
مالحظت
:
قسم من الساليداث منقىلت نصا من
بعط الكتب المعتبرة في أمراض الجهاز
الهضمي والكبد

ACUTE UPPER GI-BLEEDING
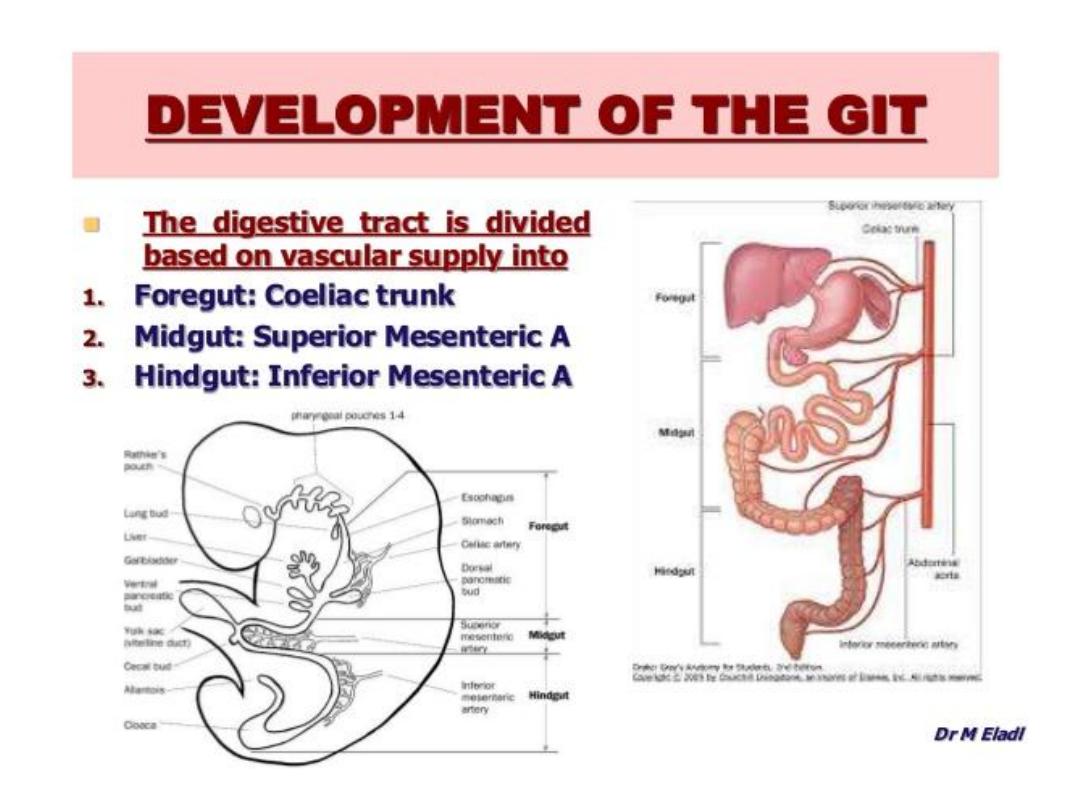
