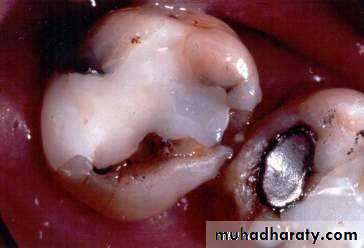
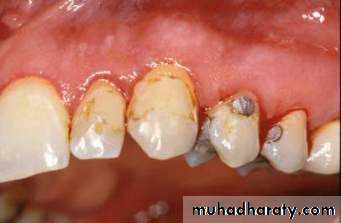
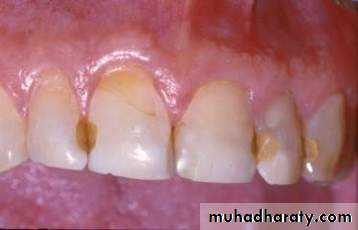
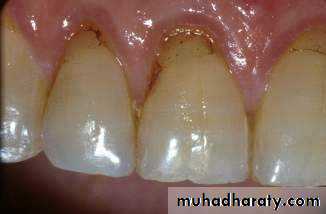
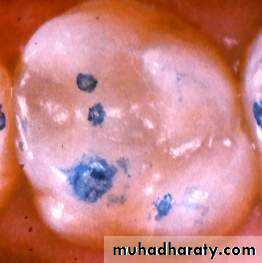
Tooth colored restorative materials
• Unfilled Resin• Composite Resin
• Porcelin (Veneers, crowns )
• Porcelin bonded metal crowns
• Glass Ionomer Cements
• Silicate Cements
Restorative Resins
• History
1871 – silicate cementsalumina-silica glass & phosphoric acid
very soluble
poor mechanical properties
1948 – acrylic resins
polymethylmethacrylate
high polymerization shrinkage
Known as ‘Unfilled acrylics’
• History
• 1962 – Bis-GMA• stronger resin
• 1969 – filled composite resin
• improved mechanical properties
• less shrinkage
• paste/paste system
• 1970’s – acid etching and microfills
• 1980’s – light curing and hybrids
• 1990’s – flowables and packables
• 2000’s – nanofills
•
• Indications
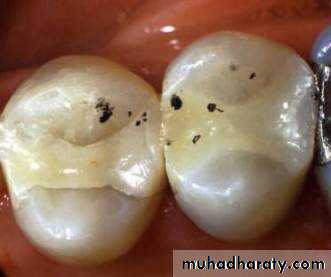
• Anterior restorations• Posterior restorations
• preventive resin
• conservative class 1 or 2
• cuspal coverage
• core Build up- materials
• Contraindications
• Large posterior restorations• Bruxism
• Poor isolation
• Advantages
• Esthetics• Conservation of tooth structure
• Adhesion to tooth structure
• Low thermal conductivity
• Alternative to amalgam
• Disadvantages
• Technique sensitivity• Polymerization shrinkage
• marginal leakage
• secondary caries
• postoperative sensitivity
• Decreased wear resistance
• Composition
• Resin matrix• Monomer
• initiator
• inhibitors
• pigments
• Inorganic filler
• glass
• quartz
• colloidal silica
• Coupling Agent
• - Silane agent
• Monomers
• Binds filler particles together• Provides “workability”
• Typical monomers
• Bisphenol A glycidyl methacrylate (Bis-GMA)
• Urethane dimethacrylate (UEDMA)
• Triethylene glycol dimethacrylate (TEGMA) Lower viscosity Diluent
• Monomers
• Bis-GMA• extremely viscous
• lowered by adding TEGDMA
• freely movable
• increases polymer conversion
• increases crosslinking
• increases shrinkage
• Monomers
• Shrinkage• 2 – 7 %
• marginal gap formation
• Filler Particles
• Crystalline quartz• larger particles
• not polishable
• Silica glass
• barium
• strontium
• Lithium
• - Polishable
• Filler Particles
• Increase fillers, increase mechanical properties
• strength
• abrasion resistance
• esthetics
• handling
• Coupling Agent
• Chemical bond• filler particle - resin matrix
• Organosilane (bifunctional molecule)
• siloxane end bonds to hydroxyl groups on filler
• methacrylate end polymerizes with resin
• Inhibitors
• Prevents spontaneous polymer formation• Extends shelf life
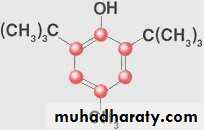
• Butylated Hydroxytoluene
• Pigments and UV Absorbers
• Pigments• metal oxides
• provide shading and opacity
• titanium and aluminum oxides
• UV absorbers
• prevent discoloration
