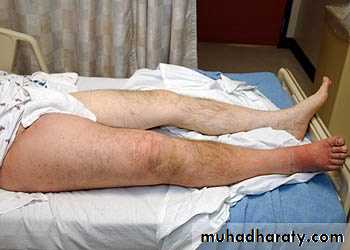
- Rarely embolizes- Causes local edema, pain, and tenderness (i.e. it is symptomatic)
- Local edema due to impaired venous drainage predisposes the involved overlying skin to infection after slight trauma leading to a condition known as varicose ulcer.
1. Superficial venous thrombosis
- May embolize, hence, is more serious.
- Usually starts in deep veins within the calf muscles.
Asymptomatic in 50% of cases
- Has higher incidence in middle aged & elderly people due to increased platelet aggregation & reduced PGI2 production by the endothelium.
2. Deep venous thrombosis (DVT)
1. Trauma, surgery, burns which usually result in:-a:Reduced physical activity leading to stasis
b:Injury to vessels
c:Release of procagulant substance from the tissue
d:Reduced t-PA activity (fibrinolysis)
2. Pregnancy & puerperal states increase coagulation factors & reduce the synthesis of antithrombotic substances.
predisposing factors:
3. Malnutrition, debilitating conditions and wasting diseases such as cancer. DVT due to these conditions is known as marantic thrombosis.
4. Inflammation of veins (thrombophlebitis) also predisposes to thrombosis.
5. Migratory thrombophlebitis is a condition that affects various veins throughout the body & is usually of obscure aetiology, but sometimes it is associated with cancer, particularly pancreatic cancer. Migratory thrombophlebitis is also known as Trosseau syndrome
thrombi may narrow or occlude the lumen of arteries such as the coronary and cerebral arteries.
Occlusion of these arteries will lead to myocardial infarction (MI) & cerebral infarction respectively.
arterial thrombi (especially, cardiac mural thromi) may embolize to any tissue, but, particularly, commonly to the brain, kidney, & spleen WHY????
B. Arterial Thrombosis
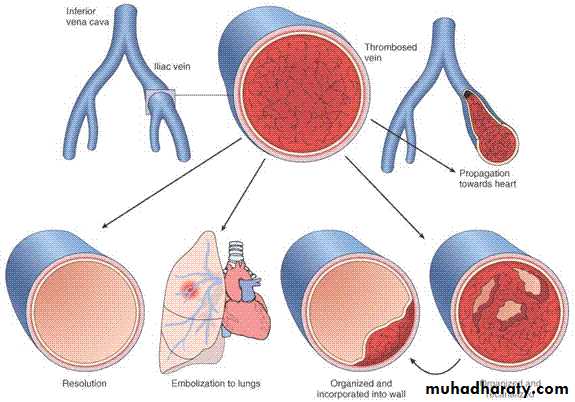
• PROPAGATION (Downstream)
• EMBOLIZATION• DISSOLUTION
• ORGANIZATION
• RECANALIZATION
FATE of THROMBI
OCCLUSIVE ARTERIAL THROMBUS
Definition:-
An embolus is a detached intravascular solid, liquid or gaseous mass that is carried by blood to sites distant from its point of origin. After traveling via the blood, the embolus can obstruct a vessel.
VIII. Embolism
-Thrombus- Platelets aggregates
- Fragment of material from ulcerating atheromatous plaque
- Fragment of a tumour
- Fat globules
- Bubbles of air
- Amniotic fluid
- Infected foreign material
- Bits of bone marrow
- Others.
Causes of embolism
Unless otherwise specified, the term embolism should be considered to mean thromboembolism. This is because thromboembolism is the commonest form of embolism.Thromboembolism
Based on its sites of origin & impaction, thromboembolism can be divided into:
a) Pulmonary thromboembolism (PTE)
b) Systemic thromboembolism
c) Crossed embolism (Paradoxical embolism)
a) Pulmonary thrombeomblism (PTE)
95% of PTE arise from thromi in the deep leg veins. The thromboembolus will travel long with the venous return & reach the right side of the heart. From there, it will go into the pulmonary trunk & pulmonary arteries. Depending on the size of the embolus and on the state of pulumonary circulation, the pulmonary embolism can have the following effects:1. (saddle embolus) causing sudden death by circulatory arrest. Sudden death, right side heart failure (cor pulmonale), or cardiovascular collapse occurs when 60% or more of the pulumonary circulation is obstructed with emboli.
