
Veterinary Clinical Pathology by Prof. Dr. Basima Albadrani
Introduction:
With new technology, in-hospital clinical diagnostic laboratories are becoming
an expected part of every veterinary hospital. Veterinary teams now have the
ability to obtain medical data in complete blood count, chemistry, blood gas,
lactate, urine, and clotting times in minutes—information that would take 1–2 days
to obtain from an outside lab. This allows us to have an excellent medical database
quickly and effectively to help evaluate and treat our patients.
Basic Equipements of clinical pathology laboratory
1. hematology and clinical chemistry instrumentation,
2. a free-arm centrifuge for spinning down serum and urine samples,
3. a microhematocrit centrifuge,
4. a
quality microscope,
5. a refrigerator with a non–frost-free freezer compartment are needed.
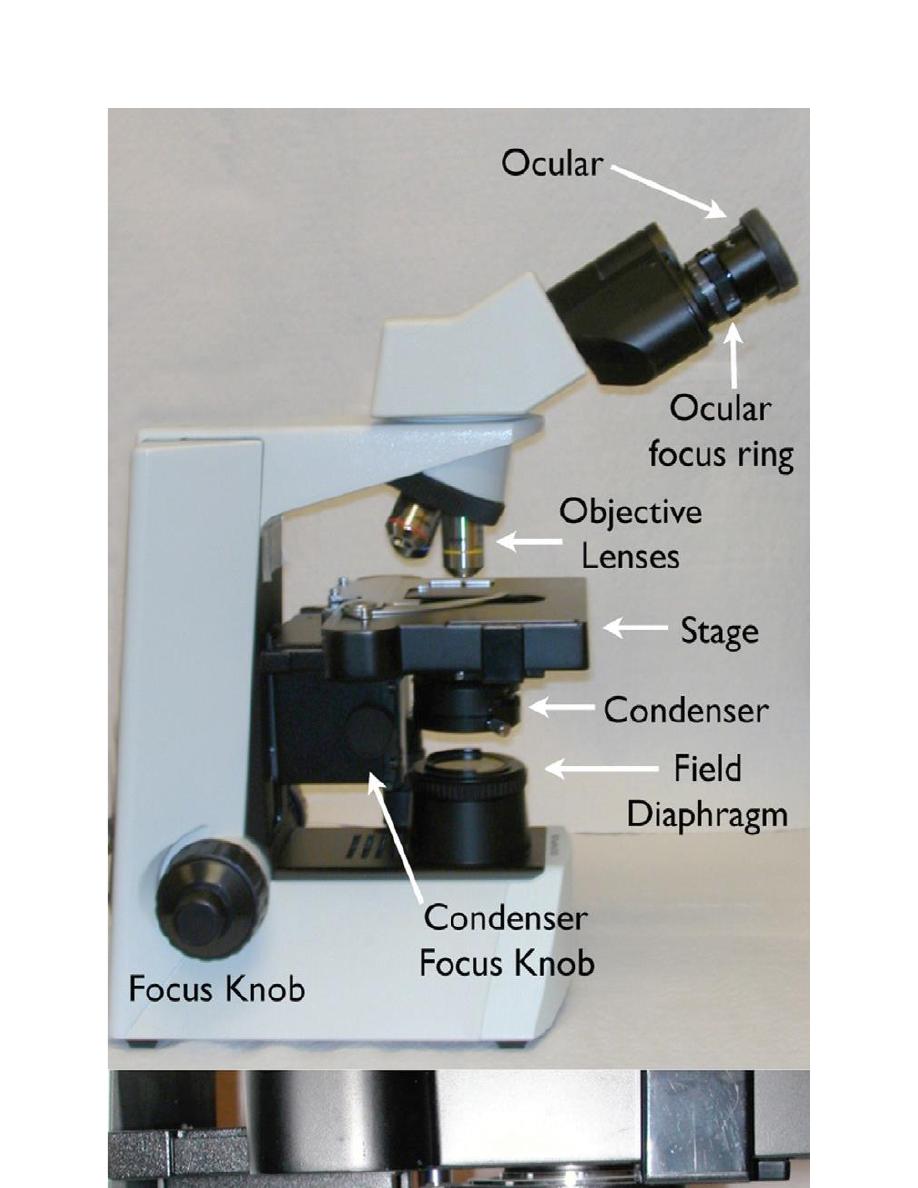
The ability to critically examine hematology and cytology specimens depends
upon the clear optics of the microscope. Figure 1.1 identifies the components of a
compound bright field microscope. The standard lenses on most light microscopes
are 10×, 40×, and 100× oil emersion. The addition of a 20× or a 50× oil
emersion lens can facilitate the rapid examination of cytology and hematology
samples. One important note is that the 40× high dry objective requires a cover
slipped slide. The optics of this objective are optimized for viewing histopathology
slides that are always cover slipped. A simple way to use the 40× objective to
view hematology and cytology slides is to use a drop of immersion oil as a
coverslip mounting medium.
Place a drop of oil on the microscope slide and place a coverslip in top of the oil.
This will allow sharp focus of the slide with the 40× objective. It is imperative
that the high dry objective does not get immersed in oil. If this inadvertently
happens, immediate cleaning with a quality lens cleaner (not xylene) and lens
paper is necessary. A good habit is to always rotate the microscope objectives in
one direction, from lowest power to highest power. This will keep the microscopist
from dragging the 40× objective through oil.
Accessory Equipment’s of clinical pathology laboratory:
1. Glasses of different shapes and sizes for carry out the biochemical test and
other measurements, e.g: test tubes, micropipettes, petrei dishes, different
volume flasks, microscopical slides, stains jars…etc.
2. Chemical solutions, Agars and microbiological media….
3. Kits for serology
4. Stains
Laboratory tests are done for a variety of reasons:
Screening tests, such as a complete blood count (CBC), may be done on
clinically normal animals when they are acquired to avoid a financial and/or
emotional commitment to a diseased animal,
to examine geriatric patients for subclinical disease, or to identify a condition
that might make an animal an anesthetic or surgical risk.
Screening tests are often done when an ill animal is first examined, especially if
systemic signs of illness are present and a specific diagnosis is not apparent
from the history and physical examination.
Tests are also done to confirm a presumptive diagnosis. A test may be repeated
or a different test may be done to confirm a test result that was previously
reported to be abnormal.
Tests may be done to assist in the determination of the severity of a disease, to
help formulate a prognosis, and to monitor the response to therapy or
progression of disease

Hematology
is the study of the cellular components of blood. Blood is
composed of both
cells
and
fluid
called
plasma
. The cells are white blood cell
(
leukocytes
), red blood cells
(erythrocytes
), and platelets
(thrombocytes
). The fluid
(plasma) component consists of
water
,
protein
,
electrolytes/
minerals
,
lipids
,
soluble carbohydrates
,
amino acids
, and other non-cellular substances such as
hormones.
Each component of blood has a specific function and can change in
response to disease states.
The
enumeration
and
morphological evaluation of the peripheral blood cells
are
essential parts of the complete blood count (CBC). An understanding of the
formation, function, and peripheral dynamics of white blood cells will allow the
veterinary technician to accurately identify and report changes in the CBC
associated with disease processes.
Components of the Complete Blood count
The
complete blood count (CBC):
is a process by which the cellular components
of the blood are evaluated. The components of the CBC provide data that is used to
determine whether there are abnormalities in
erythrocytes (RBC)
,
leukocytes
(WBC)
, and
platelets (PL)
.
Evaluation of erythrocyte:
The
packed cell volume (PCV
),
hematocrit (Hct
),
hemoglobin concentration
, and
indices
(MCV, MCH, MCHC)
that describe the size of erythrocytes and the
concentration of hemoglobin within the erythrocytes are used to evaluate
erythrocytes.
Evaluation of leukocyte:
The
white blood cell count (WBC)
and
differential leukocyte count
(
DLC
) are
used to evaluate leukocytes.

Evaluation of platelets
Total
number of platelets
and
platelets morphology
are used to evaluate platelets
The blood film evaluation: Is an essential part of the CBC and is used
1. to confirm the
numbers of RBCs, WBCS and platelets
obtained by the
automated or manual CBC
2. To fully
evaluate cell (Erythrocyte, Leukocyte, Platelets) morphology
.
3. For diagnosis and differential diagnosis of anemia.
4. to evaluation of DLC.
5. For diagnosis of some infectious diseases e.g Blood parasite infestation
(microfilariasis), blood protozoa like Babesia, Theileria., Ricketsial infection
like Anaplasmosis, Hemoplasma infection( Hemotrophic mycoplasma).
6. For diagnosis of some infectious diseases as Anthrax (Bacillus anthrasis)
staining blood film by special stain Polychrom Methylen Blue
The CBC can be automated or performed manually. The necessary items for a
manual CBC are shown in Figure 2.1. Because it can be difficult to fully
interpret the results of a CBC without a total protein, this measurement should
be included as an important component of the CBC. The total protein can be
measured by refractometer or by an in-house chemistry instrument.
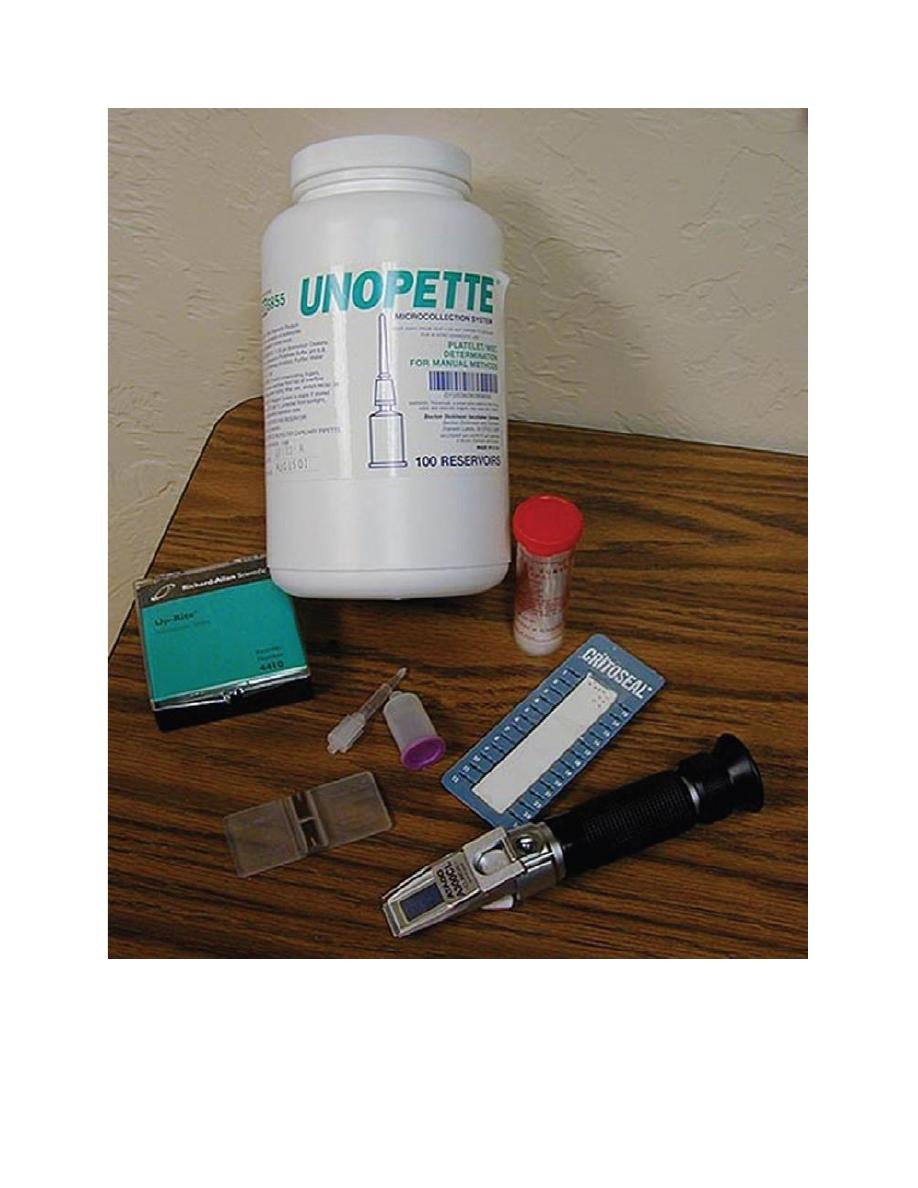
Figure 2.1. (a) Hemocytometer. (b) Refractometer. (c) Microhematocrit tube sealant. (d)
Unopette system diluent reservoir and pipette. (e) Microscope slides. (f) Microhematocrit tubes.
(g) Unopette WBC counting system.
SAMPLE COLLECTION AND HANDLING
Ethylenediaminetetraacetic acid (EDTA)
is the preferred
anticoagulant
for complete
blood count (CBC) determination in most species, but blood from some
birds, fishes
and
reptiles
hemolyzes when collected into EDTA.
In those species,
heparin
is often used as anticoagulant.
The disadvantage of heparin is that leukocytes do not stain as well (presumably
because heparin binds to leukocytes),24 and platelets generally clump more than
they do in blood collected with EDTA.
However, as discussed later, platelet aggregates and leukocyte aggregates may occur
even in properly collected EDTA-anticoagulated blood
samples.
In those cases, collection of blood using another anticoagulant (e.g.,
citrate
) may prevent the formation of cell aggregates.
Cell aggregation tends to be more pronounced as blood is cooled and
stored; consequently processing samples as rapidly as possible after
collection may minimize the formation of leukocyte and/or platelet
aggregates.
Collection of blood directly into a
vacuum tube
is preferred to collection
of blood by syringe and transfer to a vacuum tube.
This method reduces platelet clumping and clot formation in samples for CBC
determinations, as even small clots render a sample unusable. Platelet counts are
markedly reduced, and a significant reduction can sometimes occur in hematocrit
(HCT) and leukocyte counts as well.
Also, when the tube is allowed to fill based on the vacuum within the tube, the proper
sample-to-anticoagulant ratio will be present.
Inadequate sample size results in decreased HCT due to excessive EDTA solution.
care should be taken to avoid iatrogenic hemolysis, which interferes with plasma
protein, fibrinogen, and various erythrocyte measurements.
Samples should be submitted to the laboratory as
rapidly
as possible, and
blood films
should be made as soon as possible
and rapidly dried
to minimize morphologic
changes.
GROSS EXAMINATION OF BLOOD SAMPLES
Samples are checked for clots and mixed well (gently inverted 20 times)
immediately before removing aliquots for hematology procedures.
Horse erythrocytes settle especially rapidly because of
rouleaux formation
(adhesion of erythrocytes together like a stack of coins).
Blood should be examined grossly for color and evidence of erythrocyte
agglutination.
The presence of marked lipemia may result in a blood sample with a milky red
color resembling “tomato soup” when oxygenated.
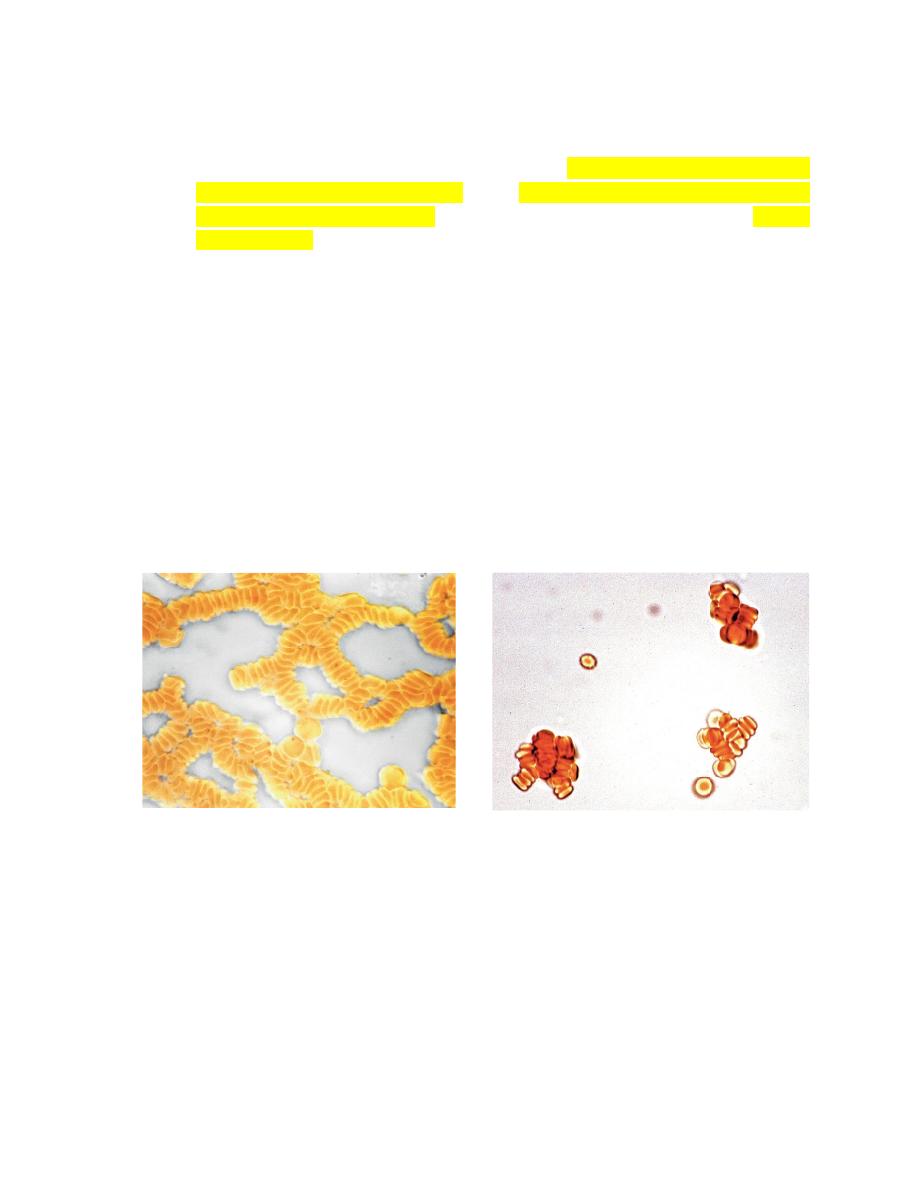
Erythrocyte Agglutination
The appearance of red granules in a well-mixed
blood sample.
