Secondary Hemostasis
Session 8 ,9 2016Objectives
1. Clotting pathways2. Anticlotting mechanisms:
Fibrinolysis
anticoagulants
The primary haemostasis (vascular spasm & platelet plugs) alone is not sufficient to close tears or cuts. When a blood vessel is severely damaged coagulation or blood clotting results in the formation of a clot (a clot is a network of thread like protein fibers called fibrin that traps blood cells, platelets and fluid). More than 50 important substances that affect blood coagulation have been found in the blood, and tissues, some that promote coagulation called procoagulants & others that inhibit coagulation called anticoagulants. Whether or not the blood will coagulate depends on the balance between these two groups of substances. The anticoagulants normally predominate & the blood does not coagulate, but when a vessel is ruptured, procoagulants in the areas of damage become activated & override the anticoagulants & then a clot (fibrin) does develop. The formation of a blood clot depends on a number of proteins found within plasma called coagulation or clotting factors. Normally the coagulation factors are in an inactive state and do not cause clotting. After injury the clotting factors are activated to produce a clot. System for naming blood-clotting factors is shown in the table a series of 12 factors was designated with Roman numerals I through XIII according to the order of their discovery & not their reaction sequence.
Table: system for naming blood-clotting factors.
FactorRoman
numberArabic
Number
Names
I
1
Fibrinogen
II
2
Prothrombin
III