2. If the embolus is very small (as in 60-80% of the cases), the pulmonary emboli will be clinically silent.
3. If the cardiorespiratory condition of the patient is poor then obstruction of a medium sized pulmonary artery by a medium-sized embolus can lead to pulmonary infarction.
4. Recurrent thromboembolism can lead to pulmonary hypertension in the long run.
- Systemic thromboembolism refers to emboli travelling within arterial circulation & impacting in the systemic arteries.
b) Systemic thromboembolism
A- Most systemic emboli (80%) arise from intracardiac mural thrombi.
two thirds of intracardiac mural thrombi are associated with left ventricular wall infarcts
and another quarter with dilated left atria secondary to rheumatic valvular heart disease.
B- The remaining (20%) of systemic emboli arise from aortic aneurysm,
thrombi on ulcerated athrosclerotic plaques,or fragmentation of valvular vegetation.
- Unlike venous emboli, which tend to lodge primarily in one vascular bed (the lung), arterial emboli can travel to a wide variety of sites.
the lower extremities (75%)
& the brain (10%),with the rest lodging in the intestines, kidney, & spleen.
The emboli may obstruct the arterial
blood flow to the tissue distal to the site of the obstruction.
This obstruction may lead to infarction.
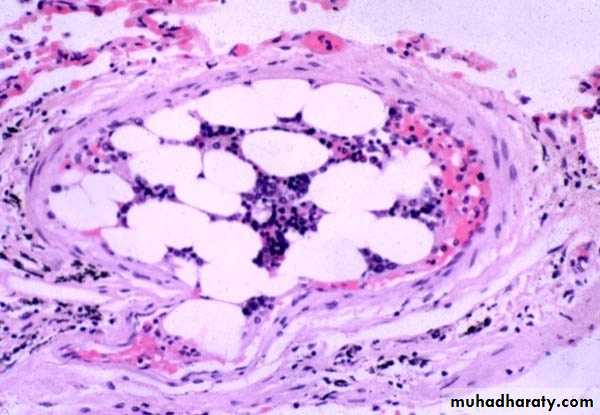
Fat embolism usually follows fracture of bones and other type of tissue injury.
After the injury, globules of fat frequently enter the circulation.it is asymptomatic in most cases and fat is removed.
Fat Embolism
But in some severe injuries the fat emboli may cause occlusion of pulmonary or cerebral microvasculature and fat embolism syndrome may result.
Fat embolism syndrome typically begins 1 to 3 days after injury during which the raised tissue pressure caused by swelling of damaged tissue forces fat into marrow sinosoid & veins.
The features of this syndrome are
a sudden onset of dyspnea, blood stained sputum,tachycardia,
mental confusion with neurologic symptoms
sometimes progress to delirium & coma.
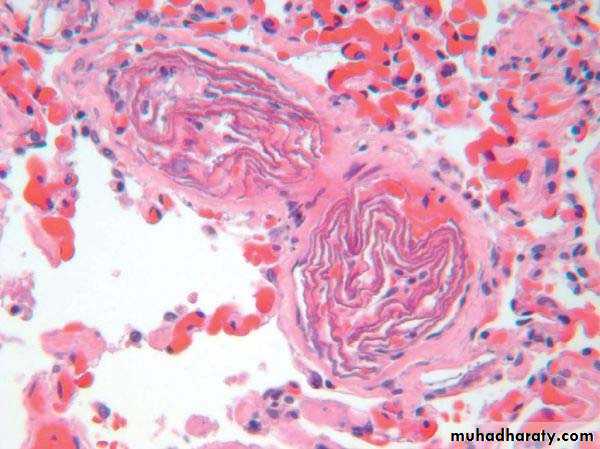
Gas bubbles within the circulation can obstruct vascular flow and cause distal ischemic injury almost as readily as thrombotic masses. Air may enter the circulation during:
5. Air embolism
• Obstetric procedures
• Chest wall injury• In deep see divers & under water construction workers.