However, it is important to differentiate
agglutination (aggregation of
erythrocytes together in clusters
) from
rouleaux (adherence of erythrocytes
together like a stack of coins
), which can be seen in the blood from healthy
horses and cats(Fig. 2-6).
Rouleaux formation is eliminated by
washing erythrocytes in physiologic saline,
but agglutination is not.
This differentiation requires centrifugation of blood, removal of plasma, and
resuspension of erythrocytes in saline.
A rapid way to differentiate rouleaux from agglutination is to mix five drops of
physiologic saline with a drop of anticoagulated blood on a glass slide and
examine it as a wet mount using a microscope. This dilution reduces rouleaux,
but agglutination is not affected.
The microscopic appearance of agglutination in a sample of washed
erythrocytes is shown (Fig. 2-7).
The presence of agglutination indicates that the erythrocytes have increased
surface-bound immunoglobulins.
FIGURE 2-
6: Microscopic rouleaux in an unstained wet mount FIGURE 2-7: Microscopic agglutination in an unstained wet
The Hemogram
The
hemogram
is the collection of
specific measurements
that allow the veterinarian to evaluate
a patient’s erythrocytes, leukocytes, and platelets. The portion of the hemogram that contains the
parameters that assess erythrocytes is the
erythrogram
and the portion that relates to the
leukocytes is the
leukogram
. Platelet parameters are often reported with the
erythrogram
.
Most
automated cell counters
will provide the following measurements:
Red cell count measured in cells/μL
Hematocrit (Hct) measured in percent (%)
Mean cell volume (MCV) measured in femtoliters (fl)
Hemoglobin (Hgb) measured in g/dL
Mean cell hemoglobin concentration (MCHC) measured in g/dL
Mean cell hemoglobin (MCH) measured in pg
Platelets numbers in cells/μL
White blood cell count in cells/μL
Leukocyte differential cell counts (DLC) in percents (%) and absolute numbers in
cells/μL
The Erythrogram
The three erythrocyte parameters in the erythrogram that are directly measured by most
in-clinic hematology instruments are:
Hemoglobin Hb
red cell count TRBC
red cell volume MCV.
The remaining red cell parameters—MCHC, Hct, and MCH—are calculated from the
measured values. As a result, if there are artifactual changes in the measured values, the
calculated values will be erroneous.
Each of these measurements can help identify and characterize the anemic patient.
The definition of
anemia
is decreased circulating erythrocytes. Therefore, an anemic
patient
will have decreased erythrocyte count, decreased Hct, and decreased Hgb
. There
are occasions in which all three of these measurements are not decreased. When this
happens, an investigation into why this discordance is present should be done. To
understand how discordant values occur, the technician must understand how these
measurements are made.
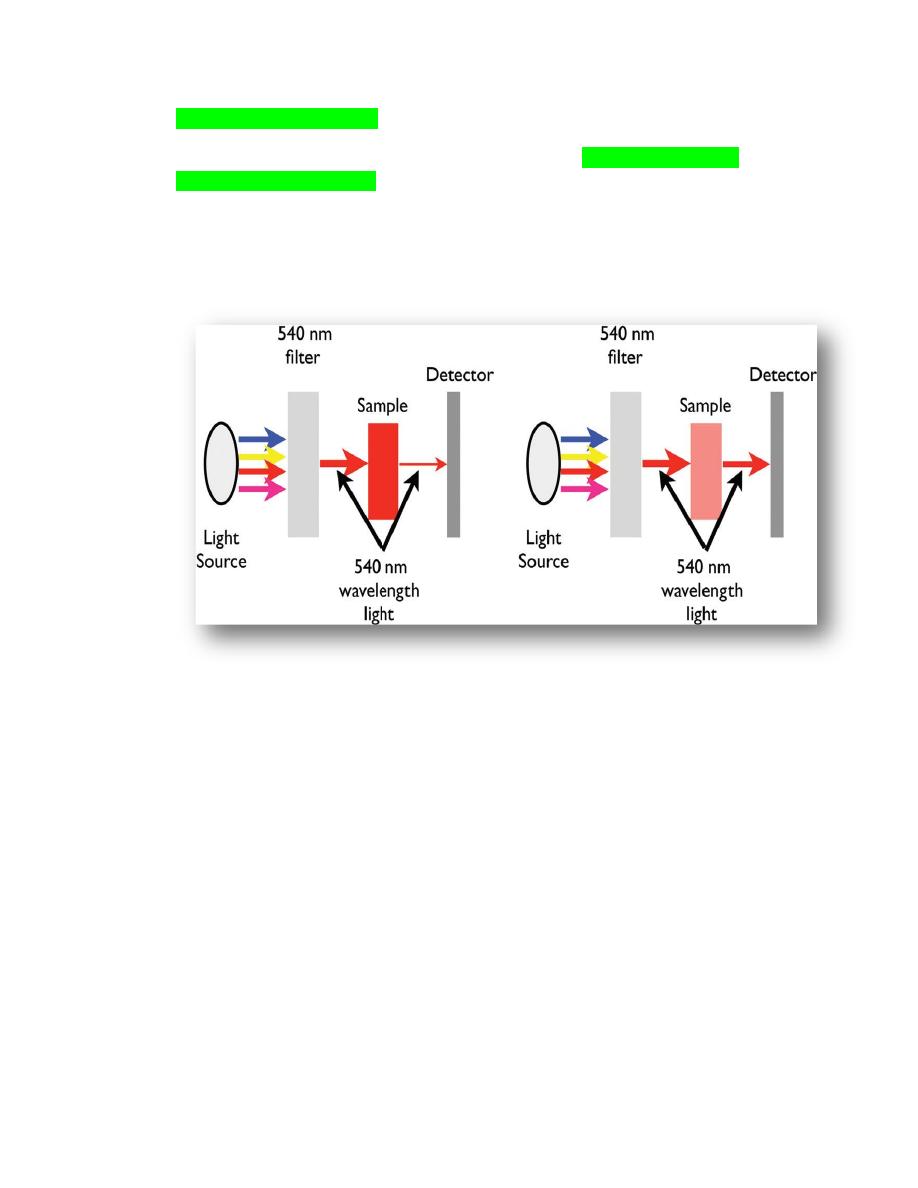
Hemoglobin measurement
The majority of in-house hematology instruments measure hemoglobin using a
spectrophotometric method. When this method is used, a fixed amount of blood is
diluted in
a lysing solution
to release the hemoglobin from the erythrocytes. Then, a
light of a specific
wavelength (540 nanometers)
is passed through the fluid containing
the released hemoglobin, and the amount that is transmitted to the photocell on the side
of the fluid is measured (Figure 2.2).
Figure 2.2.
The components of a simple
spectrophotometer. The filter allows one wavelength from
the light source to pass through, 540 nm in the case of hemoglobin measurement. The hemoglobin in the
sample will absorb the light in proportion to the amount of hemoglobin. The more hemoglobin in the
sample, the less light will pass through to the detector. Anything that will interfere with light passing
through a solution will erroneously increase the instruments measurement of hemoglobin. (a) The
sample has a high concentration of hemoglobin, absorbs a large amount of the 540 nm wavelength and,
therefore, a small amount of the light passes through the sample for detection. (b) This sample has a low
concentration of hemoglobin and, therefore, a large amount of the light passes through the sample for
detection.
The amount of hemoglobin present is inversely proportional to the amount of light that
is transmitted. The less light that passes through the fluid, the more hemoglobin will be
measured in the fluid. Therefore, anything that will decrease the amount of light that
can pass through the fluid or scatters light as it passes through the fluid will cause an
artifactual increase in the hemoglobin measurement. Artifactual changes in the
measurement of hemoglobin will directly affect the calculation of MCHC and MCH
Figure 2.3.
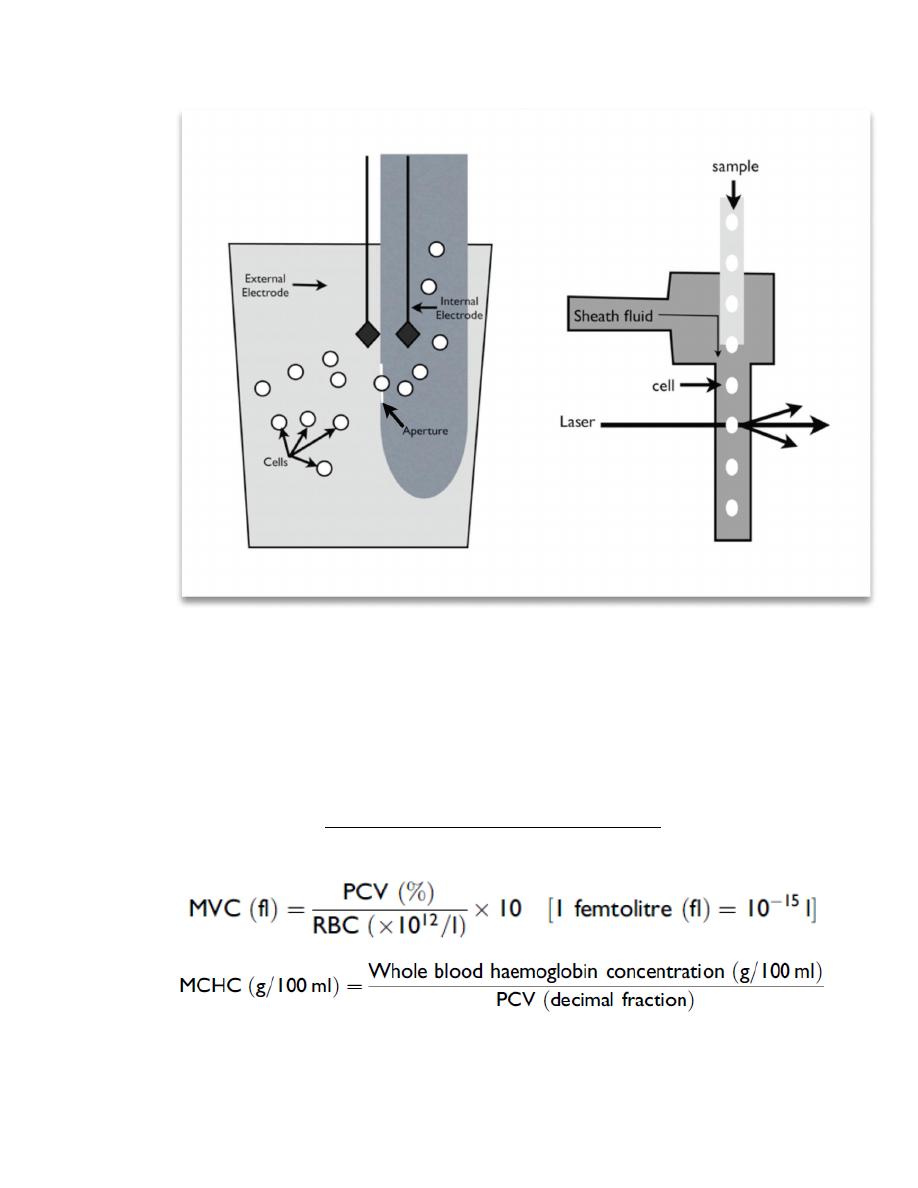
Figure 2.3. Schematic of an impedance counter (a) and a laser counter (b). (a) In the impedance counter,
an electrical current is created across a small opening (the aperture) by the external and internal
electrodes. As the cells are aspirated through the aperture, they disrupt the current in proportion to the
size of the cell. As a result, both the number and size of the cells are measured. (b) In the laser counter,
cells flow through a channel in single file and pass through a single beam of focused light. The light is
scattered to a different degree by the different types of cells.
The red cell count is a direct count of individual cells as they pass through an electrical
field (impedance) or a flow cell (laser counters). Erroneous red cell counts will affect
the Hct and MCH. The MCV is a direct measurement of cell size that is related to the
magnitude of the d measurement of theMCVwill affect the Hct and MCHC.

MICROHEMATOCRIT TUBE
EVALUATION (PCV measurement):
A microhematocrit tube is filled to about 90% of capacity with well-mixed blood and
sealed with clay at one end.
The tube is then placed in a microhematocrit centrifuge with the clay plug oriented to
the periphery of the centrifuge head and centrifuged for 5 minutes.
After centrifugation, the blood sample will be separated into three layers based on
density, with
packed erythrocytes
located at the bottom.
A white
“buffy coat”
is located above the erythrocyte layer, and the acellular
plasma
is located above the buffy coat.
When blood is submitted for a CBC, most commercial laboratories determine an
electronic HCT rather than a packed cell volume (PCV) by centrifugation.
This efficiency negates the need to centrifuge a microhematocrit tube filled with blood.
Unfortunately useful information concerning the appearance of plasma is missed unless
a serum or plasma sample is also prepared for clinical chemistry tests.
Packed Cells
The PCV is measured after centrifugation by determining the fraction of total blood
volume in a microhematocrit tube that is occupied by erythrocytes.
Leukocytes and platelets are primarily located within the buffy coat, although certain
leukocyte types may be present in the top portion of the packed erythrocyte column in
some species (e.g., neutrophils in cattle).
The
width of the buffy coat
generally correlates directly with the
total leukocyte count
.
A
large buffy coat
suggests
leukocytosis
(Fig. 2-9, A) or
thrombocytosis
, and a small
buffy coat suggests that low numbers of these cells may be present.
The
buffy coat may appear reddish
owing to the presence of a marked
reticulocytosis.
The Appearance of Plasma
Plasma is normally clear in all species.
It is nearly colorless in small animals, pigs, and sheep but light yellow in horses because
they naturally have higher bilirubin concentrations.
Plasma varies from colorless to light yellow (carotenoid pigments) in cattle, depending
on their diet.
Increased yellow coloration usually indicates increased bilirubin concentration.
This increase often occurs secondarily to anorexia (fasting hyperbilirubinemia) in horses
owing to reduced removal of unconjugated bilirubin by the liver.
Yellow plasma with a normal HCT suggests hyperbilirubinemia secondary to liver
disease.
Hyperbilirubinemia associated with a marked decrease in the HCT suggests an increased
destruction of erythrocytes; however, the concomitant occurrence of liver disease and a
nonhemolytic anemia could produce a similar finding (Fig. 2-9, B).
Red discoloration of plasma indicates the presence of free hemoglobin. This discoloration
may represent either true hemoglobinemia, resulting from intravascular hemolysis, or
hemolysis occurring after sample collection due to such causes as rough handling, fragile
cells, lipemia, or prolonged storage (Fig. 2-9, C,D).
The HCT value may help differentiate between these two possibilities, with red plasma
and a normal HCT suggesting in vitro hemolysis.
The concomitant occurrence of hemoglobinuria indicates that intravascular hemolysis has
occurred.
Plasma will also appear red following treatment with cross-linked hemoglobin blood
substitute solutions.
Lipemia is recognized as a white opaque appearance in diabetes mellitus, pancreatitis,
hypothyroidism, hyperadrenocorticism, protein-losing nephropathy, cholestasis, obesity,
and starvation may also contribute to the development of lipemia in dogs and cats.
The
platelet count
is usually reported with the erythrogram. Platelets are directly
counted in most instruments. They are counted in the same cycle as the erythrocytes.
The two populations are separated by their size. In those instruments that use
impedance, a histogram (a distribution curve) shows the size difference between the
platelets and erythrocytes. The instrument must be able to identify a valley between (a)
(b) (c) Figure 2.4.
In some cases, large platelets (macroplatelets) may interfere with the count. Large
platelets are produced when platelets are being consumed. . Platelet clumps will also
interfere with the platelet count. Small clusters of platelets are lost in the red cell
population and, therefore, not counted as platelets. The larger clusters are not counted
at all. Evaluation of the blood film is necessary for identification of macroplatelets and
platelet clumps and interpretation of the platelet count.

The Leukogram
components of the leukogram:
The total white blood cell count,
leukocyte differential, absolute leukocyte counts,
Leukocyte morphology abnormalities.
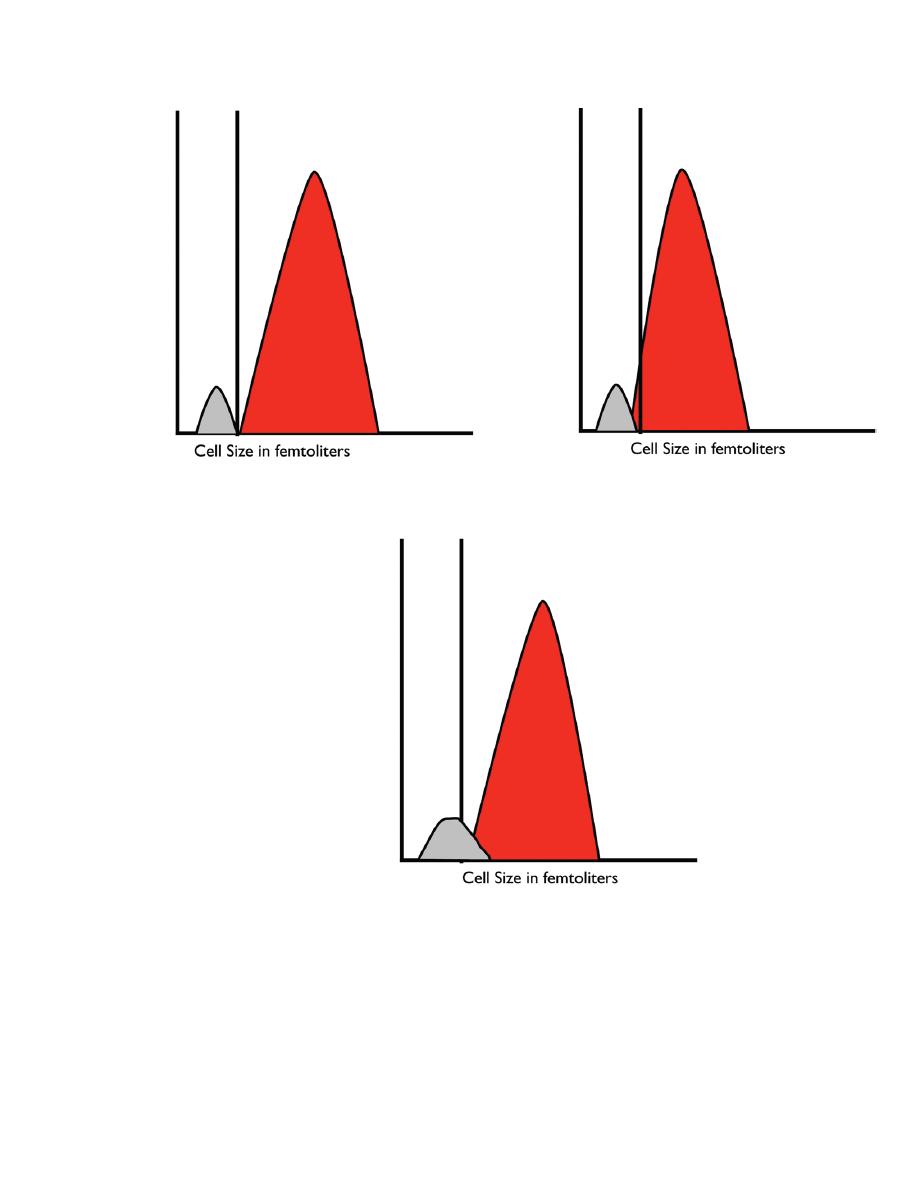
Figure 2.4. Red blood cell and platelet histograms. (a) Normal histogram showing a
well-defined ―valley‖ between the platelets and the red blood cells. (b) Histogram from
an individual with small red blood cells that extend into the platelet size ranges, making
it difficult for the instrument to accurately count the platelets. (c) Histogram from an
individual with large platelets. In this case, the larger platelets will be counted as red
blood cells and an artifactually low platelet count will be reported. The two populations
to produce an accurate platelet count.