• acts like a “sunscreen”
• Benzophenone
•
• Visible-Light Activation
• Camphorquinone
• most common photoinitiator
• absorbs blue light
• 400 – 500 nm range
• Initiator reacts with amine activator
• Forms free radicals
• Initiates addition polymerization
• Polymerization
• Initiation• production of reactive free radicals
• typically with light for restorative materials
• Propagation
• hundreds of monomer units
• polymer network
• Termination
• Classification System
• Matrix composition• Method of cure
• Filler content
• Filler particle size
• traditional ( macrofilled)
• microfilled
• small particle
• hybrid
• Newer Classification System
• Based on particle size• megafill
• 0.5–2 millimeters
• macrofill
• 10–100 microns
• midifill
• 1–10 microns
• minifill
• 0.1–1 microns
• microfill
• 0.01–0.1 microns
• nanofill
• 0.005–0.01 microns
• Most new systems
• minifillers
• Newest trend
• nanofillers
• Nanofilled Composite
• Filtek Supreme (3M ESPE)• Filler particles
• filled: 78% wgt
• nanomers
• 0.02 – 0.07 microns
• nanocluster
• act as single unit
• 0.6 – 1.4 microns
•
• Performance Factors
• Material factors• biocompatibility
• polymerization shrinkage
• wear resistance
• polish mechanisms
• placement types
• mechanical & physical properties
• Biocompatibility
• Tolerated by pulp• with good seal
• Rare allergic reactions
• HEMA
• Cytotoxicity
• short lived
• Degree of cure important
• decrease free monomer
• Polymerization Shrinkage
• Significant role in restoration failure• gap formation
• secondary caries formation
• marginal leakage
• post-operative sensitivity
• Counteract
• lower shrinkage composites
• incremental placement
• Composite Wear
• Less wear• small particle size
• less abrasion
• heavier filled
• less attrition
• non-contact areas
• 3 - 5 times less
• less surface area
• anterior location
• premolars vs. molars
• Polish Mechanisms
• Acquired polish• clinician induced
• Inherent polish
• ultimate surface
•
• Composite Selection
• Anterior/stress (Class 4)• hybrid
• mini- or midi-fill
• hybrid/microfill
• Anterior/non-stress (Class 3 or 5)
• hybrid
• mini-fill
• microfill
• Composite Selection
• Posterior composites• hybrid
• mini- or midi-fill
• reinforced microfill
•
• Composite Variants
• Packable• Flowable
• Packable Composites
• Marketed for posterior use• increase in viscosity
• better proximal contacts
• handle like amalgam?
• Subtle alteration of filler
• shape
• size
• particle distribution
• Flowable Composites
• Marketed
• class 1, 3, 5
• liner
• Particle size similar to hybrid composites
• Reduced filler content
• reduces viscosity
• Flowable Composites
• Clinical applications• preventive resin restorations
• small Class 5
• provisional repair
• composite repair
• Liners ??
•
• Future Composites
• Low-shrinking monomers• Self-adhesive ?