• In individuals in unpressurized aircraft
• Neck wounds penetrating the large veins
• Cardio thoracic surgery.
• Arterial catheterisation& intravenous infusion.
• Etc.
Generally, in excesses of 100cc is required to have a clinical effect and 300cc or more may be fatal. The bubbles act like physical obstructions and may coalesce to form a frothy mass sufficiently large to occlude major vessels.
It is a grave but uncommon, unpredictable complication of labour which may complicate vaginal delivery, caesarean delivery and abortions. It had mortality rate over 80%. The amniotic fluid containing fetal material enters via the placental bed & the ruptured uterine veins.
Amniotic fluid embolism
hypotensive shock
seizure & comapulmonary edema typically develops &
50% of the cases will develop DIC
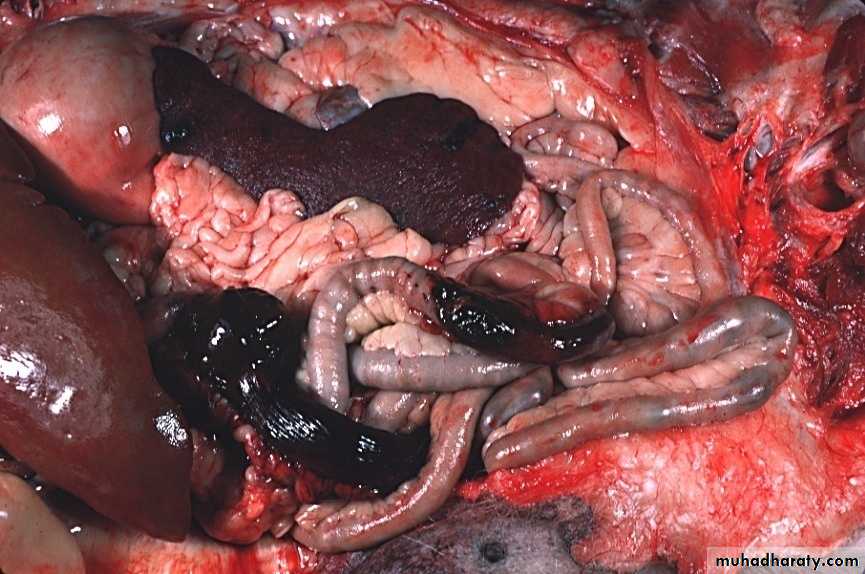
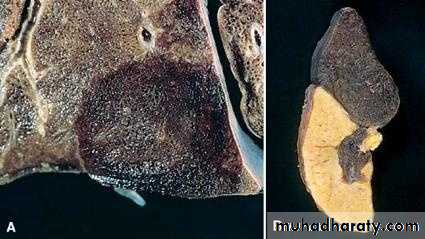
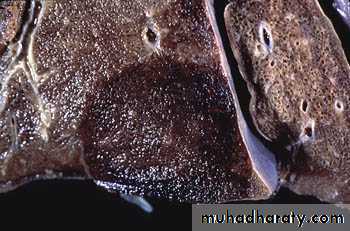
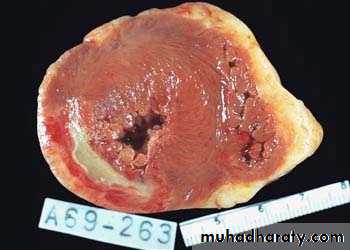
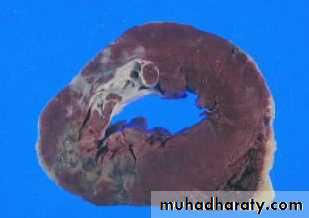
Definition: An infract is an area of ischemic necrosis caused by occlusion of either the arterial supply or venous drainage in a particular tissue.