The Blood Film
The blood film is an essential part of the complete blood count (CBC). It
can be used as an internal control for the white blood cell count,
differential, and platelet count.
BLOOD-FILM PREPARATION:
Blood films should be prepared within a couple of hours of blood sample
collection to avoid artifactual changes that will distort the morphology of
blood cells.
Blood films are prepared in various ways including
a. The Glass-Slide Blood-Film Method (Fig. 2-10).
b. Coverslip method (Fig. 2-11).
c. Automated slide spinner method.
It is essential that a monolayer of intact cells is present on the slide
So that
accurate examination
and
differential leukocyte counts
can be
performed.
If blood films are too thick, cells will be shrunken and may be difficult to
identify.
If blood films are too thin, erythrocytes will be flattened and lose their
central pallor and some leukocytes (especially lymphocytes and blast cells)
will be ruptured.
A good uniform blood film is essential to the process of blood film
examination.
There is an
―anatomy‖ to the blood film
, and each part of the blood film is
used for a specific purpose.
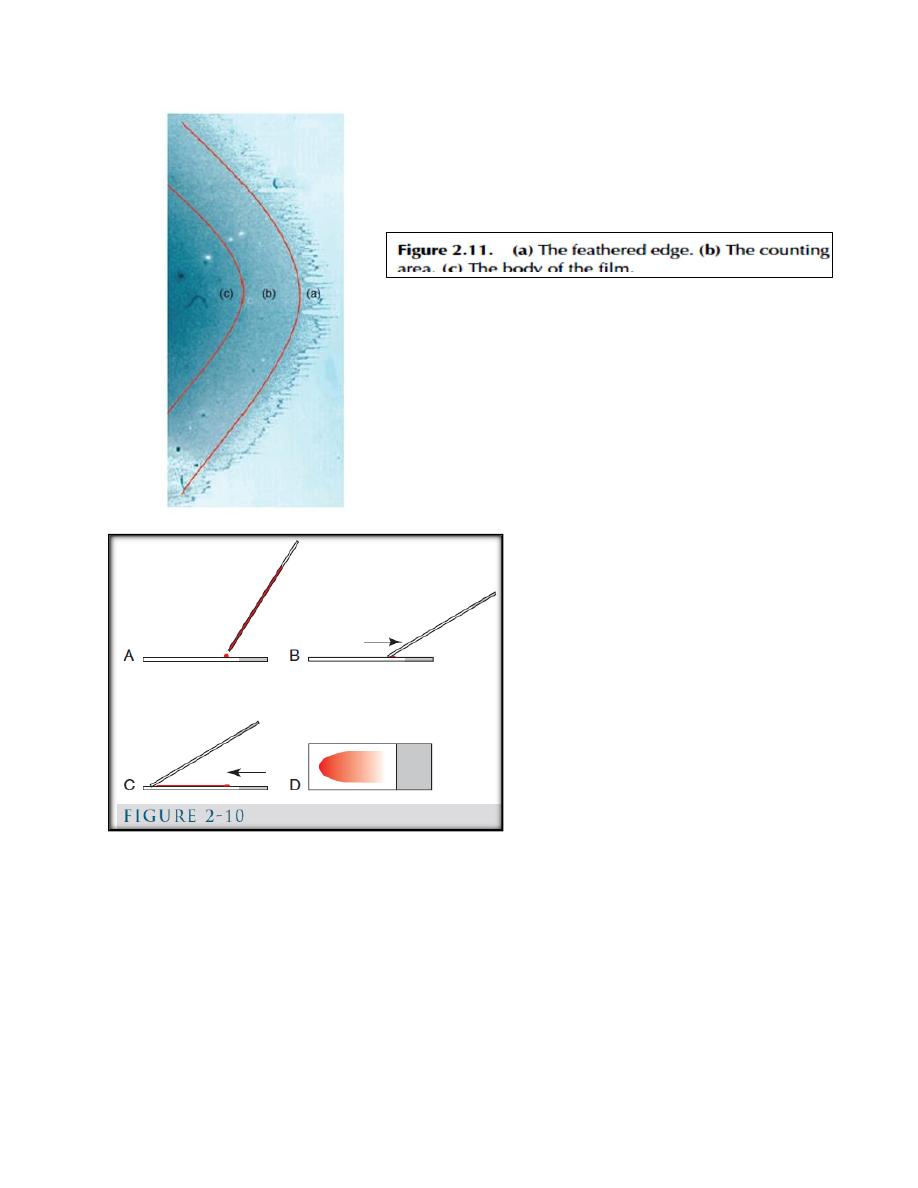
(Fig. 2-10): A, a glass slide is placed on a flat surface and a small drop of well-
mixed blood is placed on one end of the slide using a microhematocrit tube. B, a
second glass slide (spreader slide) is placed on the first slide at about a 30-degree
angle in front of the drop of blood. The spreader slide is then backed into the drop
of blood. C, as soon as the blood flows along the back side of the spreader slide,
the spreader slide is rapidly pushed forward. D, the blood film produced is thick at
the back of the slide, where the drop of blood was placed, and thin at the front
(feathered) edge of the slide.
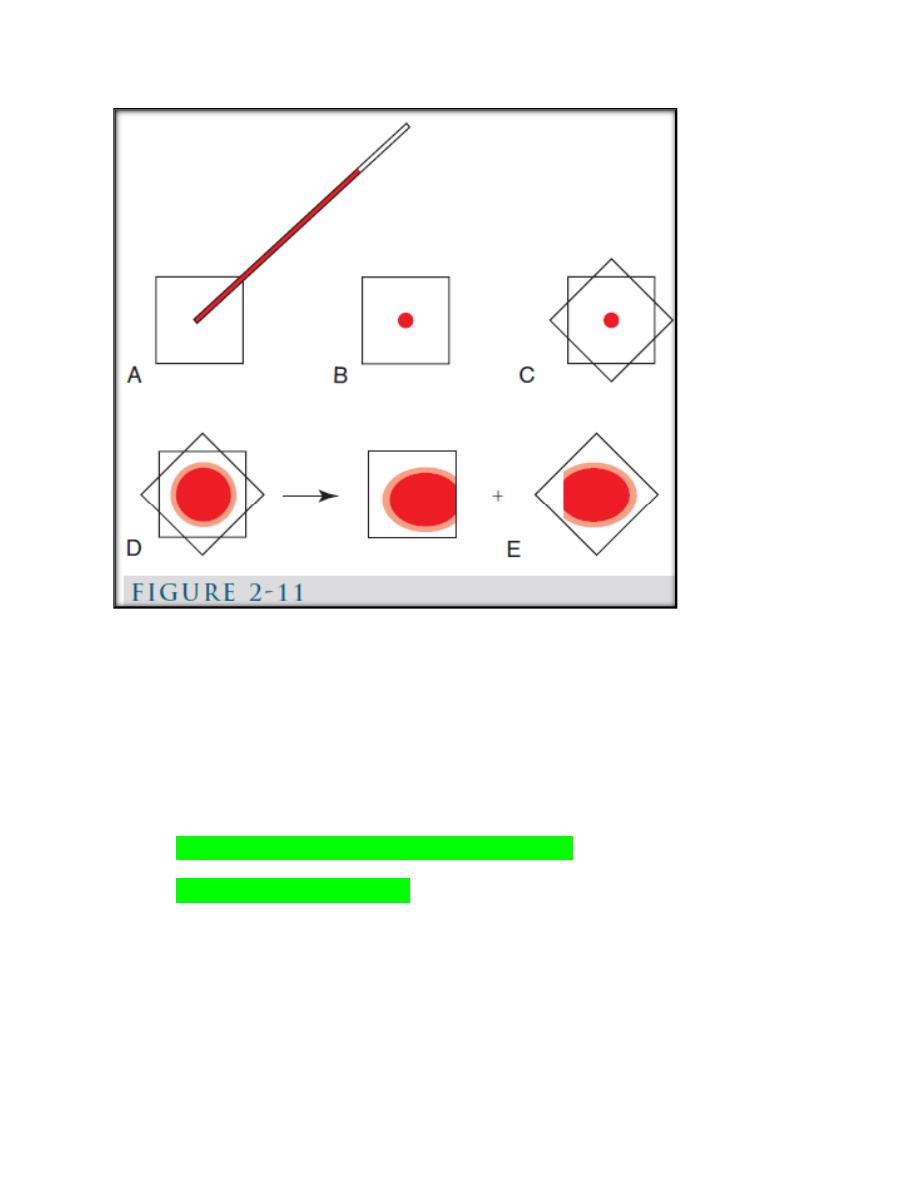
(Fig. 2-11): Coverslip blood film preparation. A, B, One clean coverslip is held
between the thumb and index finger of one hand and a small drop of blood is
placed in the middle of it using a microhematocrit tube. C, A second clean
coverslip is dropped on top of the first in a crosswise position. D, Blood spreads
evenly between the two coverslips and a feathered edge forms at the periphery. E,
the coverslips are rapidly separated by grasping an exposed corner of the top
coverslip with the other hand and pulling apart in a smooth, horizontal manner.
BLOOD-FILM STAINING PROCEDURES
Romanowsky-Type Stains
Blood films are routinely stained with a Romanowsky-type stain (e.g.,
Wright or Wright-Giemsa, Leishman, and Giemsa stain) either
manually or using an automatic slide stainer.
Romanowsky-type stains are composed of a mixture of eosin and
oxidized methylene blue (azure) dyes. The azure dyes stain acids,
resulting in blue to purple colors, and eosin stains bases, resulting in
red coloration.
These staining characteristics depend on the pH of the stains and the
rinse water as well as the nature of the cells present.
Low pH, inadequate staining time, degraded stains, or excessive
washing can result in excessively pink-staining blood films.
High pH, prolonged staining, or insufficient washing can result in
excessively bluestaining blood films.
Blood films should be
fixed in absolute methanol 1-2 minutes
within 4
hours (preferably within 1 hour) of preparation.
If the methanol contains more than 3% water, morphologic artifacts
including loss of cellular detail and vacuolation may be present.
Blood films may have an overall blue tint if stored unfixed for long
periods before staining or if the unfixed blood films are exposed to
formalin vapors, as occurs when blood films are shipped to the
laboratory in a package that also contains formalin-fixed tissue.
Blood films prepared from blood collected with heparin as the
anticoagulant have an overall magenta tint owing to the
mucopolysaccharides present.
Reticulocyte Stains
Reticulocyte stains are commercially available
stain can do so by dissolving 0.5 g of new methylene blue and 1.6 g of
potassium oxalate in 100 mL of distilled water.
Following filtration, equal volumes of blood and stain are mixed
together in a test tube and incubated at room temperature for 10 to 20
minutes.
After incubation, blood films are made and
reticulocyte counts
performed by examining 1000 erythrocytes and determining the
percentage that are reticulocytes.
The blue-staining aggregates or “reticulum” seen in reticulocytes (Fig.
2-17) does not occur as such in living cells but results from the
precipitation of ribosomal ribonucleic acid (RNA; the same RNA that
causes the bluish color seen in polychromatophilic erythrocytes) in
immature erythrocytes during the staining process.
FIGURE 2-17 Reticulocytes in cat blood. with a markedly regenerative FIGURE 2-18 Reticulocytes in the blood of a dog.
Anemia. New methylene blue reticulocyte stain.
EXAMINATION OF STAINED BLOOD FILMS
Blood films are generally examined following staining with Romanowsky-
type stains such as Wright or Wright-Giemsa.
These stains allow for the examination of erythrocyte, leukocyte, and
platelet morphology.
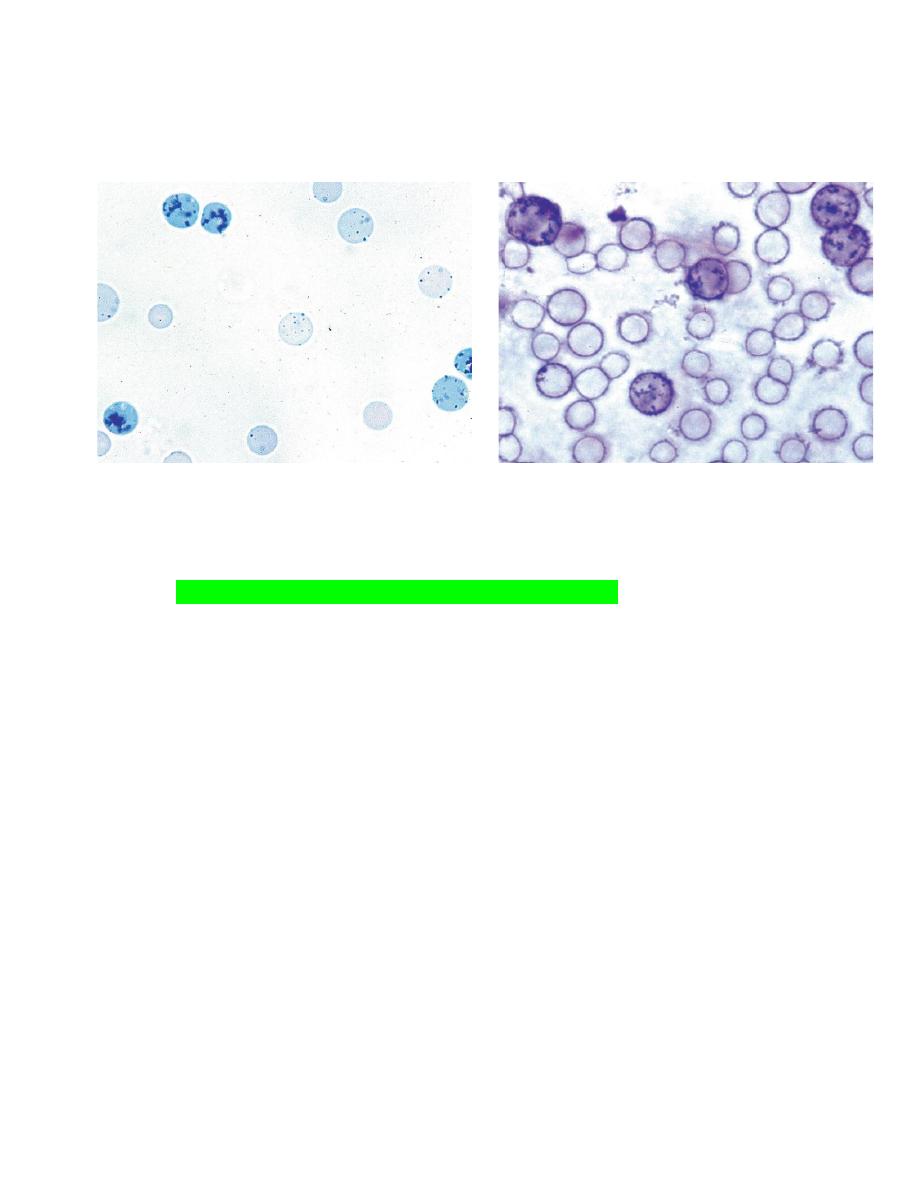
Blood films should first be scanned using a low power objective to
estimate the total leukocyte count and to look for the presence of
erythrocyte autoagglutination (Fig. 2-21), leukocyte aggregates (Fig. 2-22),
platelet aggregates (Fig. 2-23), microfilaria (Fig. 2-24), and abnormal cells
that might be missed during the differential leukocyte count.
It is particularly important that the feathered end of blood films made on
glass slides be examined.