3
Tissue thromboplastin
IV
4
Calcium
V
5
Labile factor
VII
7
Stable factor
VIII
8
Antihemophilic factor A, classic factor,
von-Willebrand factor
IX
9
Antihemophilic factor B, Christmas factor.
X
10
Stuart-Prower factor
XI
11
Antihemophilic factor C, plasma thromboplastin
XII
12
Hageman factor, glass factor
XIII
13
Fibrin-stabilizing factor
HMW-K
-
High-molecular-weight kininogen
Pre-K
-
Prekalikrein
PL, or PF3
-
Platelet phospholipids , or (Platelet factor 3)
Secondary hemostasis consists of a series of biochemical events resulting in the formation of fibrin clot. The clotting mechanism is subdivided into three interacting pathways:
The intrinsic pathway.
The extrinsic pathway.
3-The common pathway
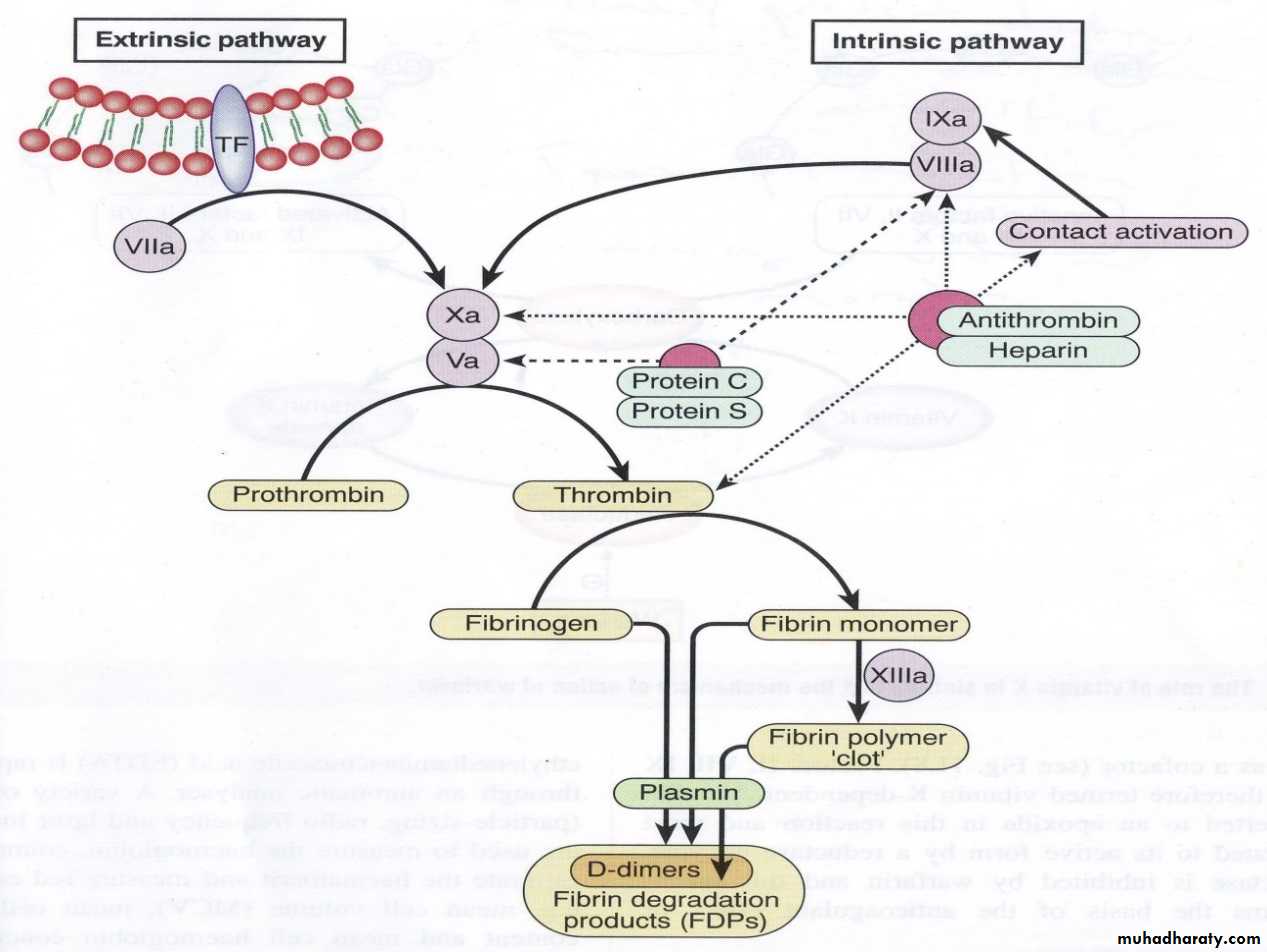
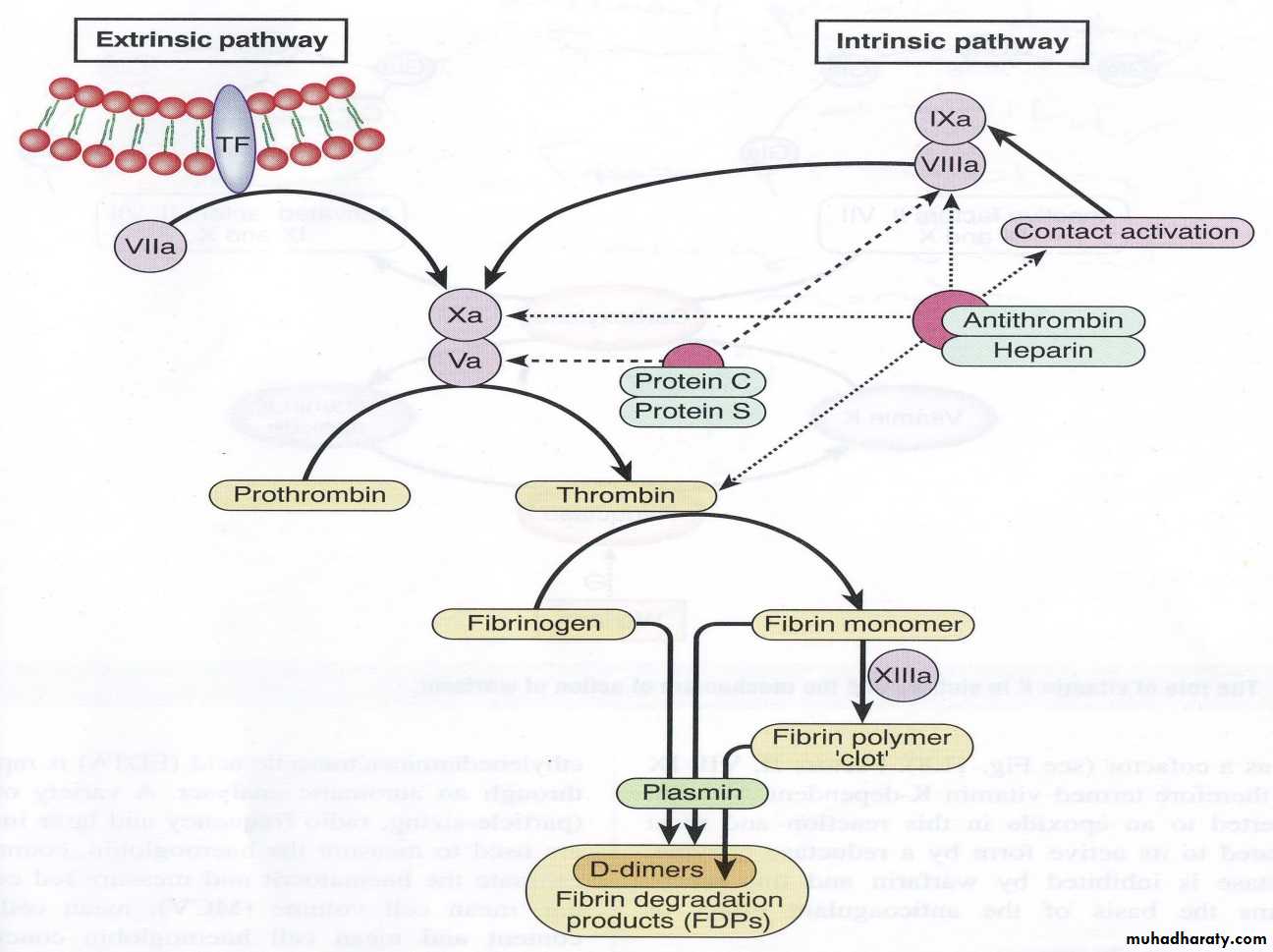
Each pathway involves reactions with a specific group of factors. Both the intrinsic & extrinsic systems eventually activate the common pathways, & influence one another. The clotting mechanism responsible for the formation of fibrin involves a cascade of reactions in which inactive enzymes are activated (a) and the activated enzymes in turn activate other inactivate enzymes. The fundamental reaction in the clotting of blood is conversion of the soluble plasma protein fibrinogen to insoluble fibrin.
Intrinsic pathway: The intrinsic pathway is named because it begins with chemicals that are inside or intrinsic to the blood. This pathway leading to the activation of Stuart Prower factor (factor X) which in turn leads to conversion of fibrinogen (factor I) into fibrin (clot) in the common pathway.