Nearly 99% of all infarcts result from thrombotic or embolic events.
IX. Infarction
• Local vasospasm
• Expansion of atheroma due to hemorrage in to athermotous plaque.• External compression of the vessels. e.g trauma
• Entrapment of vessels at hernial sacks etc.
Other mechanisms include
The development & the size of an infarct are determined by the following factors:
A. The nature of the vascular supply
B. The rate of development of occlusion
C. Suceptibility of the tissue for hypoxia
D. Oxygen content of the blood
E. The severity & duration of ischemia
A. The nature of vascular supply
The following organs have a dual blood supply.- Lung
→ pulumonary artery
→ Bronchial artery
- Liver
→ hepatic artery
→ Portal vein
• Hand & forearm
→ Radial arteries
→ Ulnar arteries.
The effect of such a dual blood supply is that if there is obsrtuction of one of the arterial supplies, the other one may offset the rapid occurrence of infarction in these organs unlike the renal & splenic circulations which have end arterial supply.
Infarction caused by venous thrombosis is more likely to occur in organs with single venous outflow channels, such as testis &ovary.
B: Rate of development occlusion
Slowly developing occlusions are less likely to cause infraction
C: Tissue suceptibility to hypoxia:
The susceptibility of a tissue to hypoxia influences the likelihood of infarction. Neurons undergo irreversible damage when deprived of their blood supply for only 3 to 4 minutes.Myocardial cells die after 20-30 minutes of ischemia. Fibroblasts are more resistant, especially those in the myocardium.
D: Oxygen content of blood
Partial obstruction of the flow of blood in an anaemic or cyanotic patient may lead to tissue infarction.E: The severity & duration of ischemia.
Infarcts are classified depening on:
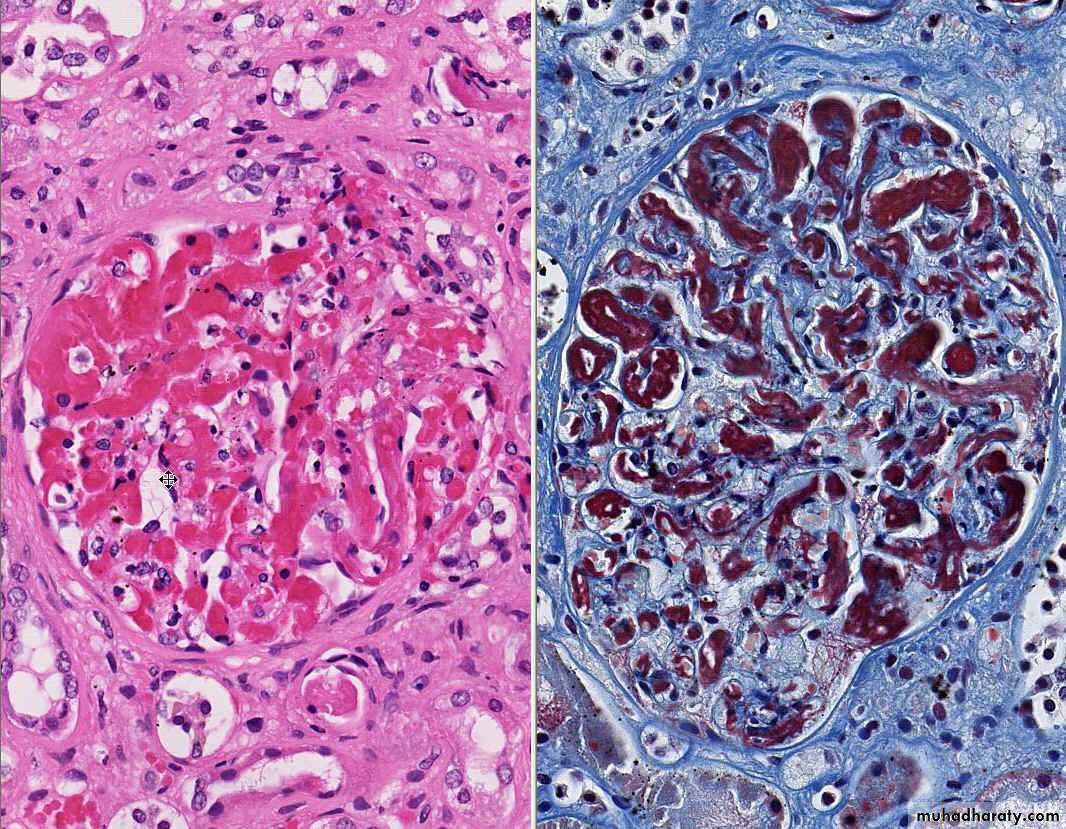
A) the basis of their colour (reflecting the amount of haemorrhage) into:1. Hemorrhagic (Red) infarcts
2. Anemic (White) infarcts
B) the presence or absence of microbial infection into:
1. Septic infarcts
2. Bland infarcts
Types of infarcts
a) Venous occlusions as in ovarian torsion
b) Loose tissues such as the lung which allow blood to collect in infarct zone.c) Tissues with dual circulations (eg. the lung), permitting flow of blood from unobstructed vessel in to necrotic zone.
d) In tissues that were previously congested because of sluggish outflow of blood.
e) When blood flow is reestablished to a site of previous arterial occlusion & necrosis.
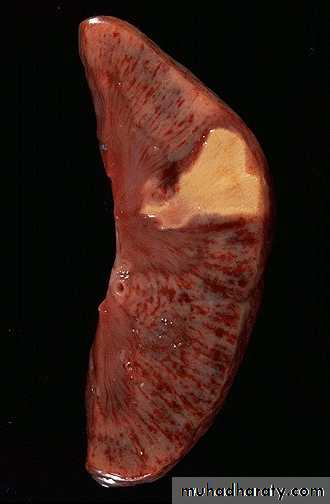
1. Red infarcts occur in
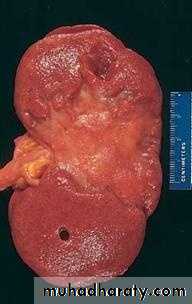