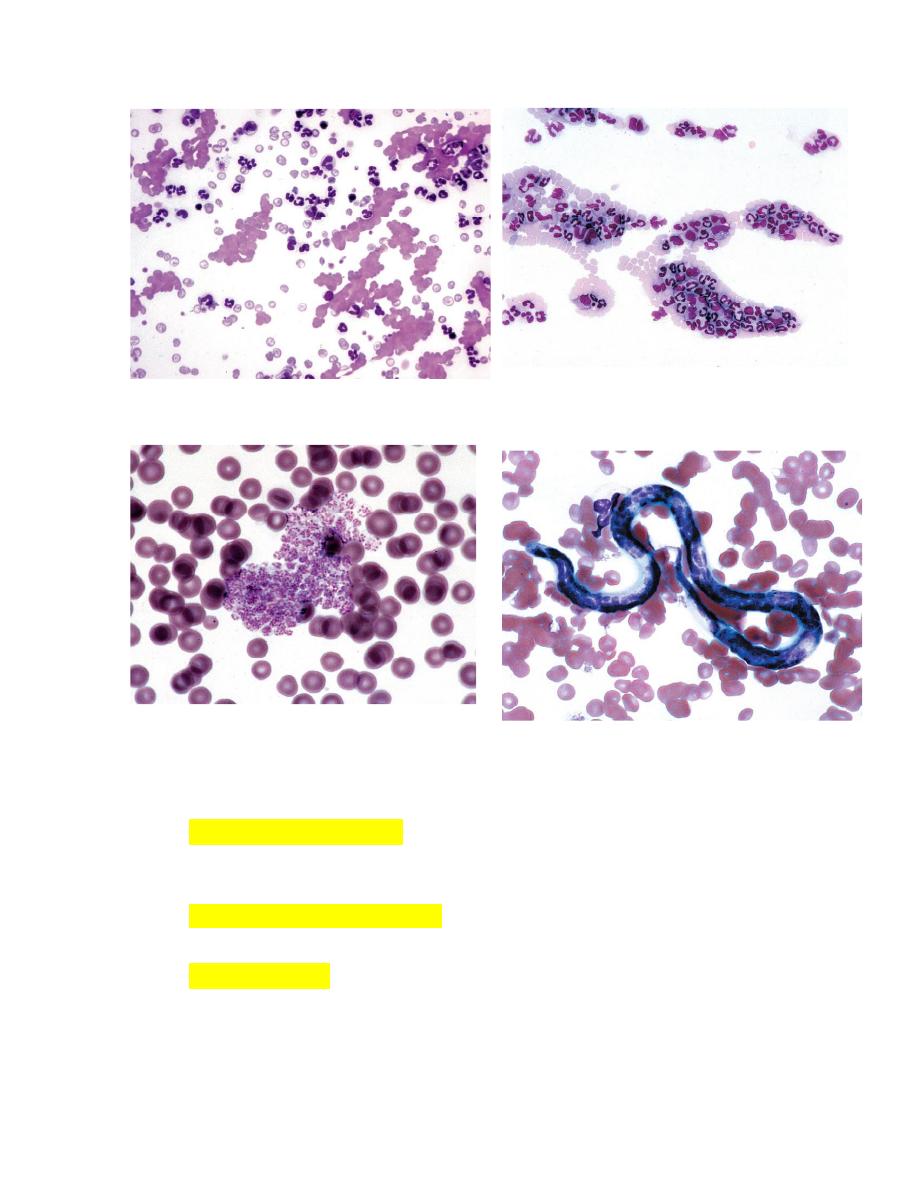
FIGURE 2-21Autoagglutination of erythrocytes FIGURE 2-22 Leukocyte aggregate in blood
FIGURE 2-23
Platelet aggregate in blood from a cow. FIGURE 2-24 Dirofilaria immitis microfilaria
The total leukocyte count in blood (cells per microliter) may be estimated
by determining the average number of leukocytes present per field and
multiplying by 100 to 150.
A differential leukocyte count is done by identifying 100- 200
consecutive leukocytes using a 40× or 50× objective.
Platelet numbers may be estimated by multiplying the average number
per field by 15,000 to 20,000 to get the approximate number of platelets
per microliter of blood.
After the count is complete,
the percentage of each leukocyte type
present is calculated and
multiplied by the total leukocyte count
to get the
absolute number
of each cell type present per microliter of blood.
It is the absolute number of each leukocyte type that is important. relative
values (percentages) can be misleading when the total leukocyte count is
abnormal.
Let us consider two dogs, one with 7% lymphocytes and a total leukocyte
count of 40,000/μL and the other with 70% lymphocytes and a total
leukocyte count of 4000/μL. The first would be said to have a
―relative
‖
lymphopenia
and the second would be said to have a
―relative
‖
lymphocytosis
, but they would both have the same normal absolute
lymphocyte count (2800/μL).
Erythrocyte Morphology
Erythrocyte morphology should be examined and recorded as either
normal or abnormal
.
Erythrocytes on blood films from normal horses, cats, and pigs often
exhibit
rouleaux formation
FIGURE 4-3
; those from normal horses and
cats may contain a low percentage of
small, spherical nuclear remnants
called Howell-Jolly bodies.
Rouleaux and
the
presence of Howell-Jolly bodies should be recorded on
the hematology form when they appear in blood films from species in
which these are not normal findings.
Additional observations regarding erythrocyte morphology, such as the
degree of
polychromasia (presence of polychromatophilic erythrocytes),
anisocytosis (variation in erythrocytes size), and poikilocytosis
(abnormal shape of erythrocytes) should also be made.
Polychromatophilic erythrocytes are reticulocytes that stain bluish-red
because of the combined presence of hemoglobin (red-staining) and
ribosomes (blue-staining).
Abnormal erythrocyte shapes should be classified as specifically as
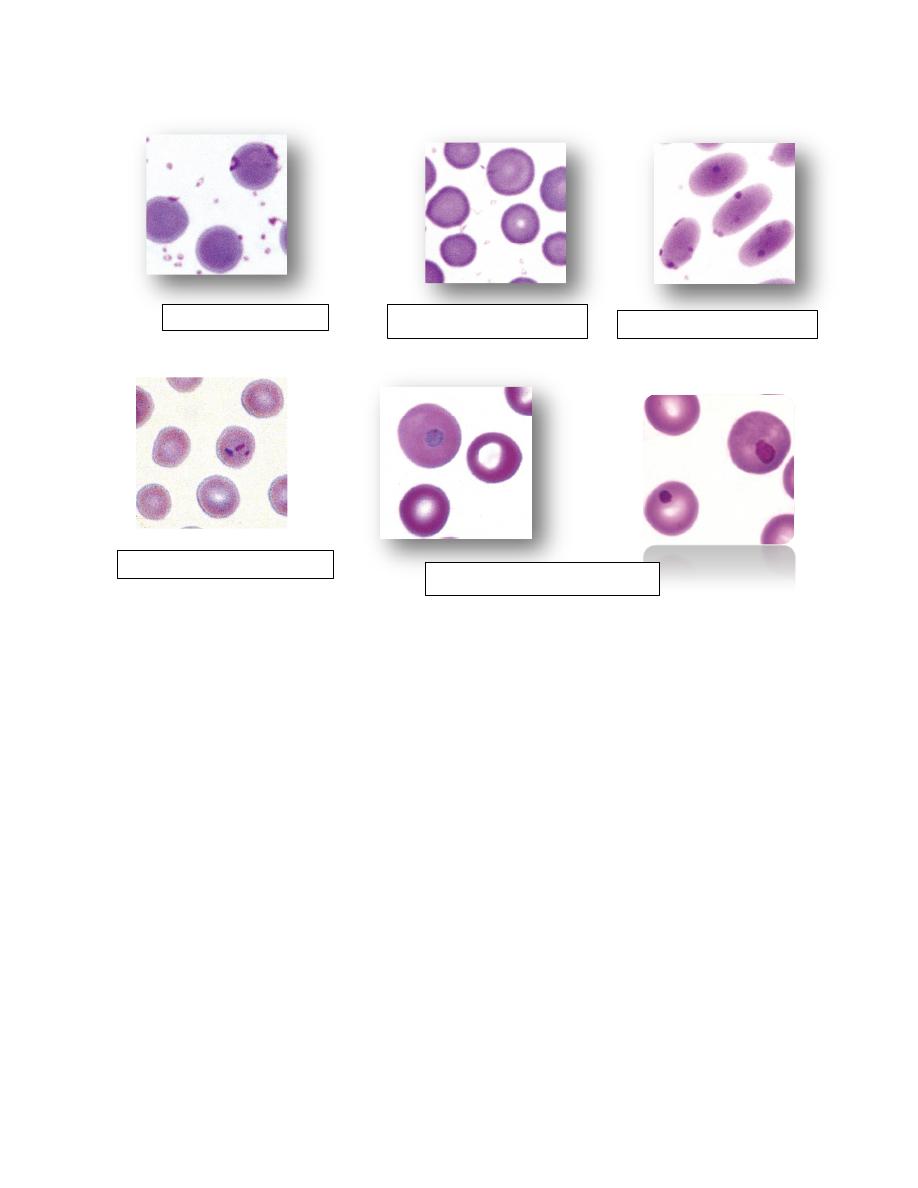
possible, because specific shape abnormalities can help to determine the
nature of a disorder that may be present.

Examples of
abnormal erythrocyte morphology
include echinocytes,
acanthocytes, schistocytes, keratocytes, dacryocytes, elliptocytes,
eccentrocytes, and spherocytes.
Infectious Agents or Inclusions of Blood Cells
Blood films are examined for the presence of infectious agents or
intracellular inclusions using the 100× objective.
Infectious agents or inclusions that may be seen in blood cells include
Howell-Jolly bodies, Heinz bodies (unstained), basophilic stippling,
canine distemper inclusions, siderotic inclusions, Döhle bodies,
Babesia species, Cytauxzoon felis, hemotrophic Mycoplasma
(formerly Haemobartonella) species, Ehrlichia species, Anaplasma
species, Hepatozoon species, and Theileria species.
Microfilariae (nematode larvae)
that might be observed include
Dirofilaria immitis (see Fig. 2-24) and Dirofilaria repens in dogs,
cats, and wild canids, Dipetalonema reconditum in dogs, and Setaria
species in cattle and horses.
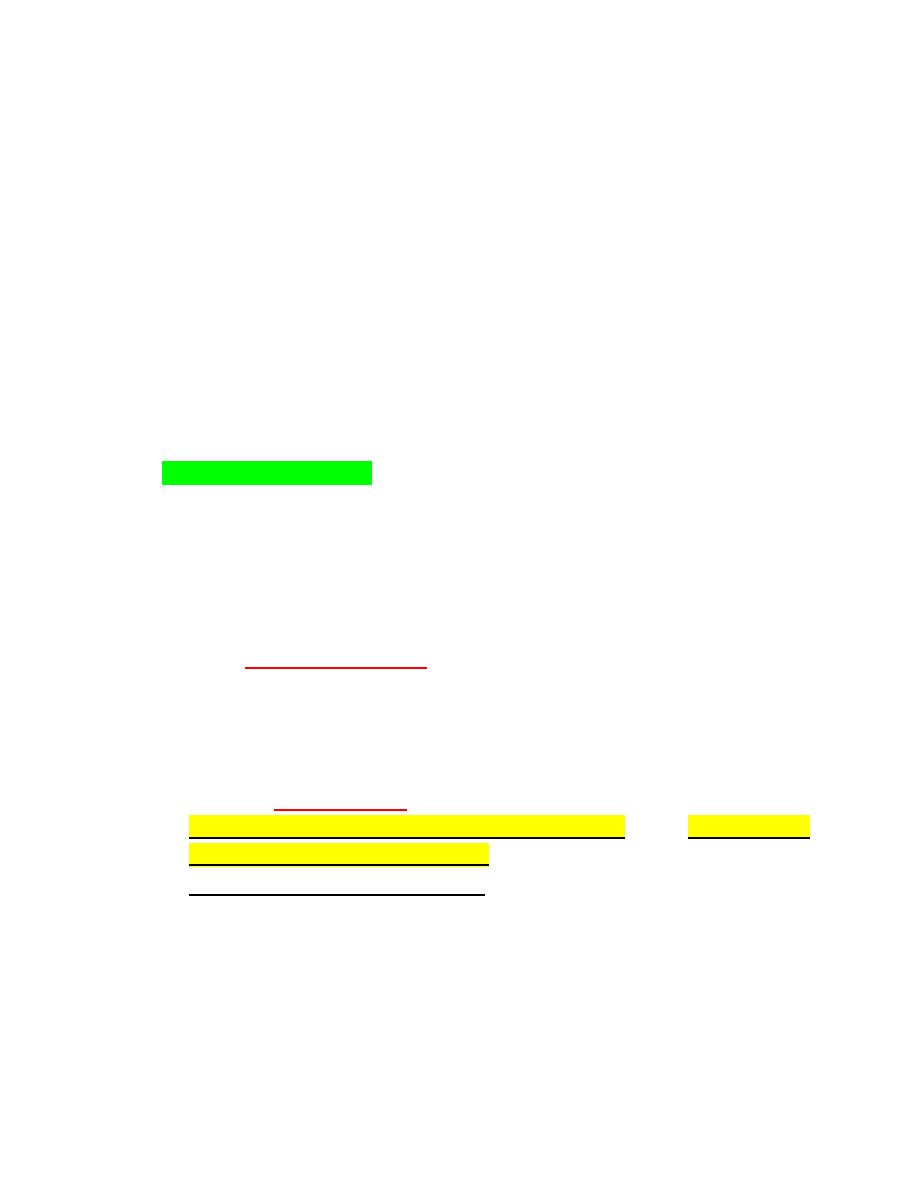
Various
Trypanosoma species
may be seen in blood. These elongated,
flagellated protozoa cause important diseases of livestockbut the
species seen in cattle (T. theileri) (Fig. 2-36). Many dogs are infected
with T. cruzi in the United States, but organisms are rarely seen in
blood and most cases are subclinical (Fig. 2-37). When present,
clinical forms of disease have principally involved heart or neural
dysfunction.
Various
bacterial species
may be present in blood films. It is
important to verify that these are not contaminants, especially
during the staining procedure. The presence of phagocytized
bacteria
within neutrophils indicates that the bacteria are likely of clinical
significance. Spirochetes have been seen in blood from dogs with
Borrelia infections
.
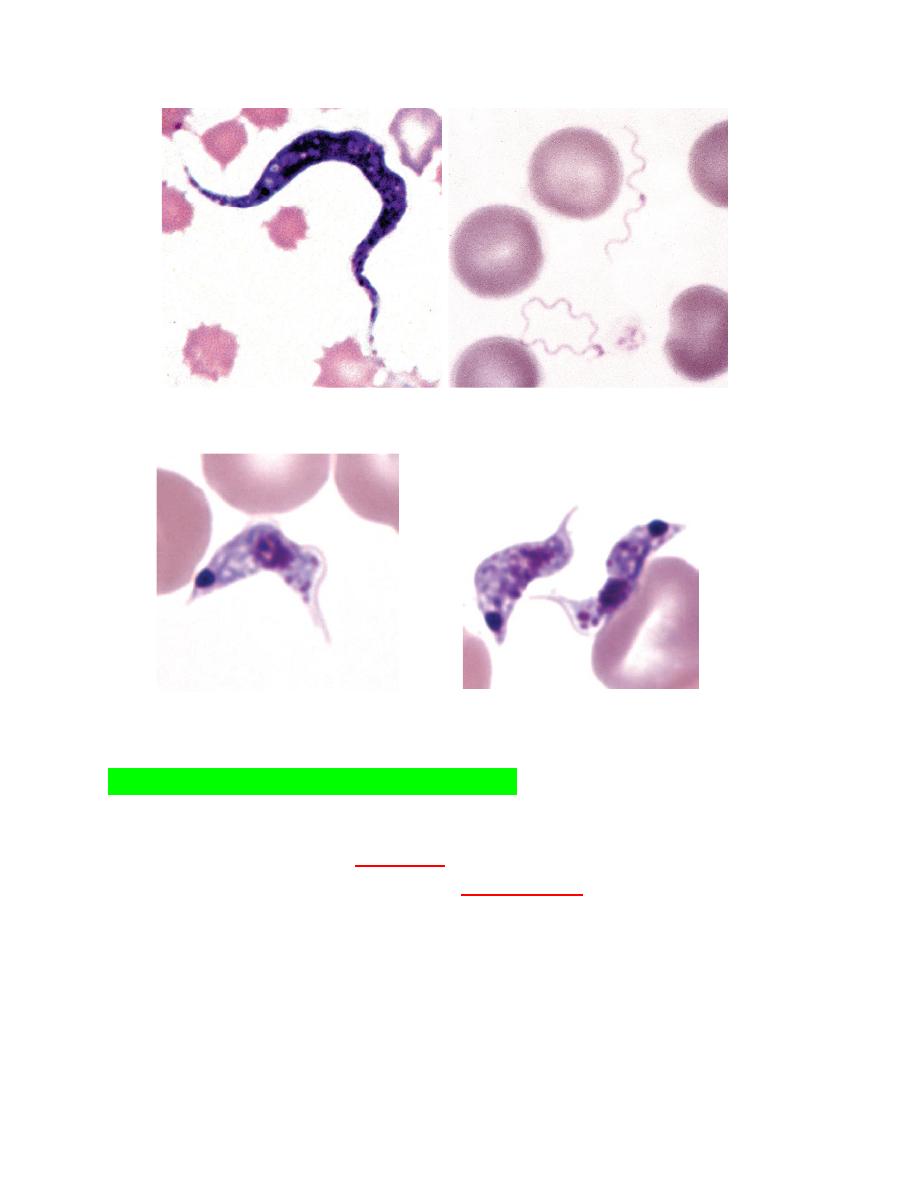
FIGURE 2-36Trypanosoma theileri FIGURE 2-38 Two Borrelia spirochetes
FIGURE 2-37 A, B, Trypanosoma cruzi
NORMAL ERYTHROCYTES
Morphology
Erythrocytes from all mammals are anucleated, and most are in the shape of
biconcave discs called
discocytes
(Figs. 4-1, 4-2).
The biconcave shape results in the
central pallor
of erythrocytes observed
in stained blood films.
Among common domestic animals, biconcavity and central pallor are most
pronounced in dogs (Figs. 4-5), which also have the largest rythrocytes.