Vascular injury causes exposure of collagen of damaged blood vessels which in turn activate Hageman factor or glass factor (factor XII) and finally there is activation of factor X which in turn activate common pathway and formation of fibrin which stops the bleeding. Clotting can occur in 1- 6minutes by intrinsic pathway.
2. Extrinsic pathway:
The name extrinsic is derived from the fact that activation of this pathway requires a factor not normally present in the blood but in the most cells of the body. This factor is known as tissue factor or tissue thromboplastin (factor III).Vascular injury cause the release of tissue factor which is in turn cause activation of Stuart Prower factor ( the first step in the common pathway) followed the conversion of fibrinogen into fibrin. Clotting can occur in 15 seconds by extrinsic pathway.
Interaction between extrinsic and intrinsic pathway: a. the end result of both intrinsic and extrinsic pathway is to activate Stuart Prower factor after the blood vessels rupture. B.Clotting is initiated by both pathways simultaneously.C.an important difference between the two pathway that in severe tissue trauma, the intrinsic pathway requiring 1-6 minute to cause clotting (slow process) while extrinsic requiring 15 seconds(rapid process) i.e. The intrinsic pathway requires more time to be activated than the extrinsic pathway.
3.Common pathway: This is activated by both intrinsic and extrinsic pathways.
Intrinsic
IXa, VIIIa; PF3; Ca++
X Xa
VIIa, activated tissue factor (IIIa);
Ca++; PF3
Extrinsic
The common pathway consists of 5 different steps:
1. Activation of Stuart Prower factor by both intrinsic and extrinsic pathways.
2. Formatoin of prothrombin activator by activated factor X(X a).
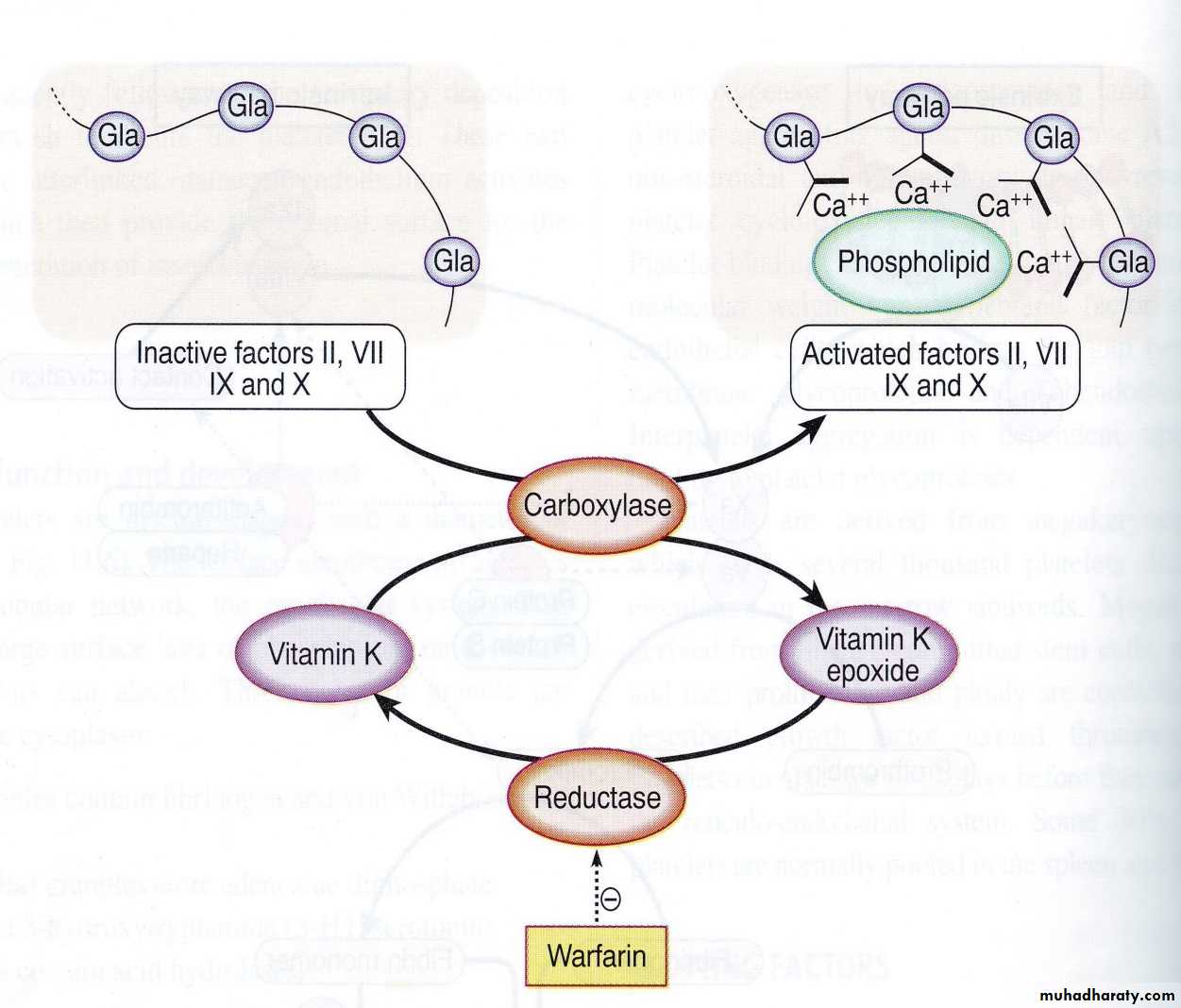
3. Conversion of prothrombin (factor II) into thrombin by prothrombin activator. Prothrombin is a α-globulin plasma protein, & is formed continually by the liver cells. Normal concentration in plasma is 15mg/dL. Vitamin K is required by the liver for normal formation of prothrombin. In liver disease, there is lack of vitamin K that prevents normal prothrombin synthesis can decrease the level of prothrombin in plasma & bleeding tendency results.
4. Conversion of fibrinogen into fibrin by thrombin.
5. Stabilization of fibrin: The fibrin fibers adhere to damaged surfaces of blood vessels and there by prevents blood loss. The fibrin (clot) is initially a loose mesh of interlacing strands i.e. the resultant clot (fibrin) is weak & can be broken apart with ease. Activated – stabilizing factor (XIIIa) convert weak soluble fibrin into a dense, light, stable, insoluble fibrin (clot).