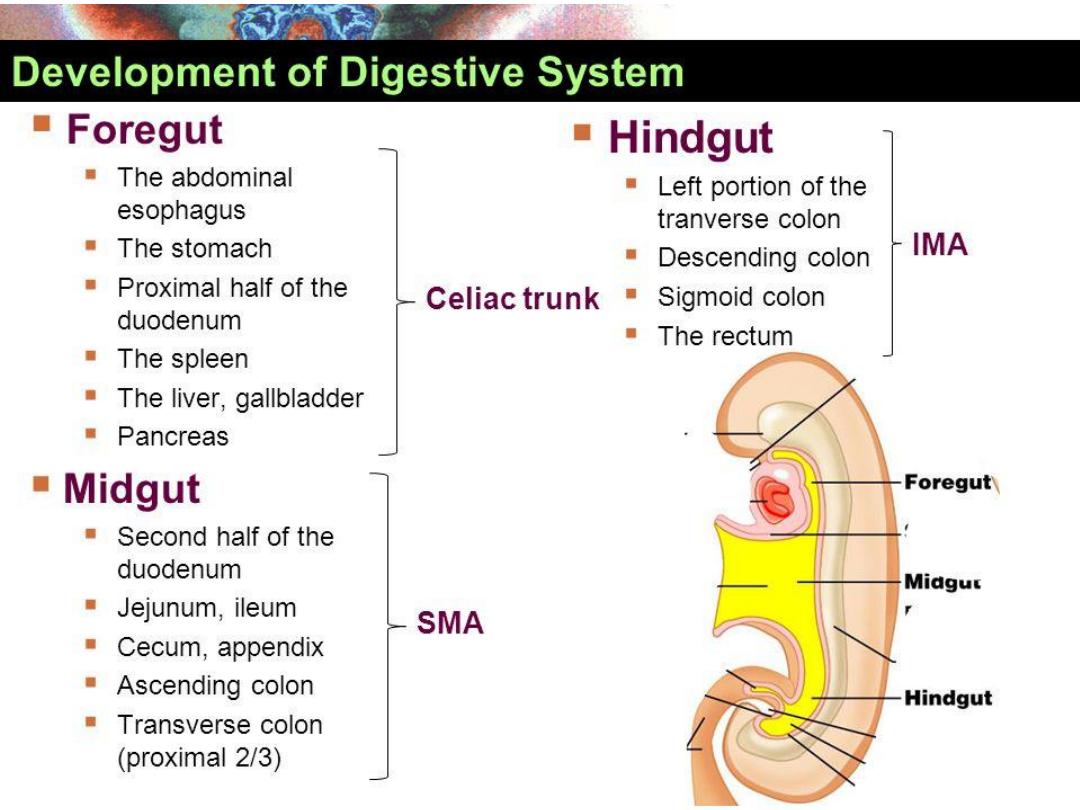

BOUNERIES FORE, MID AND HAND
GUT BLOOD SUPPLY
Foregut:
Coeliac Trunk (T12).