a) Arterial occlusion in organs with a single arterial blood supply.b) Solid organs such as the heart, spleen, & kidney, where the solidity of the tissue limits the amount of hemorrage that can percolate or seep in to the area of ischemic necrosis from the nearby capillaries.
2. White infarcts occur in:
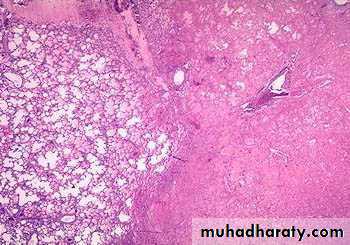
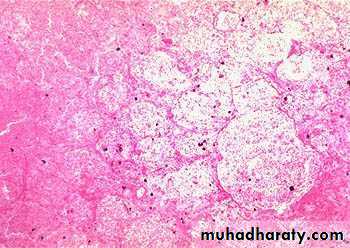
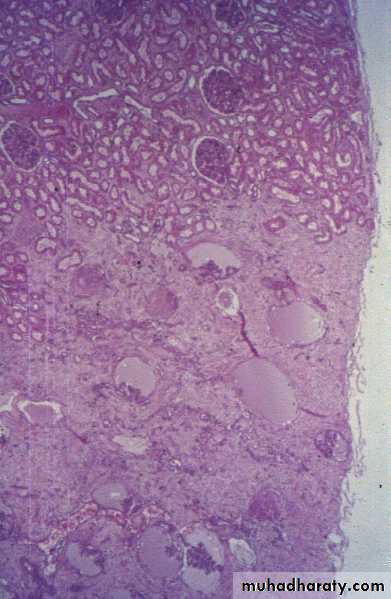
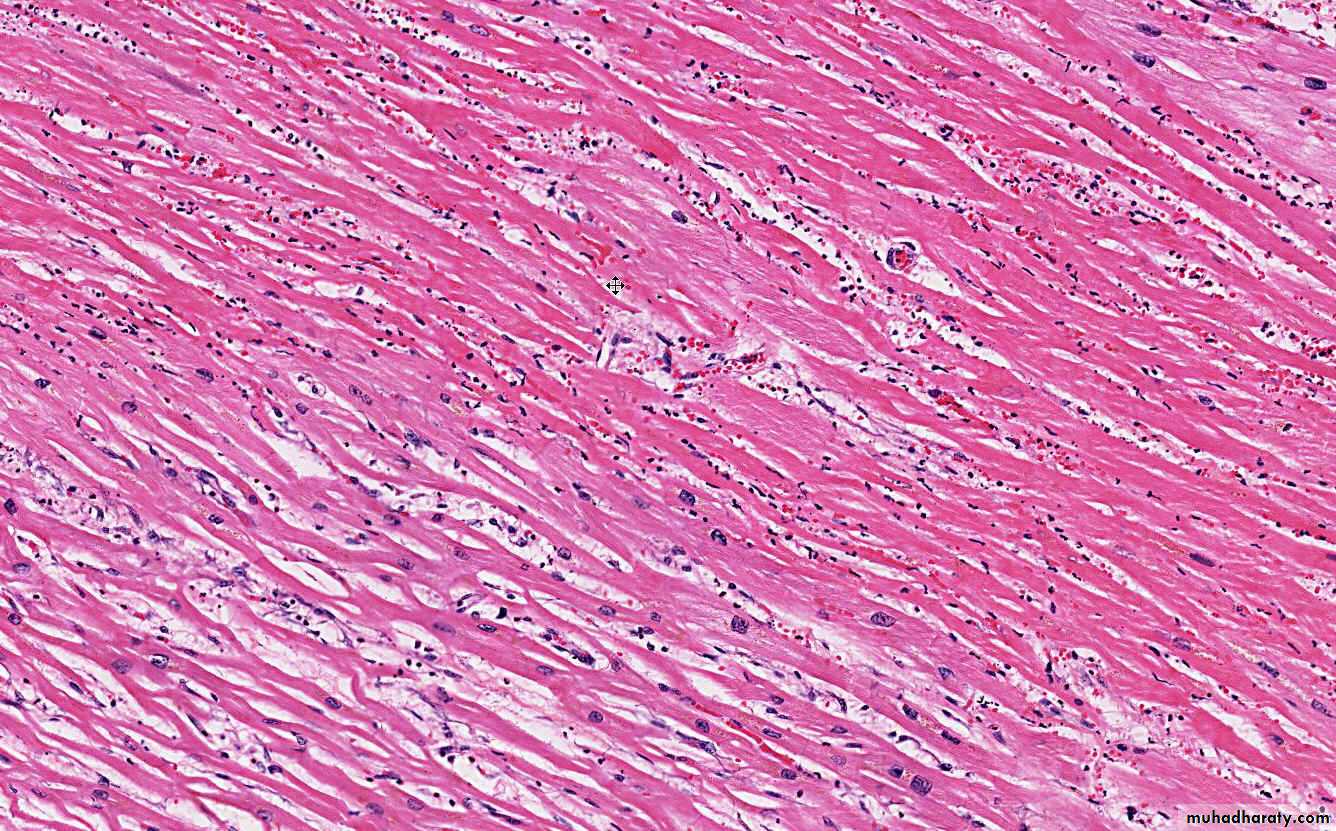
Morphology of infarctsGross: All infarcts are wedge-shaped with the occluded vessel at the apex and the periphery of the organ forming the base of the wedge. The infarction will induce inflammation in the tissue surrounding the area of infarction. Following inflammation, some of the infarcts may show recovery, however, most are ultimately replaced with scars except in the brain.
Microscopy:
The dominant histologic feature of infarction is ischemic coagulative necrosis. The brain is an exception to this generalization, where liquifactive necrosis is common.Amniotic Fluid Embolism
HEART
X. Disseminated Intravascular Coagulation (DIC)
Definition: -DIC is an acute, or chronic thrombohemorrhagic disorder occurring as a result of progressive activation of coagulation pathway secondary to a variety of diseases resulting in failure of all components of hemostasis. Hence the other term for DIC is consumption coagoulopathy.Etiology and Pathogensis.