If the blood is drawn into an untreated glass test tube it will clot within 6-10 minutes. If it is drawn into siliconized containers or into a nonwettable plastic test tube, it will remain fluid for a period of more than 1 hour.i.e does not clot. In the untreated glass test tube there is activated of factor XII (glass factor or contact factor) which initiate the intrinsic clotting mechanism. On the other hand siliconized containers prevents contact activation of factor XII so no initation of intrinsic pathway.
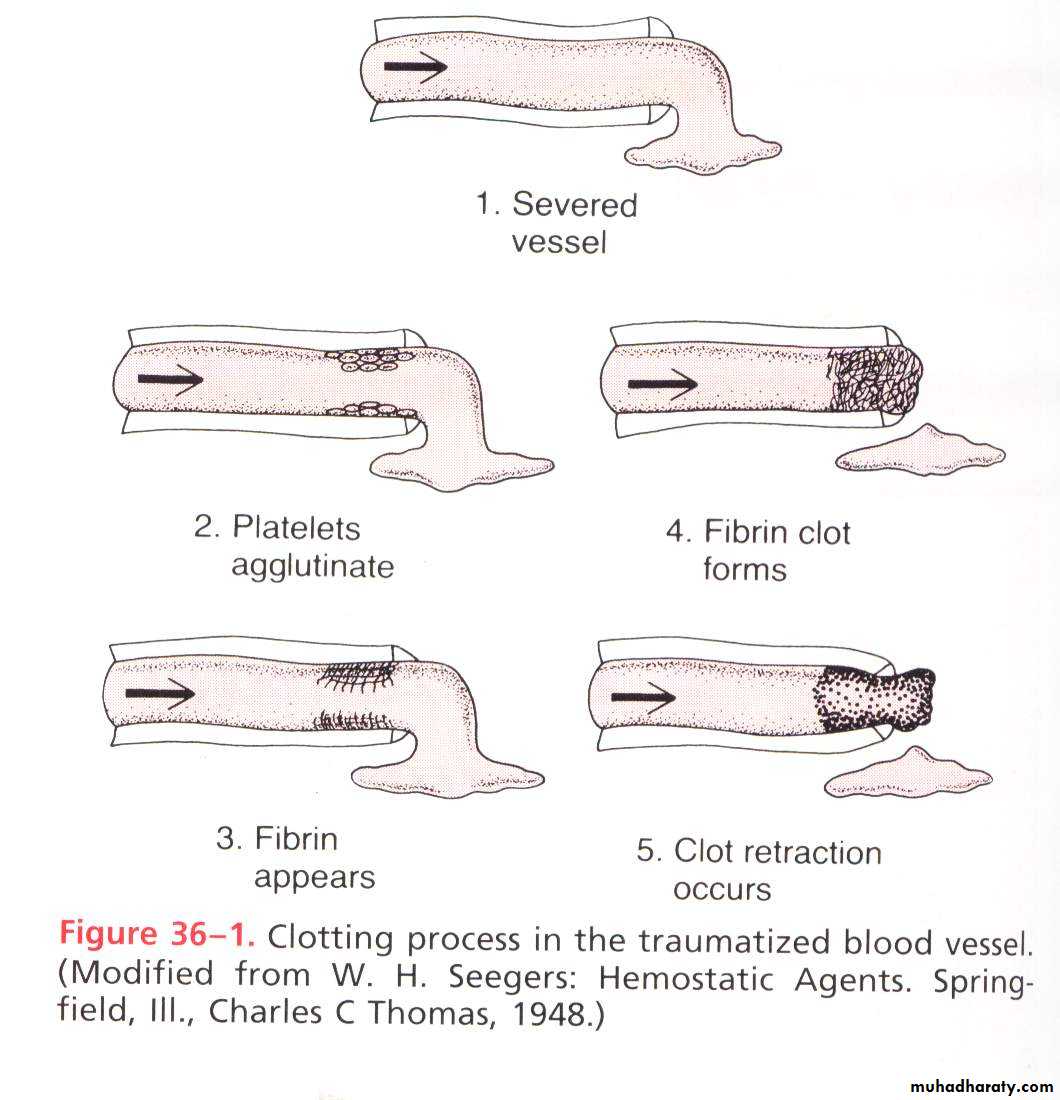
Clot Retraction
Within 3-6 minutes after rupture of a blood vessel, if the vessel opening is not too large the entire opening or broken end of the vessel is filled with clot. After 20 minutes to an hour, the clot retracts.Once the clot has formed, it begins to condense into a denser, compact structure through a process called clot retraction, which in turn closes the vessel wall further platelets play an important role in the retraction because it contain contractile protein action & myosin. As the clot condenses, a fluid called serum is squeezed out of it. Serum is plasma from which fibrinogen & some of clotting factors have been removed. Serum cannot clot because of lack of these factors. As the clot retracts, the edges of the broken blood vessels are pulled together, thus possibly contributing to the ultimate state of hemostasis.Role of calcium in clotting mechanism:
Ca is required for promotion of reactions in the intrinsic & extrinsic pathways; therefore in the absence of Ca++ blood clotting does not occur. Clotting of blood is prevented by reducing the concentration of Ca++ either by deionizing Ca++ by reacting it with substances such as citrate ions or by precipitating the Ca++ with substances such as oxalate ion.Role of vitamin k in clotting mechanism:
Vitamin K is fat-soluble vitamin required for synthesis of prothrombin, stable factor, (antihemophilic factor B or Christmas factor), Stuart Prower factor, and protein C (in fibrinolytic system). Deficiencies of vit. K can lead to severe bleeding tendencies and ineffective coagulation. Vit. K is present in many foods, especially green vegetables. Vit. K