Midgut:
Superior mesenteric artery ( at L1 level).
Hindgut:
Inferior mesenteric artery (L3)
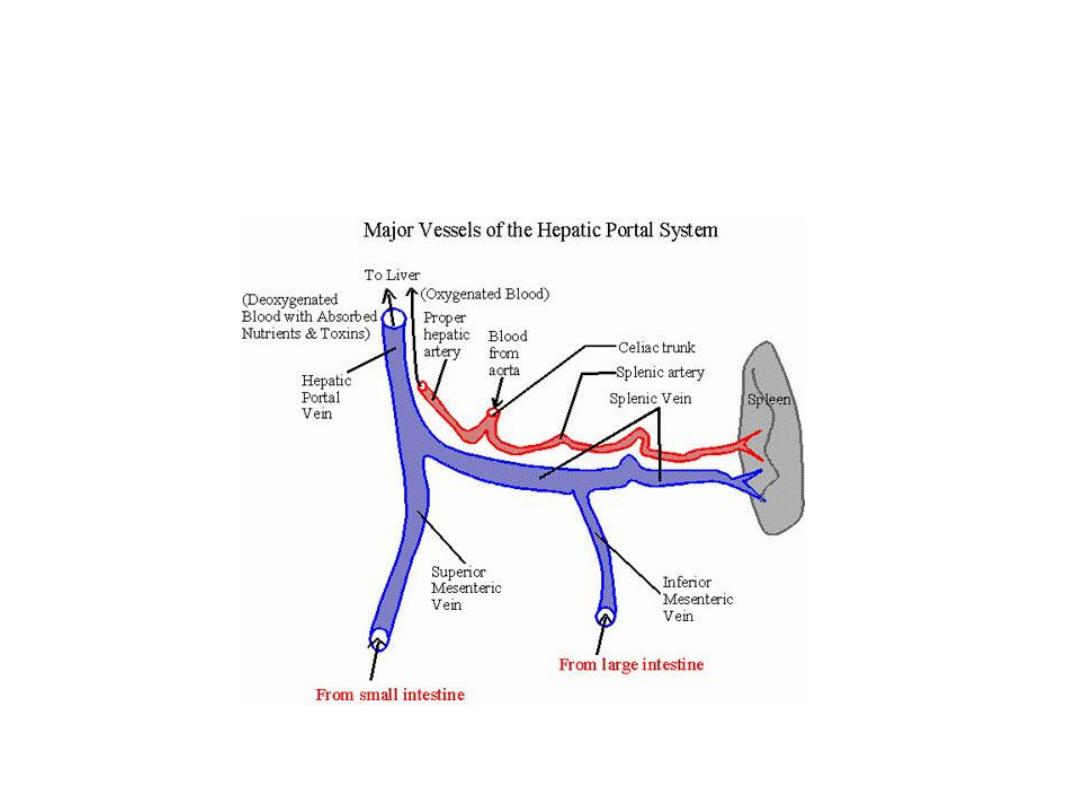