DIC follows massive or prolonged release of soluble tissue factors & /or endothelial-derived thromboplastin into the circulation with generalized (pathologic) activation of coagulation system.DIC results from pathologic activation of the extrinsic &/or intrinsic pathways of coagulation
or impairment of clot inhibiting influences by different causes.
Two major mechanisms activating the coagulation pathway to cause DIC are:
(1) release of tissue factor or thromboplastic substance into the circulation
(2) widespread injury to the endothelial cells.
1. Tissue thromboplastin substance may be derived from a variety of sources such as:
A: Massive trauma, severe burns & extensive surgery.
B: Obstetric conditions in which thromboplastin derived from the placenta, dead retained fetus, or amniotic fluid may enter the circulation. .
C: Cancers such as acute promyelocytic leukaemia, adenocarcinoma of the lung
D: Gram negative sepsis (an important cause of DIC) in which bacterial endoxins cause release of tissue factor from monocytes.-
2. Endothelial injury: Widespread endothelial injury may result from:
- Deposition of antigen-antibody complexes as it occurs in systemic lupus erythematosus- Extreme temperature eg. Heat stroke, burns
- Hypoxia, acidosis, shock
First, there is a widespread deposition of fibrin within the microcirculation. This may lead to ischemia of the more severely affected or more vulnerable organ
and hemolytic anemia resulting from fragmentation of red cells as they squeeze through the narrowed microvasculature (Microangiopathic haemolytic anaemia).
Clinical Course
*Second, a hemorrhagic diathesis may dominate the clinical picture because of consumption of the coagulation factors and increased fibrinolysis.extensive skin & mucus membrane bleeding
and haemorrhage from multiple sites, usually from surgical incision, vein punctures, or catheter sites.
Respiratory symptoms such as dyspnea, cyanosis may occur.
They may present with convulsion & coma in the case of CNS bleeding
or with acute renal failure with oliguria.
Clinically,
include thrombocytopenia secondary to platelets aggregation in the thrombus,
prolonged PT, PTT, thrombin time &reduced fibrinogen from depleted coagulation proteins.
There is also increased fibrin degradation product (FDP) from intense fibrinolysis.
Laboratory manifestations
Definition: Shock is a state in which there is failure of the circulatory system to maintain adequate cellular perfusion resulting in widespread reduction in delivery of oxygen & other nutrients to tissues.XI. Shock
reduction either in cardiac out put
or in the effective circulating blood volume.-The end results are hypotension followed by impaired tissue perfusion and cellular hypoxia.
Adequate organ perfusion depends on arterial blood pressure (BP) which, in turn, depends on:
1. Cardiac output (CO)
2. Peripheral vascular resistance (PVR)
Shock can be divided into:
A. Hypovolemic shockB. Cardiogenic shock
C. septic shock
Classification of shock
A. Hypovolemic shockDefinition: This is shock caused by reduced blood volume.
Causes of hypovolumic shock include:
a) Haemorrhage
b) Diarrhoea & vomiting
c) Burns
d) Trauma
e) etc
The effect of haemorrhage depends on the rate and amount of blood loss. Hypovolumic shock is the most common type of shock in clinical medicine .
loss of 25% or more of the blood volume results in significant hypovolemia.
Definition: This is shock that results from severe depression of cardiac performance. It primarily results from pump failure [myocardial failure].
o Usually pulmonary oedema coexists.
Causes of cardiogenic shock can be divided into:
A. Myopathic
B. Mechanical
B. Cardiogenic shock
A) Myopathic causes of cardiogenic shock include:1. Acute myocardial infraction.