Other species do not consistently exhibit central pallor in erythrocytes on
stained blood films. The apparent benefit of the biconcave shape is that it
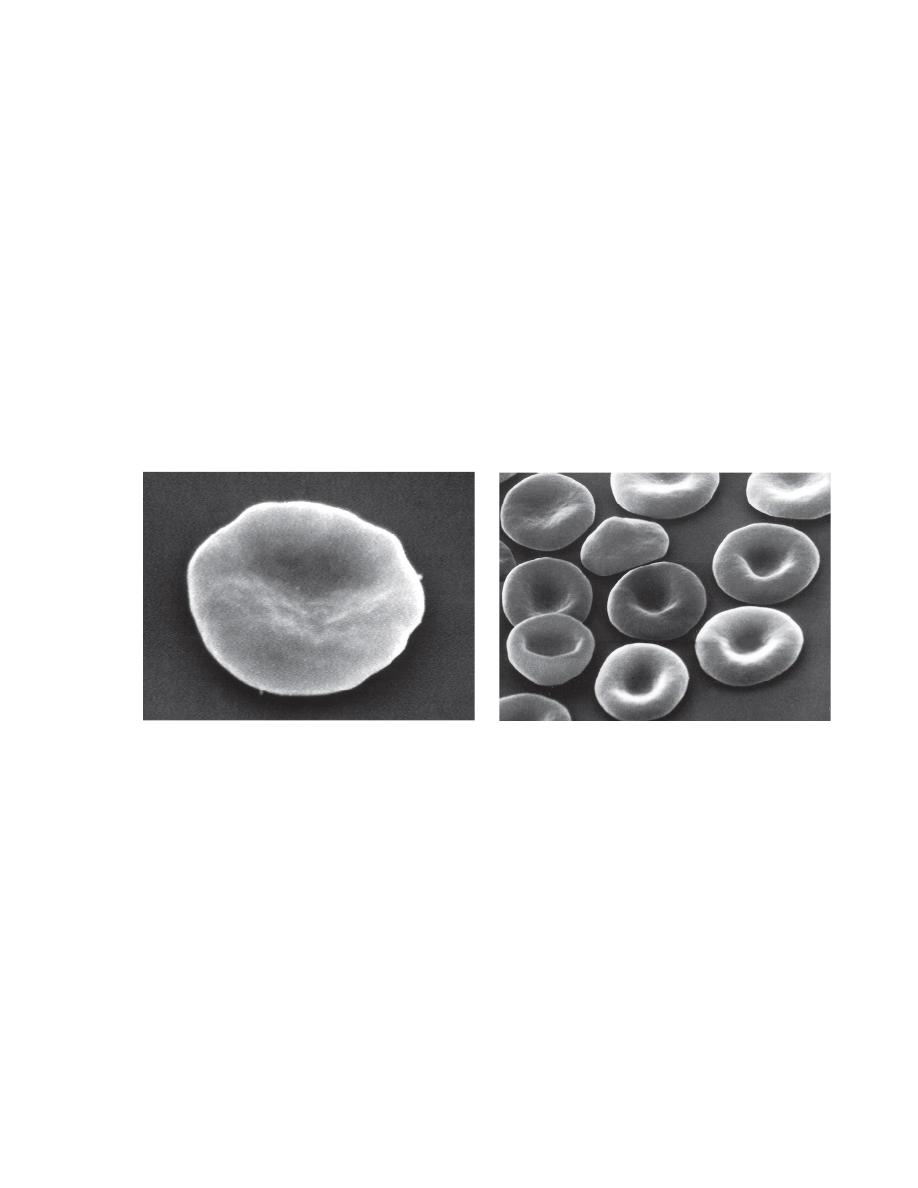
gives erythrocytes high surface area volume ratios and allows for
deformations that must take place as they circulate.
Erythrocytes from
goats
generally have a
flat surface
with little surface
depression; a variety of irregularly
shaped erythrocytes (poikilocytes
) may
be present in clinically normal goats (Fig. 4-4).
Erythrocytes from animals in the Camelidae family (camels, llamas, vicuñas,
and alpacas) are anucleated, thin, elliptical cells termed elliptocytes or
valocytes (Fig. 4-6). They are not biconcave in shape and are minimally
deformable.
Erythrocytes from birds (Fig. 4-7), reptiles, and amphibians are also
elliptical in shape, but they contain nuclei and are larger than mammalian
erythrocytes.
FIGURE 4-1 Scanning electron photomicrograph of a normal FIGURE 4-2 Scanning electron photomicrograph of a
horse erythrocyte called a discocyte normal dog erythrocyte called discocyte.
FIGURE 4-3Most horse erythrocytes are adhered together like stacks of coins (rouleaux)
FIGURE 4-4 Poikilocytes in blood from a normal goat. FIGURE 4-5 dog erythrocytes exhibit central pallor
FIGURE 4-6 Elliptocytes in blood from a normal camel FIGURE 4-7 Nucleated erythrocytes of bird blood

ABNORMAL ERYTHROCYTE MORPHOLOGY
Rouleaux
1. Erythrocytes on blood films from healthy horses, cats, and pigs often exhibit
rouleaux (aggregations of erythrocytes grouped together like a stack of
coins) formations (see Fig. 4-17).
2. Rouleaux formation depends on both the nature of the erythrocytes and the
composition of plasma.
3. Rouleaux formation also depends on the presence of high-molecular weight
proteins in plasma.
4. Increased concentrations of globulin proteins—including fibrinogen,
haptoglobin, and immunoglobulins
5. rouleaux formation in association with inflammatory conditions.
6. Prominent rouleaux formation results in rapid erythrocyte sedimentation
(ESR) in whole blood samples allowed to stand undisturbed.
7. Increased sedimentation rates after 1 hour were suggestive of increased
globulins in plasma, as typically seen with inflammation.
8. Unfortunately the sedimentation rate increases as the hematocrit decreases.
Agglutination
1. Aggregation or clumping of erythrocytes in clusters (not in chains, as in
rouleaux) is termed agglutination (Fig. 4-18).
2. Agglutination is caused by the occurrence of immunoglobulins (IgM) bound
to erythrocyte surfaces.
3. Agglutination did not occur if blood was collected in heparin or citrate.
4. High-dose unfractionated heparin treatment in horses also causes
erythrocyte agglutination by an undefined mechanism.
FIGURE 4-17
Rouleaux
FIGURE 4-18
Erythrocyte agglutination

Polychromasia
1. The presence of bluish-red erythrocytes in stained blood films is called
polychromasia (Fig. 4-19).
2. Polychromatophilic erythrocytes are reticulocytes that stain bluish-red owing
to the combined presence of hemoglobin (red staining) and individual
ribosomes and polyribosomes (blue staining).
3. Slight polychromasia may be present in normal cats, but many normal cats
exhibit no polychromasia in stained blood films.
4. Polychromasia is absent in stained blood films from normal cattle, sheep,
goats, and horses because reticulocytes with sufficient RNA to impart a
bluish color are not normally present in the blood in these species.
5. Low numbers of polychromatophilic erythrocytes are usually seen in blood
from normal dogs and pigs.
6. Increased polychromasia is usually present in regenerative anemias
because many reticulocytes stain bluish-red with routine blood stains (see
Fig. 4-19).
7. The presence of a regenerative response suggests that the anemia results
from either increased erythrocyte destruction or hemorrhage.
8. A nonregenerative anemia generally indicates that the anemia is the result of
decreased erythrocyte production.
FIGURE 4-19 Polypolychromasia
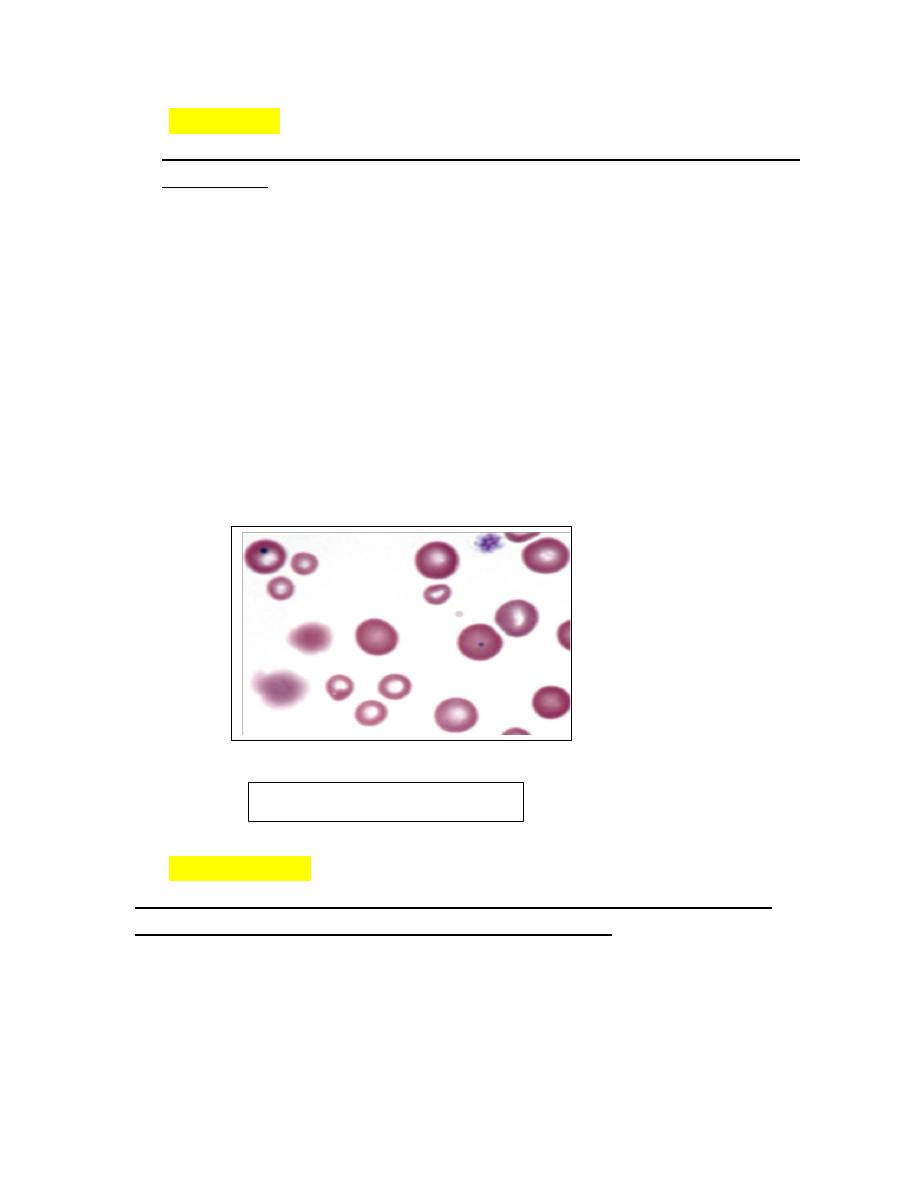
Anisocytosis
1. Variation in erythrocyte diameters (size) in stained blood films is called
anisocytosis (see Fig. 4-23, B).
2. It is greater in normal cattle than in other normal domestic animals.
3. Anisocytosis is increased when different populations of cells are present, as
can occur following a transfusion .
4. Anisocytosis may occur when substantial numbers of smaller than normal
cells are produced, as occurs with iron deficiency, or when substantial
numbers of larger than normal cells are produced, as occurs when increased
numbers of reticulocytes are produced.
5. Consequently increased anisocytosis is usually present in regenerative
anemia.
6. Anisocytosis without polychromasia may be seen in horses with intensely
regenerative anemia.
Hypochromasia
1. The presence of erythrocytes with decreased hemoglobin concentration
and increased central pallor is called hypochromasia (Figs. 4-26 )
2. Not only is the center of the cell paler than normal but the diameter of the area
of central pallor is increased relative to the red-staining periphery of the cell.
3. Erythrocytes from dogs, ruminants, and pigs with iron deficiency anemia often
appear hypochromic on stained blood smears.
Fig. 4-23, B). Anisocytosis
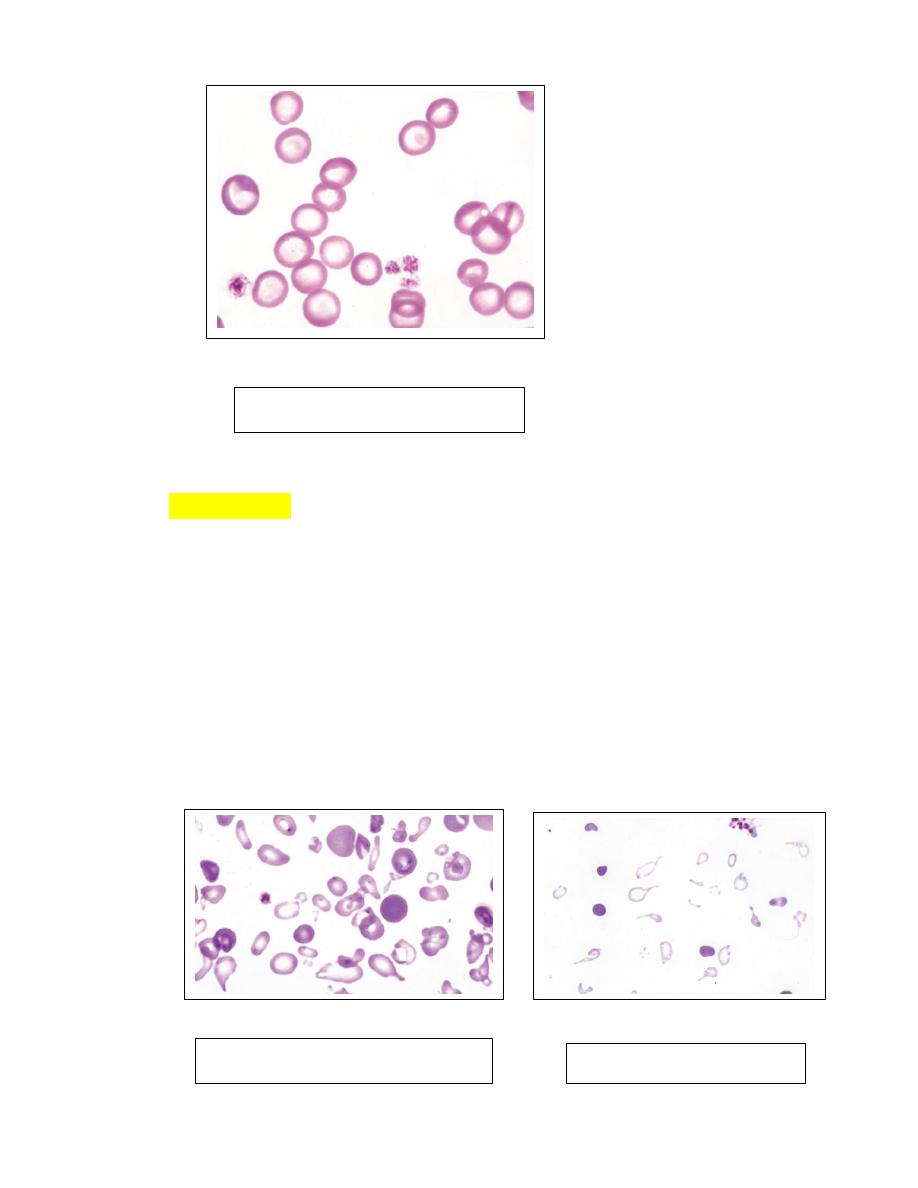
Poikilocytosis
1.
Erythrocytes can assume a wide variety of shapes. Poikilocytosis is a general
term used to describe the presence of abnormally shaped erythrocytes (
Fig. 28-
29).
2. Poikilocytosis may be present in clinically normal goats and young cattle.
3. In some instances, these shapes appear to be related to the hemoglobin types
present.
4. Poikilocytosis forms in various disorders associated with erythrocyte
fragmentation, including disseminated intravascular coagulation (DIC), liver
disease, myeloid neoplasms, myelofibrosis, glomerulonephritis, and
hemangiosarcoma (dogs).
FIGURE 4-26
Hypochromic erythrocytes
FIGURE 4-28
poikilocytosis and hypochromasia
FIGURE 4-29
Poikilocytosis
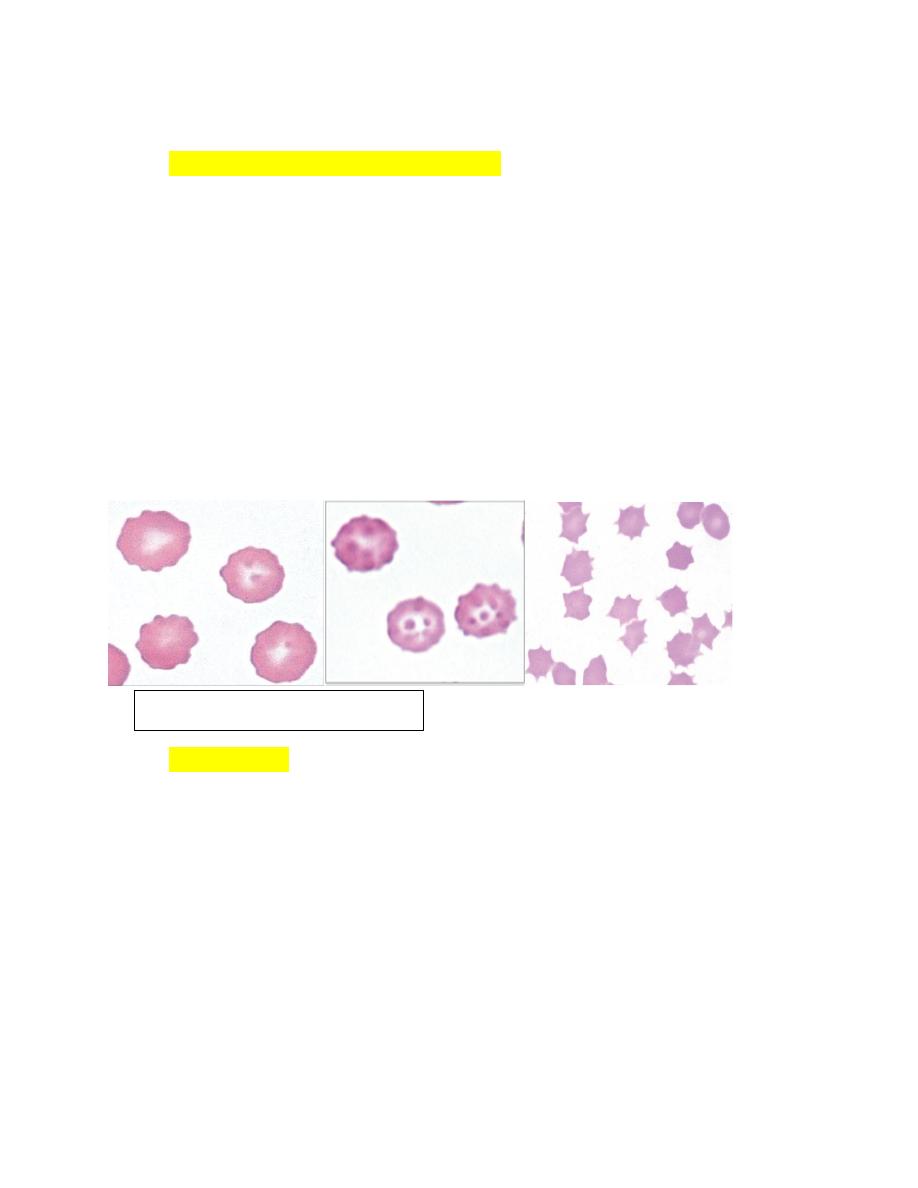
Echinocytes (Crenated Erythrocytes)
1. Echinocytes are spiculated erythrocytes in which the spicules are relatively
evenly spaced and of similar size.