is produced by intestinal bacteria and those bacteria can be killed by oral antibiotics, resulting in insufficient coagulation ability. New born lack these intestinal bacteria & vit. K injection is routinely given to infant at birth. Infant can also obtain vit. K from food such as milk. Because cow’s milk contain more vit. K than does human milk, breast-fed infants is more susceptible to hemorrhage than bottle-fed infants. Vitamin K deficiency can result in hemorrhages such as frequent nosebleeds.
Lec.10 2016
Anticloting mechanismsThe tending of blood to clot is balanced in vivo by limiting reactions that tend to prevent clotting inside the blood vessels and to break down any clots that do form when a vascular break occurs. Initiation of coagulation must remain localized to the area of injury, as activating clotting factors that leak into the general circulation may cause severe clotting problems in the blood. Types of anticlotting mechanisms:
fibrinolysis.
anticoagulants
Despite the presence of these inhibitory mechanisms, activated clotting factors occasionally leak from the sites of injury and form clots at other sites in the body. This hazard is present during major surgical operations and may cause clots in the brain (strokes) the lungs (embolism), the heart (infarction) and other organs (thrombosis).
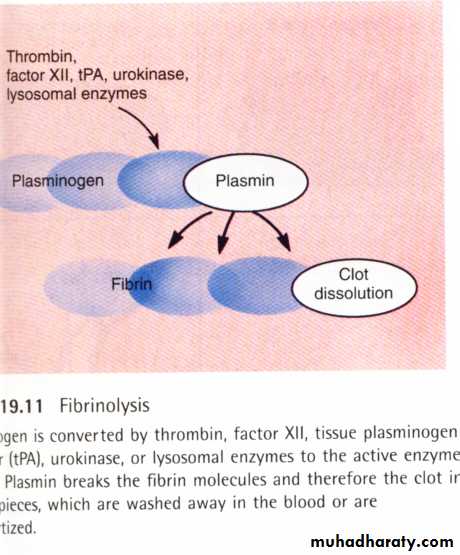
Fibrinolysis: Fibrinolysis: means lysis of blood clots. Blood contains proteolytic enzymes known as the “fibrinolytic system” which dissolve the fibrin .The major protein of the fibrinolytic system is plasminogen which is also known as fibrinolysin which present in an inactive form in plasma in a concentration of 10 – 20 mg/dL. Plasminogen is a2-globulin synthesized in the liver. When a clot is formed, the injured tissues and vascular endothelium, very slowly release a powerful activator called tissue plasminogen activator (TP-A) that converts plasminogen into plasmin. Plasmin lyses fibrin fibers; fibrinogen; factor V; VIII; prothrombin & factor XII. Plasmin interacts with fibrin & degrades large fibrin polymers into small ineffective fragments which are rapidly cleared from circulation by the liver.