Portal vein anatomy
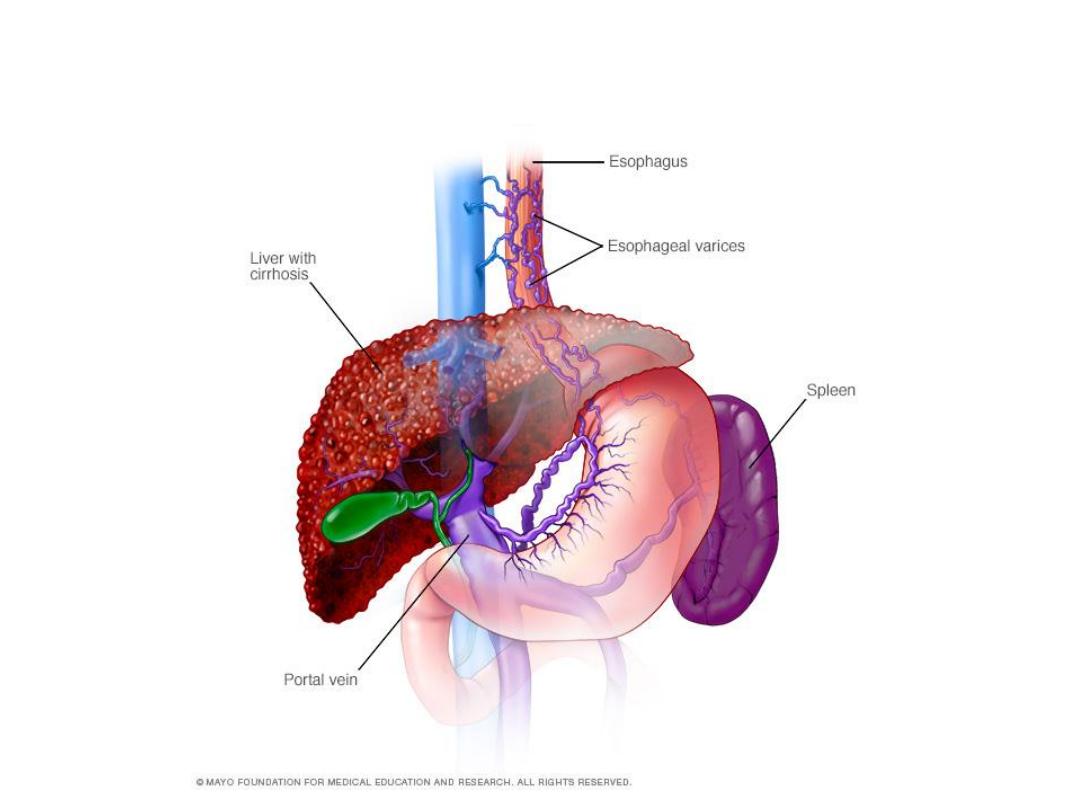
Varices formation
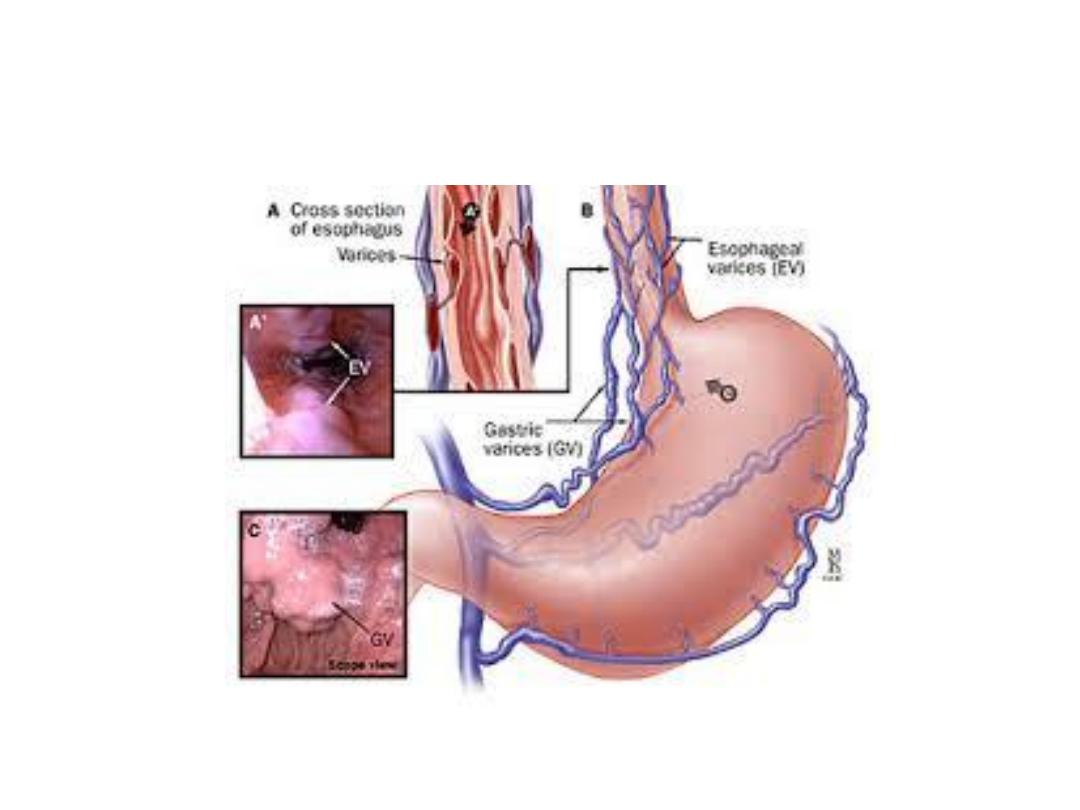
Mechanism of varices formation