2. Spicules may be sharp or blunt.
3. When observed in stained blood films, echinocytosis is usually an artifact that
results from
a. excess EDTA,
b. improper smear preparation,
c. or prolonged sample storage before blood film preparation.
4. The appearance of the echinocytes can vary depending on the thickness of the
blood film (Fig. 4-37).
5. They are common in normal pig blood smears.
Acanthocytes
1. Erythrocytes with irregularly spaced, variably sized spicules are called
acanthocytes or spur cells (Fig. 4-43).
2. Acanthocytes form when erythrocyte membranes contain excess cholesterol
compared to phospholipids.
3. If cholesterol and phospholipids are increased to a similar degree, codocyte
formation is more likely than acanthocyte formation.
4. Acanthocytes have been recognized in animals with liver disease.
possibly due
to alterations in plasma lipid composition, which can alter erythrocyte lipid
composition.
5. Marked acanthocytosis is reported to occur in young goats and some young
cattle.
FIGURE 4-37
Echinocytes in blood

6. Acanthocytosis of young goats occurs as a result of the presence of
hemoglobin C at this early stage of development.
Keratocytes
1. Erythrocytes containing one or more intact holes are called prekeratocytes,
and erythrocytes with ruptured holes are called keratocytes (Figs. 4-46).
2. the hole results in the formation of one or two projections.
3. Keratocytes have been recognized in various disorders including iron-
deficiency
anemia,
liver
disorders,
doxorubicin
toxicity
in
cats,myelodysplastic syndrome, and in various disorders in dogs having
concomitant echinocytosis or acanthocytosis.
Stomatocytes
1. Mouth like erythrocytes that have oval or elongated areas of central
pallor when viewed in stained blood films are called stomatocytes (Fig.
4-48).
2. They most often occur as artifacts in thick blood film preparations.
FIGURE 4-43 Acanthocytes
FIGURE 4-47 Keratocytes in blood
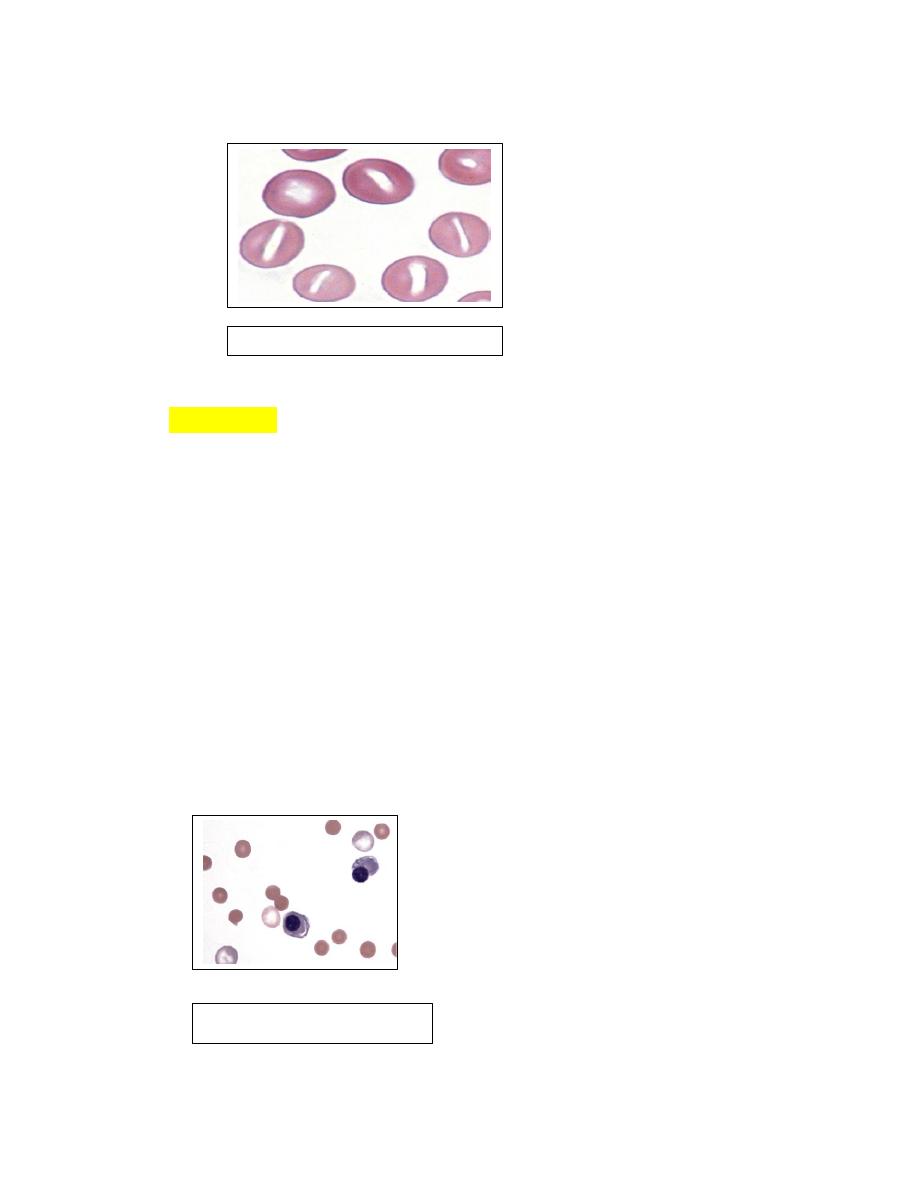
Spherocytes
1. Spherical erythrocytes that result from cell swelling and/or loss of cell
membrane are referred to as spherocytes.
2. Spherocytes lack central pallor and have smaller diameters than normal on
stained blood films (Fig. 4-50).
3. Spherical erythrocytes with slight indentations on one side may be called
stomatospherocytes
4. Spherocytes and stomatospherocytes occur most frequently in association
with immune-mediated hemolytic anemia in dogs.
5. Other potential causes of spherocyte formation include snake
envenomations, bee stings, zinc toxicity, erythrocyte parasites,
transfusionof stored blood, and a familial dyserythropoiesis in dogs.
Spherocytes have been reported in cattle with anaplasmosis and Theileria
buffeli infection and in horses with cutaneous burns.
FIGURE 4-48 Stomatocytes
FIGURE 4-50
Spherocytes
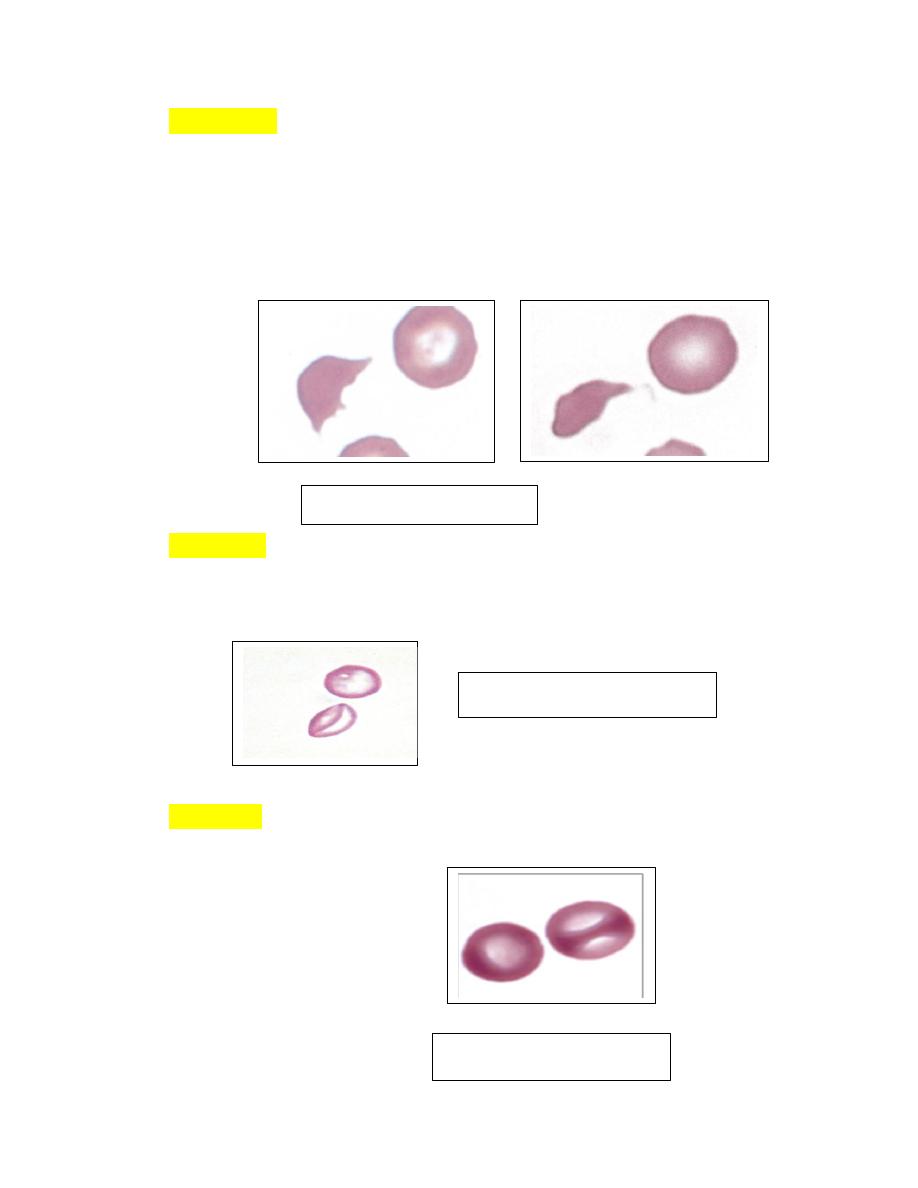
Schistocytes
1. Erythrocyte fragments with pointed extremities are called schistocytes or
schizocytes (Fig. 4-52), and they are smaller than normal discocytes.
2. Schistocytes have also been seen in severe iron-deficiency anemia
myelofibrosis, heart failure,glomerulonephritis, hemolytic uremic syndrome,
hemophagocytic histiocytic disorders, hemangiosarcoma and
dirofilariasis in
dogs.
LeptocytesThese cells are thin, often hypochromic-appearing erythrocytes
with increased membrane-to-volume ratios. Some leptocytes appear folded
(Fig. 4-56 ).
knizocytes (Fig. 4-57) that give the impression that the erythrocyte has a
central bar of hemoglobin
FIGURE 4-52 Schistocytes
FIGURE 4-56 Leptocytes
FIGURE 4-56 knizocytes
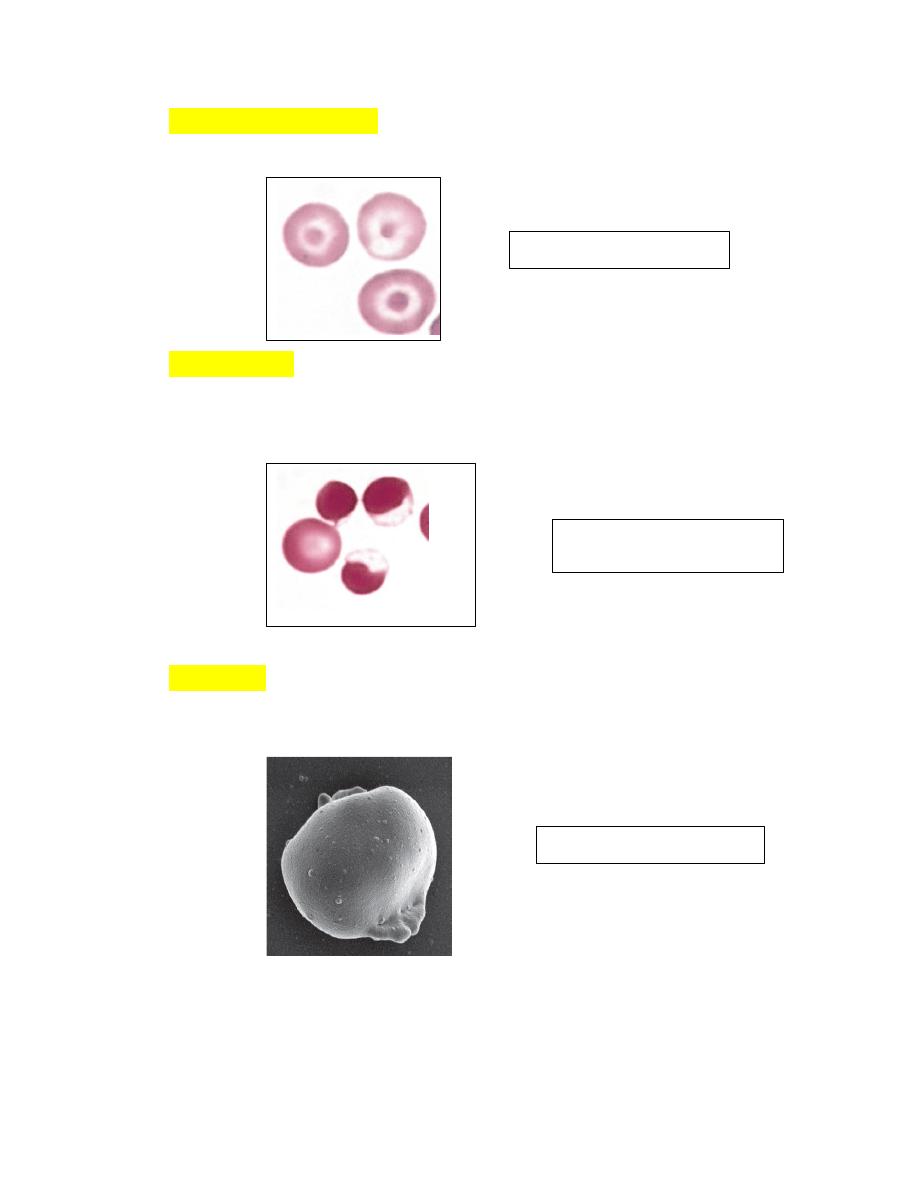
Codocytes (target cells) (Fig. 4-58) are bell-shaped cells that exhibit a
central density or ―bull’s eye‖ in stained blood films.
EccentrocytesAn erythrocyte in which the hemoglobin is localized to part
of the cell, leaving a hemoglobin-poor area visible in the remaining part of
the cell, is termed an eccentrocyte (Figs. 4-59).
PyknocytesIrregularly spherical erythrocytes with small cytoplasmic
projections are called pyknocytes (see Figs4-60).
FIGURE 4-58 Codocytes
FIGURE 4-59
Eccentrocytes
FIGURE 4-60 pyknocyte
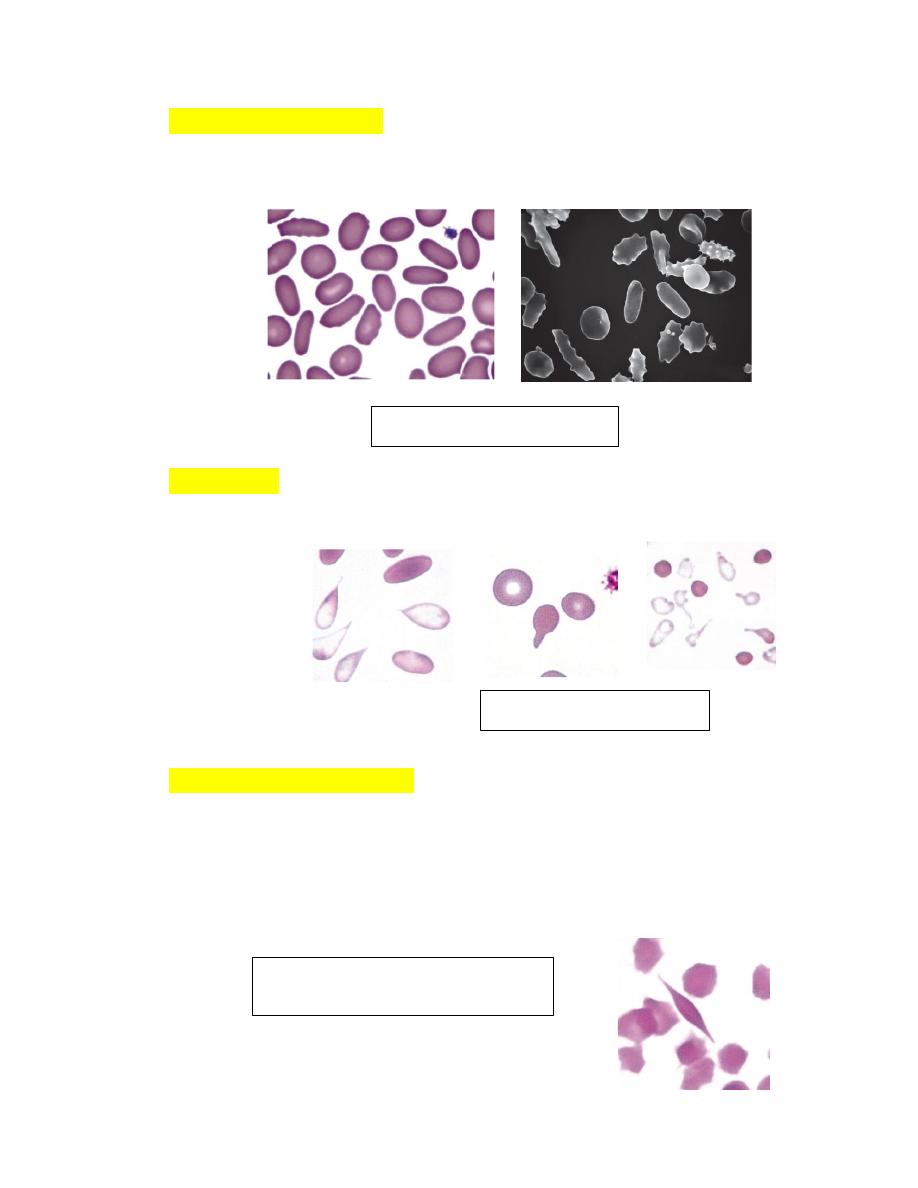
Elliptocytes (Ovalocytes)Erythrocytes from nonmammals and animals in
the Camelidae family are elliptical or oval in shape (see Fig. 4-66). They
are generally flat rather than biconcave.