Tissue Plasminogen activator
(TP-A)Plasminogen plasmin
(inactive) ( active)fibrin--lysis----liver
"Schematic diagram for “Fibrinolytic System”The fibrinolytic & coagulation systems are interrelated under normal conditions. In rare conditions if the fibrinolytic system is activated without the activation of the coagulation system, this may result in severe bleeding problems. On the other hand if clotting mechanism is activated without activation of fibrinolytic system this may result in clotting disorders or thrombosis.
2. Anticoagulants
To prevent inappropriate activation of the clotting cascade, natural inhibitors of the clotting system are present which are called anticoagulants.Anticoagulants are chemical substances in the blood that prevent coagulation or clotting mechanism .Types:
1. Intravascular anticoagulants (i.e. prevention of blood clotting in the normal vascular system). Examples:
a. Antithrombin III: is a plasma protein produced by the liver & inactivates thrombin.
b. Heparin: produced by basophils ,mast cells and endothelial cells.
Activates antithrombin III which inhibits clotting. It is widely used in medical practice to prevent intravascular clotting.
c. Prostacylin: is a prostaglandin derivative produced by endothelial cells. is a vasodilator & inhibit the release of clotting factor from platelets.
d. Thrombodulin: produced by endothelial cells and will bind to thrombin which activates protein C which inactivates several clotting factors.
e. Plasma Ca++ in vivo, a low plasma Ca++ level enough to interfere with blood clotting.
2. Anticoagulants that can be used outside the body: such as in blood transfusions and laboratory blood tests. Examples:
a-Heparin.
b-EDTA (ethylenediamine Tetra acetic) prevents clot formation by binding to Ca++ making them inaccessible for clotting reactions.
c-Citrate e.g. sodium citrate same action of EDTA. Citrate is not toxic to the body.
d-Oxalate ion: which precipitate Ca++ . Oxalate combines with Ca++ and form insoluble salt. Oxalate is toxic to the body.
e-Blood bank anticoagulants. Examples: a. Acid-citrate dextrose. b.citrate – phosphate dextros
3. Anticoagulant for clinical use: Various anticoagulants have been developed for this purpose. The ones most useful clinically are:
a. Heparin as an intravenous anticoagulant. B.Cumarins such as warfarin: prevent clot formation by suppressing the production of vit. K dependent coagulation factor II, VII, and IX & X by the liver.
Heparins and cumarins are anticoagulants used for the treatment of venous thrombosis (formation of clots inside blood vessels).
Anticoagulants
To prevent inappropriate activation of the clotting cascade, natural inhibitors of the clotting system are present:
Antithrombin III is a protein produced by the liver which has inhibitory activity against factors IIa, VIIa, IXa, Xa, and XIa, especially in the presence of heparin so it is a protease inhibitor. It is has a weak inhibitory activity against thrombin and factor Xa. When antithrombin binds to heparin this inhibitory activity is markedly accelerated and this forms the basis of the anticoagulant of heparin. Congenital deficiency of antithrombin is associated with a predisposion to venous thromboembolism, such patients may be relatively resistant to anticoagulation with heparin because of the low level of antithrombin which is necessary for heparin to produce it is anticoagulant effect.
Protein C is a vitamin K dependant factor produced by the liver, when activated by interaction with protein S, it inhibits factor Va and VIIIa. Thus a deficiency of either protein C or S results in aprothrombotic state due to reduced inhibition of activated factor V and VIII.
Deficiency of either factor is usually inherited in an autosomal fashion. These natural inhibitors are powerful control points in the positive feedback cascade of clotting, and abnormalities in their function result in a tendency to thrombosis.
Anticoagulant therapy
HeparinStandard or unfractionated heparin (SH) produces its anticoagulant effect by potentiating the activity of antithrombin which inhibits the procoagulant enzymic activity of factors IIa,VIIa,IXa,Xa and XIa. The more recently developed low molecular weight heparins (LMWH) augment antithrombin activity especially against factor Xa . LMWH does not prolong the APTT unlike SH and if plasma level needs to be measured this is done by use of a specific anti-Xa based assay