• Composite Curing
• Original composites (Chemical cured)• UV- Light curing
• Visible light curing
• - “ Dual curing”
• Light curing
• Quartz- Tungsten Halogen (QTH)• Light- emitting diode (LED)
• Plasma Arc
• Laser
•
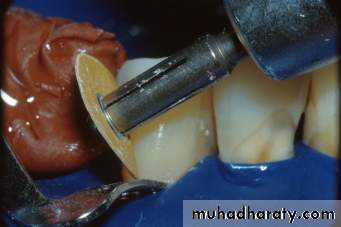
Acid Etching / Conditioning
• Theories of Adhesion
• Mechanical• • micromechanical interlocking
• Adsorption - chemical bonds
• • Primary - ionic and covalent
• • Secondary - hydrogen, van der Waals
• Acid Etchants / Conditioners
• Citric acid
• lactic acid
• Maleic acid
• EDTA
• Phosphoric acid - a strong inorganic acid
• (30% - 50%) most commonly used for etching
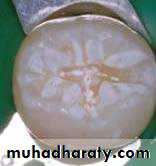
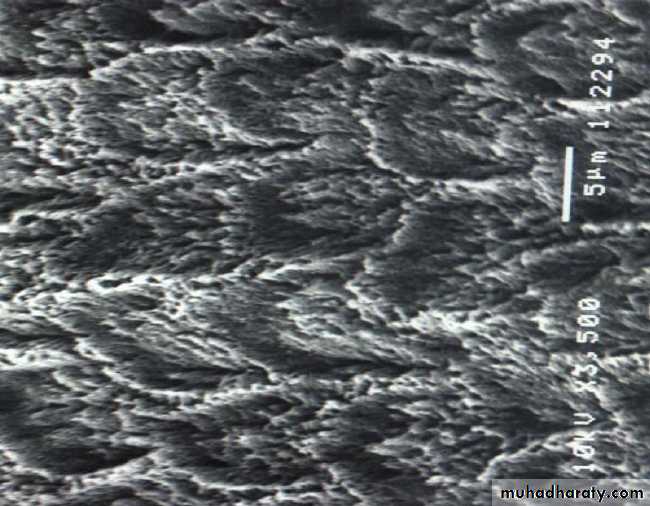
• Conditioning Enamel
• Removes 10 microns of surface and creates microporous layer• Three etching patterns
• - Type I – Core etching
• - Type II – Periphery etching
• - Type III – Mixed patterns
• Resin tags
• - Macrotags
• - Microtags
• Etched Enamel
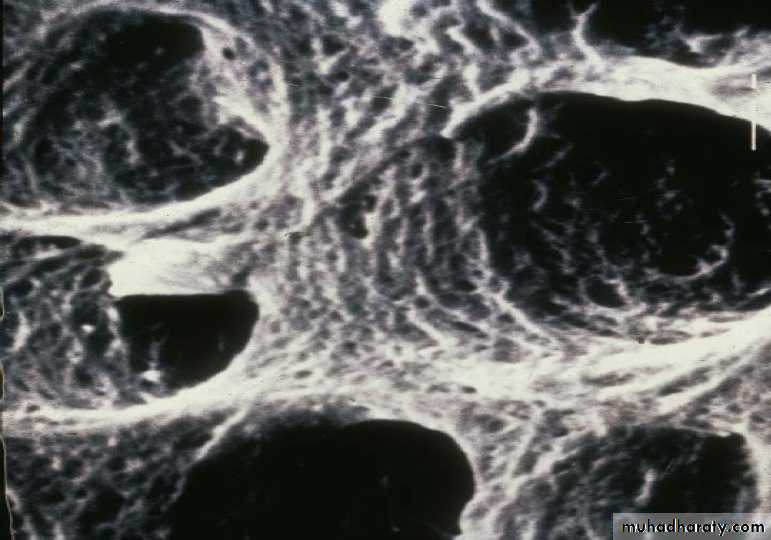
• Conditioning Dentine
• Chemical alteration of dentine• Objective is to remove the smear layer
• Demineralizing the dentine to expose a microporous scaffold of collagen fibrils
• (Hybrid layer)
• Conditioned Dentine
• Acid Etching
• Enamel• Selective Demineralization
• Increases surface area
• Increases life of composite
• Decreases marginal staining
• Decreases secondary caries
• Decreases post-operative sensitivity
• Permits efficient wetting by
• hydrophobic resin
• Tag formation in microporosities
• Dentine
• Demineralizes dentine surface
• Opens dentinal tubules
• Exposes collagen
• Conditions dentine for better wetting of the primer