DacryocytesThese erythrocytes are teardrop-shaped with single elongated
or pointed extremities (Fig. 4-67).
Drepanocytes (Sickle Cells)These fusiform or spindle-shaped erythrocytes
were first recognized in deer blood in 1840 and in a human with sickle cell
anemia in 1910. Drepanocytes are often observed in blood from normal
deer (Figs. 4-68).
FIGURE 4-66 Elliptocytosis
FIGURE 4-67 Dacryocytes
FIGURE 4-68
Drepanocytes and
fusiform erythrocytes in a deer and
goat
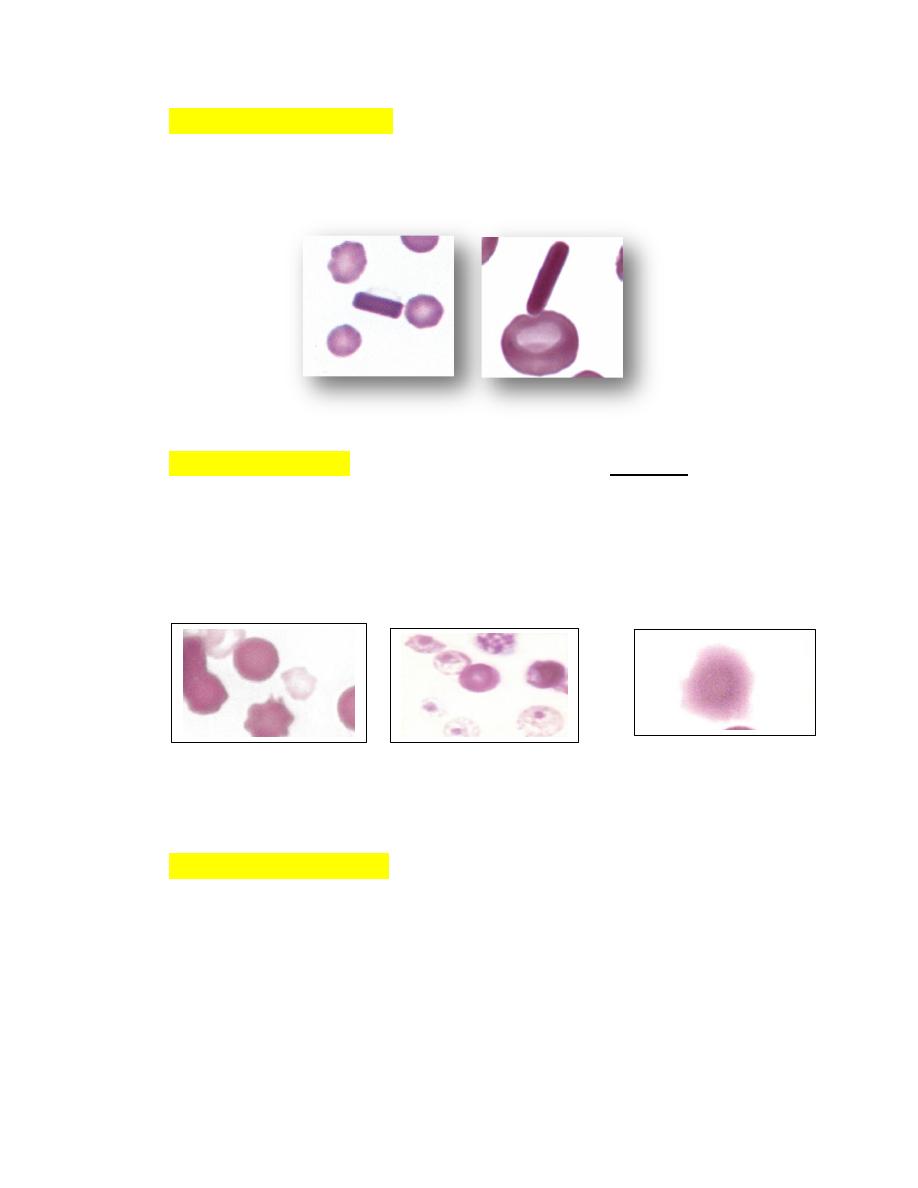
Crystallized HemoglobinThe presence of large hemoglobin crystals within
erythrocytes is occasionally recognized in blood films from cats and dogs
(Fig. 4-70) and frequently in the blood of llamas and alpacas (Fig. 4-70).
FIGURE 4-70 Hemoglobin crystals in stained blood films
Lysed ErythrocytesThe presence of erythrocyte “ghosts” in peripheral
blood films indicates that the cells lysed prior to blood film preparation
(Fig. 4-72). the presence of erythrocyte ghosts indicates either recent
intravascular hemolysis or in vitro hemolysis in the blood tube after
collection.
FIGURE 4-72 Lysed erythrocytes in blood films.
Nucleated ErythrocytesRubricytes and metarubricytes (Fig. 4-75) are
seldom present in the blood of normal adult mammals, although low
numbers may occur in some normal dogs and cats. Metarubricytosis
(generally called normoblastemia in human hematology) is often seen in
blood in association with aregenerative anemia; however, their presence
does not necessarily indicate that a regenerative response is present.
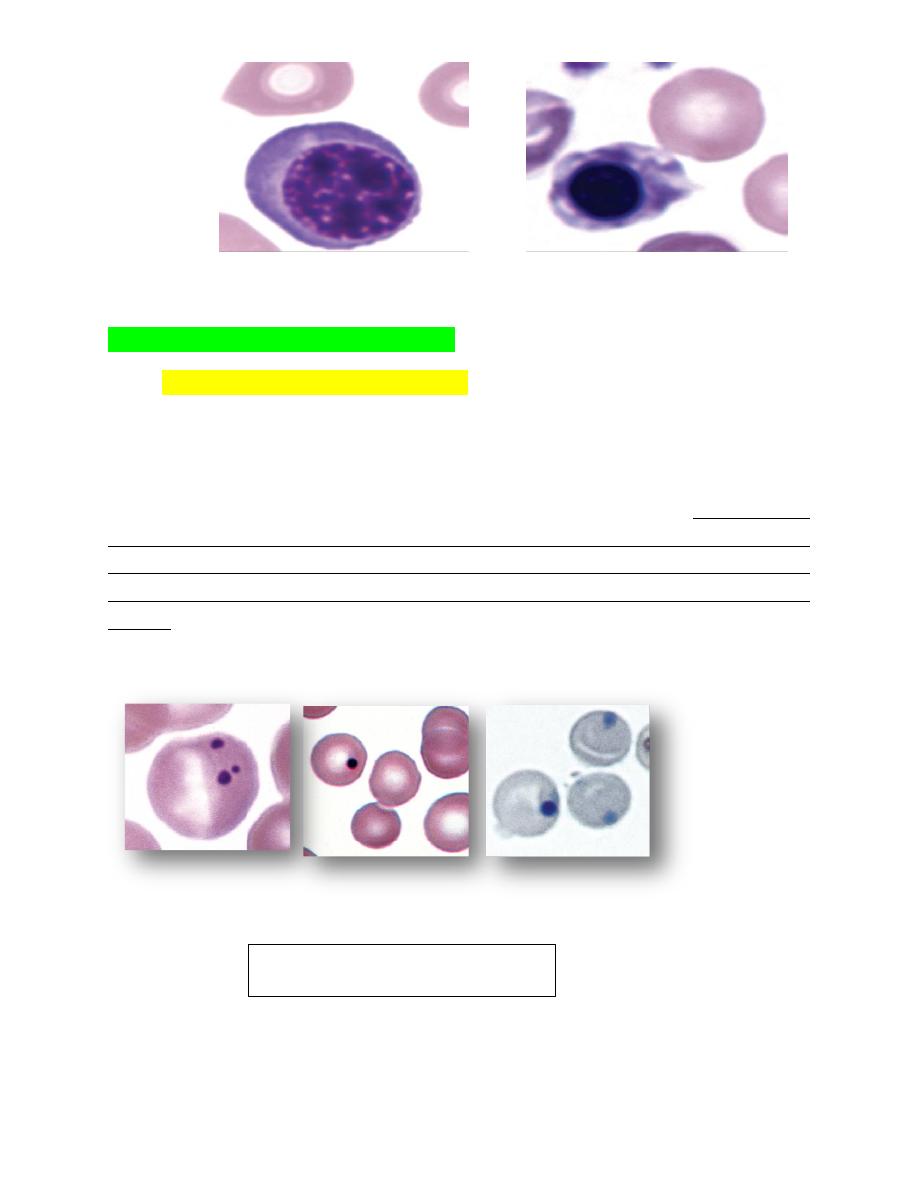
FIGURE 4-75 Nucleated erythroid cells and nuclear fragmentation
INCLUSIONS OF ERYTHROCYTES
1. Howell-Jolly Bodies (Micronuclei)
These small spherical nuclear remnants form in the bone marrow following
nuclear fragmentation or rupture of the nuclear membrane, with nuclear material
left behindwhen the nucleus is expelled They have generally been called Howell-
Jolly bodies by hematologists and micronuclei by toxicologists. They may be
present in low numbers in erythrocytes of normal horses and cats. They are often
present in association with regenerative anemia or following splenectomy in other
species. Howell-Jolly bodies may be increased in animals receiving glucocorticoid
therapy. Howell-Jolly bodies, which
stain dark blue with Romanowsky-type stains
(Fig. 4-76).
FIGURE 4-76 Howell-Jolly bodies

Heinz Bodies
These inclusions are large aggregates of oxidized, precipitated hemoglobin
attached to the internal surfaces of erythrocyte membranes appearing as pale
―spots‖ within erythrocytes. In contrast to Howell-Jolly bodies, which stain dark
blue,
Heinz bodies stain red to pale pink with Romanowsky-type stains
(Fig. 4-78
A). They can also be visualized as dark refractile inclusions in new methylene blue
wet mount preparations. Heinz bodies stain lighter blue than Howell-Jolly bodies
when stained with reticulocyte stains (Brilliant crysel blue stain)
When
intravascular hemolysis occurs, they may be visible as red inclusions within
erythrocyte ghosts (Fig. 4-78 B).
In contrast to other domestic animal species, normal cats may have up to 5% Heinz
bodies within their erythrocytes. Increased numbers of Heinz bodies have been
FIGURE 4-78 A: Heinz bodies in blood from a cat appearing as pale “spots”
within erythrocytes.
FIGURE 4-78 B: Heinz bodies in blood from a cat. New methylene blue
reticulocyte stain.
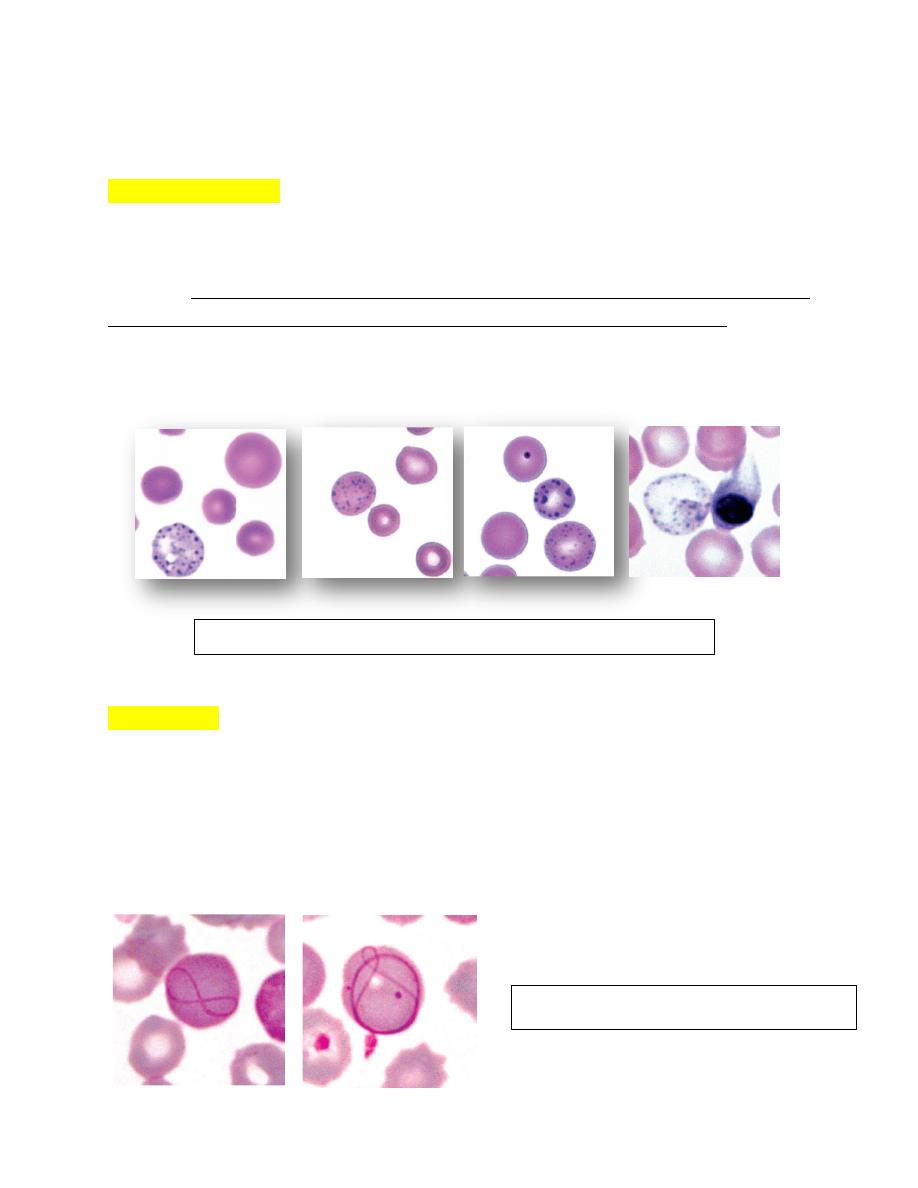
seen in kittens fed fish-based diets and in cats fed commercial soft-moist diets
containing propylene glycol.
Heinz body associated with hemolytic anemia.
Basophilic Stippling
Reticulocytes usually stain as polychromatophilic erythrocytes with Romanowsky-
type blood stains owing to the presence of dispersed ribosomes and polyribosomes;
however, sometimes the ribosomes and polyribosomes aggregate together, forming
blue-staining punctate inclusions referred to as basophilic stippling (Fig. 4-
81).Diffuse basophilic stippling commonly occurs in regenerative anemia in
ruminants. It may be prominent in any species in the presence of lead poisoning.
Cabot Rings
Cabot rings are reddish purple-staining thread like loops or figure-eight structures
that are primarily found in reticulocytes in humans.
Cabot rings have been bserved
in normal camel and llama erythrocytes stained with reticulocyte stains (Fig. 4-84).
FIGURE 4-81 Diffuse and focal basophilic stippling
FIGURE 4-84 Cabot rings in erythrocytes
Cabot rings in erythrocytes
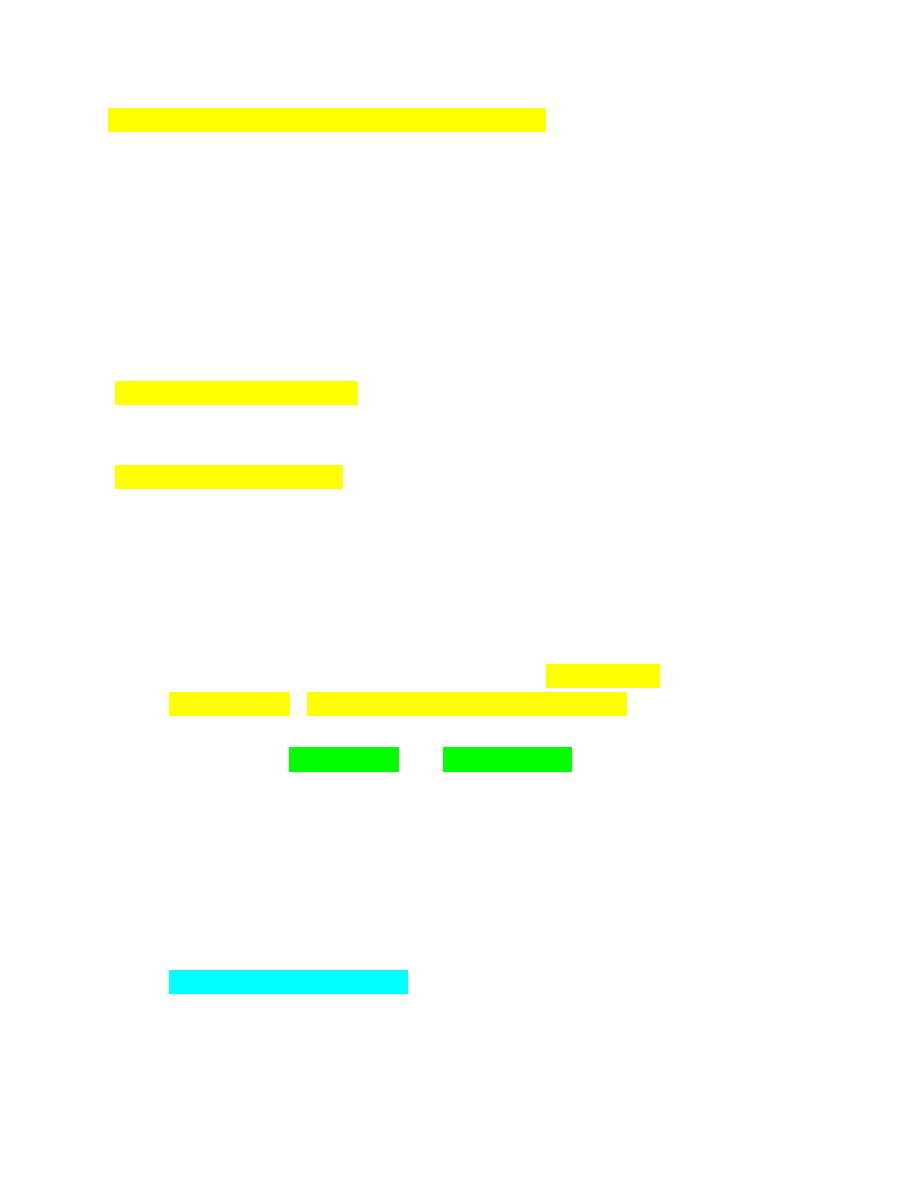
INFECTIOUS AGENTS OF ERYTHROCYTES
1. A number of infectious agents are recognized to occur in or on erythrocytes.
These include
intracellular protozoal parasites
Babesia species
Theileria species
Cytauxzoon felis)
Intracellular rickettsial organisms
(Anaplasma species)
A. Erythrocytic anaplasma
a. Anaplasma marginali and Anaplasma centrali (cattle)
b. Anaplasma ovis and Anaplasma hercei (sheep)
B. Leukocytic anaplasma
Anaplasma phagocytophilia
Ehrlichia spp.