2. Mycocarditis
3. Dilated cardiomyopathy/hypertrophic cardiomyopathy
B) Mechanical
a) Aortic stenosis, hypertrophic cardiomyopathyb) Aortic or mitral regurgitation
c) Arrhythmia
3) Anaphylactic shock
- Initiated by generalized IgE – mediated hypersensitivity response, associated with systemic vasodilatation & increased vascular permeability.Bactermia is the presence of viable bacteria in the blood as evidenced by blood culture.
Septicemia is systemic infection due the presence of microbes and their toxin the blood.
Definition: This is a kind of shock caused by systemic microbial infection, most commonly by gram – negative infection
Or It can be defined as sepsis with
1. Hypotention
2. Organ dysfunction, &
3. Unresponsiveness to fluid administration.
Septic shock
Pathogenesis of septic shock:It results from the spread & expansion of an initially localized infection
like pneumonia into the blood stream.
Most causes of septic shock (~70%) are caused by endotoxin-producing gram-negative bacilli, hence the term endotoxic shock
Endotoxins are bacterial wall lipopolyschardes (LPS) released when cell walls are degraded.
The mononuclear phagocytes respond to LPS by producing TNF which, in turn, induces IL – 1 synthesis. TNF & IL-1 both act on endothelial cells to produce further cytokines
- septicshock by acting on:
→ The heart – causing decreased myocardial contractility which results in low cardiac output,→ Blood vessel – causing systemic vasodilation
The mediators also cause widespread endothelial injury & activation of the coagulation system resulting in DIC, &
→ Lung – causing alveolar capillary damage resulting in adult respiratory distress syndrome (ARDS).
Stages of shock
Uncorrected shock passes through 3 important stages:
1) An initial nonprogressive phase
o It is also called a period of early compensatory period, during which compensatory mechanisms are activated & perfusion of vital organs maintained.
2. Progressive stage (Established shock)
- This is characterized by tissue hypoperfusion- There is a widespread tissue hypoxia.
- Anaerobic gycolysis results in excessive lactic acid production.
3. An irreversible stage
- A stage at which, even if hemodynamic disorders are corrected survival is not possible.- Transition to irreversible damage is mediated via various mechanisms.
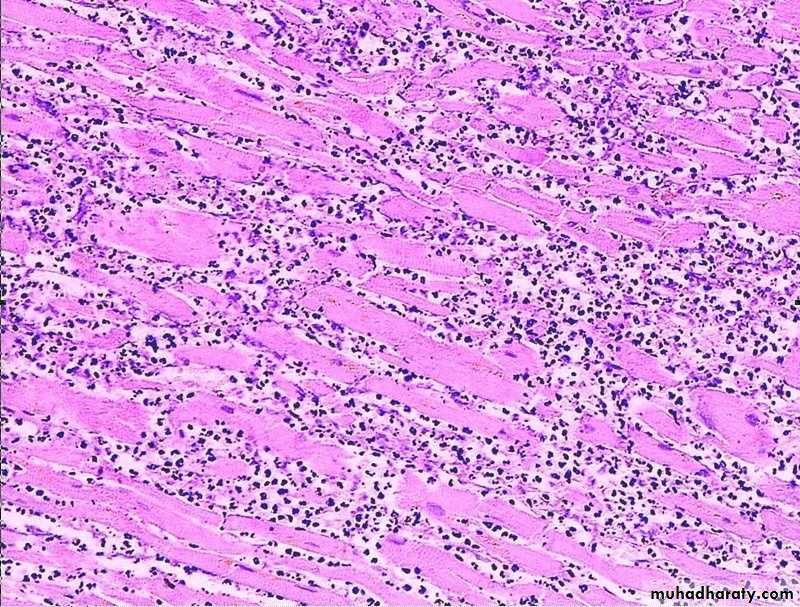
Morphology of septic shock:
All organs are affected in severe shock. In shock, there is widespread tissue hypoperfusion involving various organs such as the heart, brain, & kidney.widespread hypoxic tissue necrosis.
The widespread tissue necrosis manifests as multiple organ dysfunction [MODS].
And lungs may show ARDS or Shock lung.
MYOCARDIAL NECROSIS
ATN
DIC