Epicellular Mycoplasma species
c. ( Hemotrophic Mycoplasma e.g. Mycoplasma heamofelies, Mycoplasma
heamocanis, Mycoplasma hemolama, Mycoplasma wenyonii ).
1. The erythrocyte protozoal organisms (piroplasms) of the order
Piroplasmida (Babesia species Theileria species) each have a nucleus
within their cytoplasm.
2. In contrast to Plasmodium and Haemoproteus genera, these organisms do
not form pigment in infected erythrocytes even though they also consume
hemoglobin.
3. The rickettsia and mycoplasma organisms are bacteria and therefore lack
nuclei.
4. These infectious agents generally cause mild to severe hemolytic anemia
depending on the pathogenicity of the organism and the susceptibility of the
host.
5. Distemper virus inclusions may also be seen in dog erythrocytes.
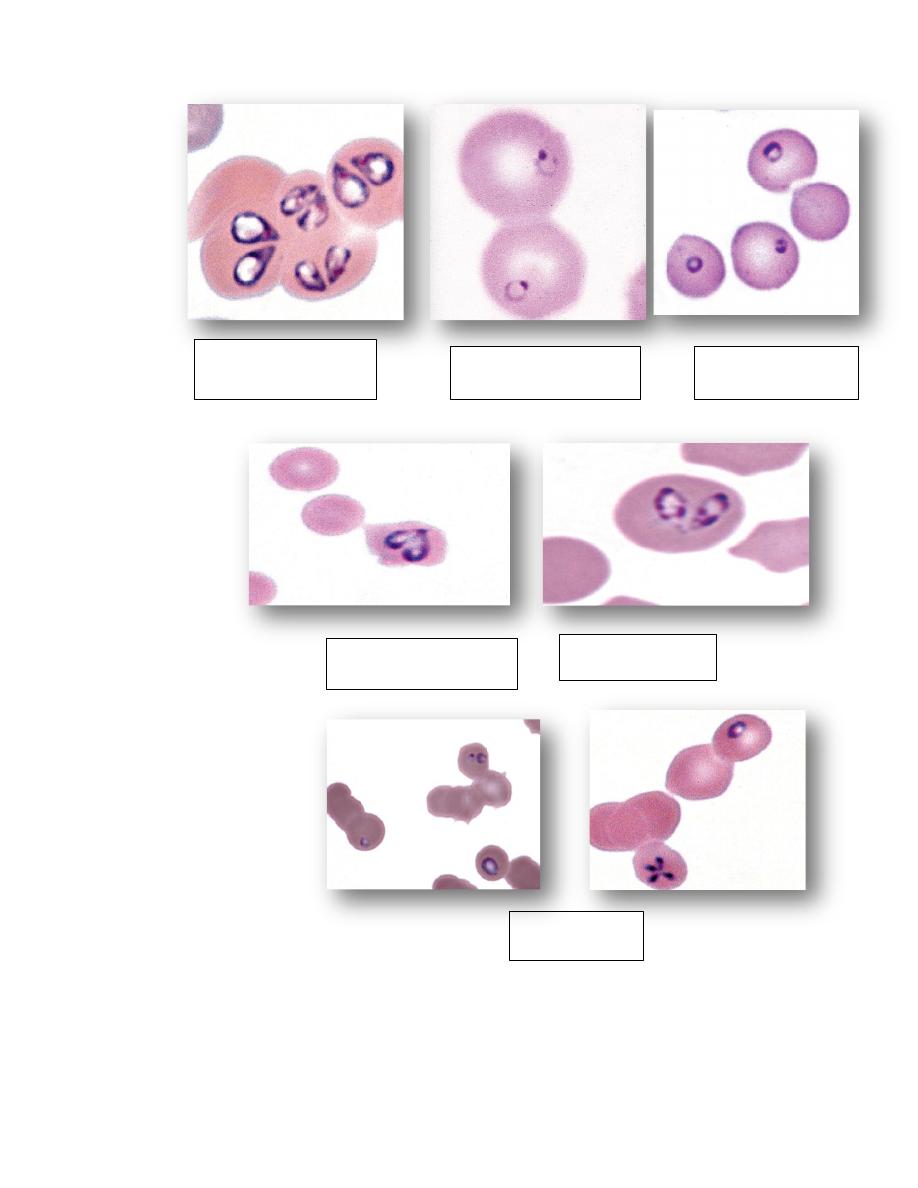
Two pear-shaped
Babesia canis
Single small Babesia
gibsoni in dog
Single Babesia felis
Babesia bigemina cow
Babesia caballi
Horse
Babesia equi
Horse

Theileria buffeli in cow
Theileria cervi in deer
Cytauxzoon felis in cat
Anaplasma marginale in cow
Anaplasma ovis in goat
Mycoplasma haemofelis
Mycoplasma haemocanis
Mycoplasma suis
Mycoplasma ovis
Mycoplasma wenyonii
Mycoplasma haemolama
Bartonella henselae in cat
Distemper inclusions in dog
erythrocytes

Anemia and DIFFERENTIAL DIAGNOSIS OF ANEMIA
True or absolute anemia is defined as a decrease in erythrocyte mass within
the body. HCT, hemoglobin, and RBC count values are usually below their
reference intervals.
the anemia can sometimes be masked by concomitant dehydration.
Low erythrocyte parameters may also be present in blood when the total-
body erythrocyte mass is normal (relative anemia).
This can result from overhydration resulting in erythrocyte dilution and from
splenic sequestration of erythrocytes as occurs with splenic relaxation during
anesthesia, heparin-induced erythrocyte agglutination in horses, and various
causes of splenomegaly.
Anemia is a condition, not a diagnosis. Anemia is classifiedin various ways
to assist in determining its specific cause so that effective therapy can be
given.
In addition to past history, presenting complaints, and laboratory findings,
results of other test procedures (e.g., diagnostic imaging) are important in
reaching a final diagnosis.
Anemia may occur following blood loss ( Hemorrhagic anemia), increased
erythrocyte destruction ( Hemolytic anemia), or decreased erythrocyte
production ( Aplastic anemia) .
Factors that can be useful in categorizing anemia into these broad causes
(and often into more specific causes) include:
a. reticulocyte counts, erythrocyte indices, erythrocyte morphology on stained
blood films,
b. the appearance of the plasma,
c. plasma protein concentration,
d. serum iron measurements,
e. serum bilirubin determination,
f. direct antiglobulin test,
g. and bone marrow evaluation.
Anemia may also develop as a result of the expansion ofthe vascular space
faster than the expansion of the total-body erythrocyte mass.

This hemodilution contributes to the anemia of the neonate and to the mild
anemia that develops during pregnancy in most domestic animals, the horse
being an exception.
Classification of Anemia using Erythrocyte Indices
An anemia can also be classified using the MCV and MCHC values to assist
in determining its cause.
The terms used to indicate size are macrocytic (increased MCV),
normocytic (normal MCV), and microcytic (decreased MCV).
The terms used to describe MCHC values are normochromic (normal
MCHC) and hypochromic (decreased MCHC).
Anemias are not classified as hyperchromic because high MCHC values are
artifacts.
Causes of Hemolytic Anemias in Domestic Animals
1. Immune-mediated erythrocyte destruction: Primary or autoimmune
hemolytic anemia (common in dogs); neonatal isoerythrolysis (primarily
horses and cats); lupus erythematosus (primarily dogs); incompatible blood
transfusions; drugs, including penicillin (horses), cephalosporins (dogs),
levamisole (dogs), sulfonamides (horses and dogs), pirimicarb (insecticide in
dogs), and propylthiouracil (cats)
2. Erythrocyte parasites (may have an immune-mediated component): Anaplasma
spp. (ruminants), erythrocytic Mycoplasma spp. (except horses), Babesia spp.,
Cytauxzoon felis, Theileria spp. (ruminants)
3. Other infectious agents (may have an immune-mediated component): Leptospira
and Clostridium spp. (primarily ruminants and horses), FeLV (seldom hemolytic),
equine infectious anemia virus (multifactorial, also with decreased production),
Sarcocystis spp. (cattle and sheep), Trypanosoma spp. (primarily outside the
United States)
4. Chemicals and plants (most are oxidants): Onions, red maple (horses), Brassica
spp. (ruminants), lush winter rye (cattle), copper (sheep and goats), phenothiazine
(horses), acetaminophen (cats and dogs), methylene blue (cats and dogs),
benzocaine (cats and dogs), phenazopyridine (cats), methionine (cats), vitamin K
(dogs), propylene glycol (cats), naphthalene (dogs?), zinc (dogs and ruminants),
indole (experimental in cattle and horses), tryptophan (experimental in horses),
crude oil (marine birds), venoms (snakes, bees,wasps, and spiders)
5. Fragmentation: Disseminated intravascular coagulation (primarily dogs),
dirofilariasis (especially posterior vena cava syndrome) in dogs, hemangiosarcoma
(dogs), vasculitis, hemolytic uremia syndrome.
6. Hypo-osmolality: Hypotonic fluid administration, water intoxication (primarily
in cattle)
7. Hypophosphatemia: Postparturient hemoglobinuria (cattle), ketoacidotic diabetic
animals following insulin therapy (cats and dogs), hepatic lipidosis (cats).
8. Hereditary erythrocyte defects: Pyruvate kinase deficiency (dogs and cats),
phosphofructokinase deficiency (dogs), glucose-6-phosphate dehydrogenase
deficiency (horses), hereditary stomatocytosis (mild anemia in dogs),
erythropoietic porphyria (cattle and cats), , erythrocyte flavin adenine dinucleotide
deficiency in horses (methemoglobinemia and sometimes mild anemia), hereditary
spherocytosis in cattle.
9. Miscellaneous: Liver failure (horses), selenium deficiency in cattle grazing on
St. Augustine grass, postparturient hemoglobinuria in cattle not associated with
hypophosphatemia
Causes of Blood-Loss Anemias in Domestic Animals
1. Trauma: Accidents, fights, gastrointestinal foreign bodies, surgery
2. Parasites: Hookworms, fleas, blood-sucking lice, Haemonchus spp. (small
ruminants), liver flukes, Coccidia spp.
3. Coagulation disorders: Vitamin K deficiency, sweet clover (dicoumarol)
toxicity (cattle), rodenticide toxicity, bracken fern toxicity (cattle and sheep),
disseminated intravascularcoagulation, inherited coagulation factor deficiencies.
4. Platelet disorders: Thrombocytopenia and inherited platelet function defects
5. Neoplasia: Gastric tumors including carcinomas, and lymphoma; transitional
cell carcinoma and transitional cell papilloma of urogenital system; and ruptured
hemangioma, hemangiosarcoma, and adrenal gland tumors with bleeding into body
cavities and tissues
6. Gastrointestinal ulcers: Glucocorticoids, nonsteroidal anti-inflammatory drugs,
mast cell tumors, gastrinoma, stress, metabolic diseases (uremia, liver failure,
hypoadrenocorticism)
7. Vascular abnormalities: Arteriovenous fistula and vascular ectasia in the
gastrointestinal or urogenital tracts
8.
Phenylephrine-induced
hemorrhage:
Presumably
associated
with
hypertension in aged horses treated for nephrosplenic entrapment of the large
colon.
Anemias Resulting from Decreased Erythrocyte Production in Domestic
Animals
1. Chronic renal disease: Primarily lack of erythropoietin
2. Endocrine deficiencies: Hypothyroidism, hypoadrenocorticism,
hypopituitarism, hypoandrogenism
3. Inflammatory disease: Inflammation and neoplasia
4. Cytotoxic damage to the marrow: Bracken fern poisoning (cattle), cytotoxic
anticancer drugs, estrogen toxicity (dogs and ferrets), chloramphenicol (cats,
usually not anemic), phenylbutazone (dogs), trimethoprim-sulfadiazine (dogs),
radiation, albendazole (dogs, cats, alpacas), griseofulvin (cats), trichloroethylene
(cattle)
5. Infectious agents: Ehrlichia spp. (dogs, horses, and cats), FeLV,
nonbloodsucking trichostrongyloid parasites (ruminants), parvovirus (pups)
6. Immune-mediated: Nonregenerative anemia, selective erythroid aplasia,
continued treatment with recombinant human erythropoietin, idiopathic aplastic
anemia (?)
7. Congenital/inherited: Foals and dogs?
8. Myelophthisis: Myeloid leukemias, lymphoid leukemias, myelodysplastic
syndromes,
multiple
myeloma,
myelofibrosis,
osteosclerosis,
metastatic
lymphomas, metastatic mast cell tumors.
ERYTHROCYTOSIS (POLYCYTHEMIA)
Erythrocytosis refers to an increase in HCT, hemoglobin, and RBC count above
the normal reference interval.
Relative Erythrocytosis
1. Splenic contraction: Excitement, exercise, pain (primarily in horses, dogs, and
cats)
2. Dehydration: Water loss, water deprivation, shock with fluid shift into tissues
Absolute Erythrocytosis
1. Primary erythrocytosis: A myeloproliferative neoplasm in adult dogs and cats
2. Familial erythrocytosis in young Jersey cattle: Etiology unknown
3. Hypoxemia with compensatory increased erythropoietin production: Chronic
lung disease, heart disease with right-to-left shunting of blood, chronic
methemoglobinemia (rare in dogs and cats)
4. Inappropriate erythropoietin production: Renal lesions (primarily tumors),
nonrenal erythropoietin secreting tumors (rare).
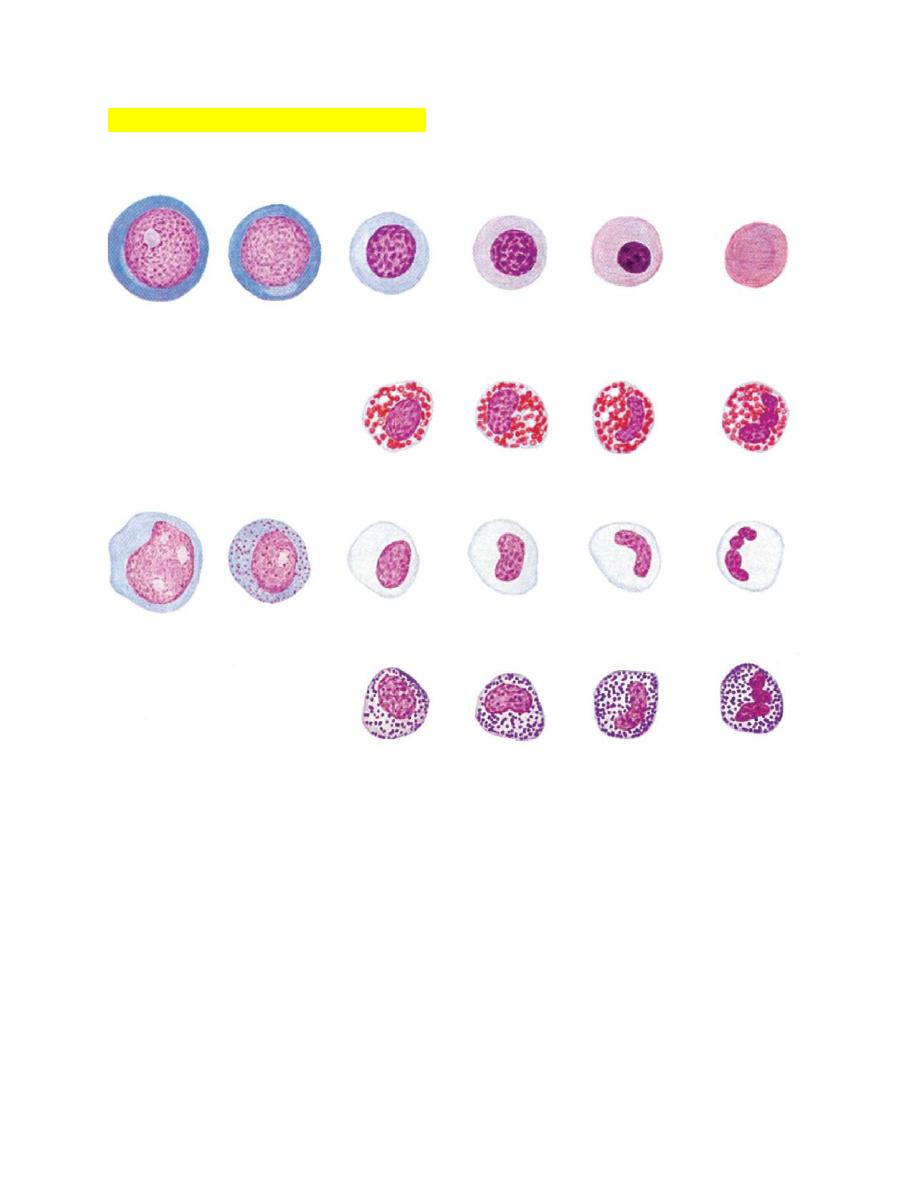
Evaluation of Leukocytic Disorders
FIGURE 3-10 Maturation of canine erythroid and granulocytic cells as they
appear in Wright-Giemsa-stained bone marrow aspirate smears.