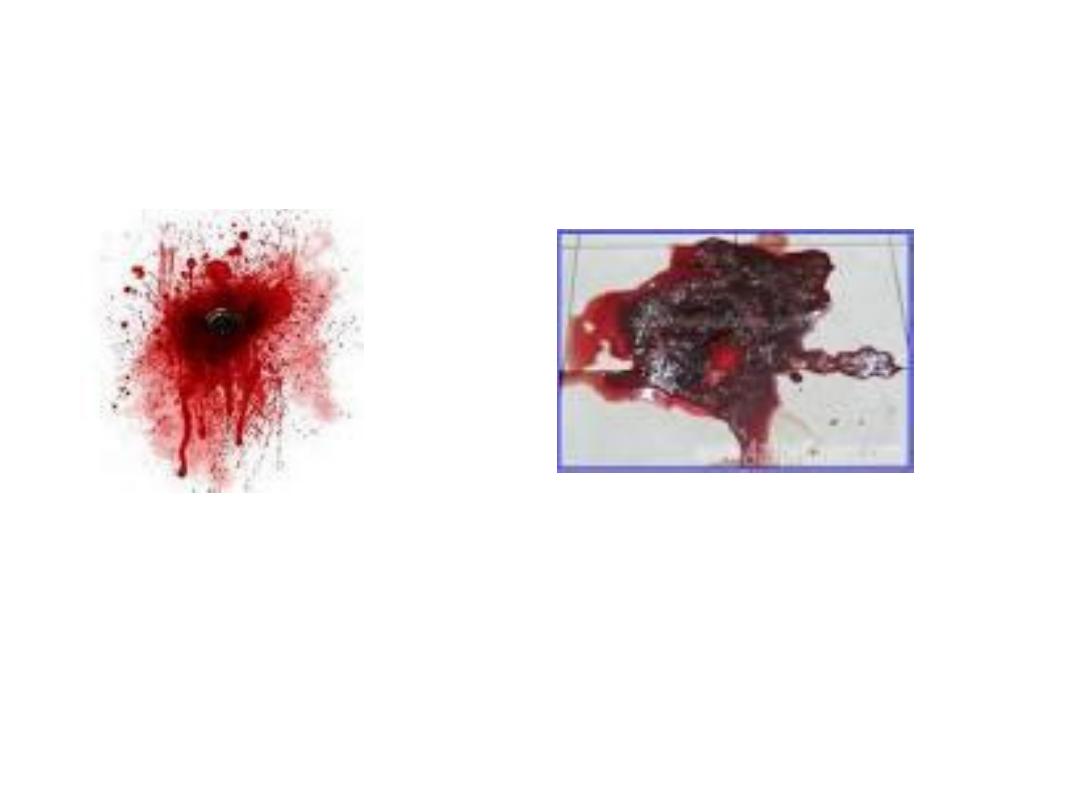
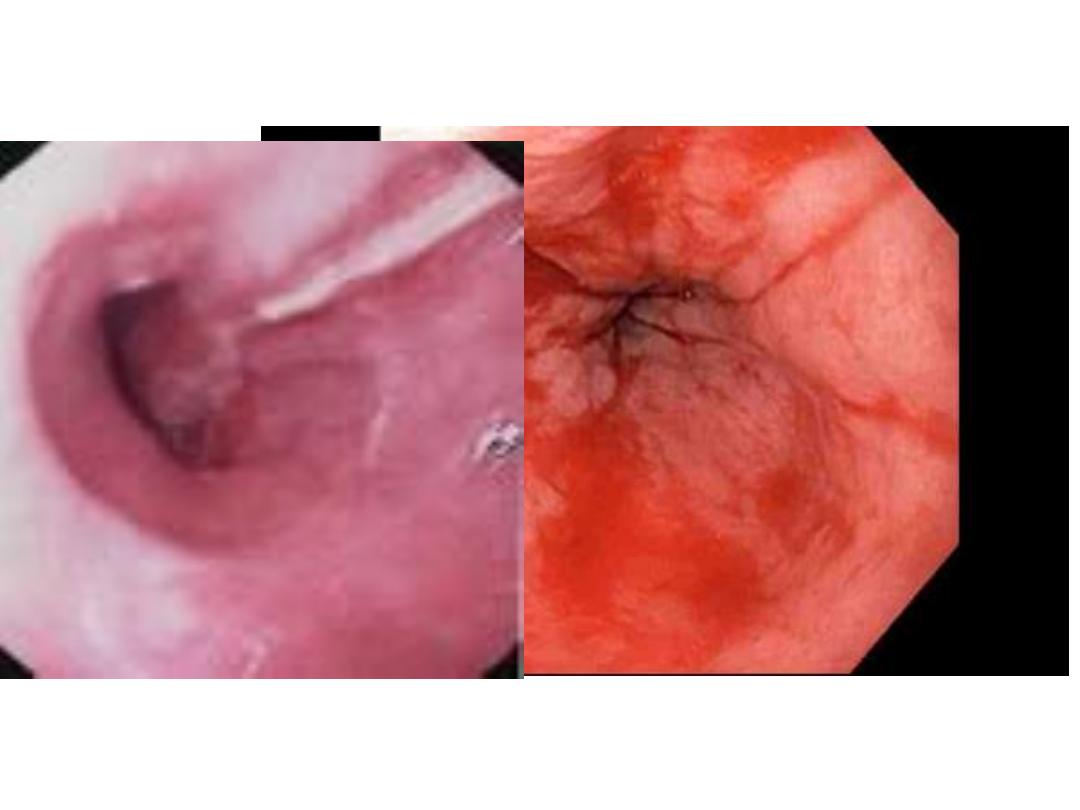
HEMATEMESIS

CAFFEE GROUND

MELENA STOOL
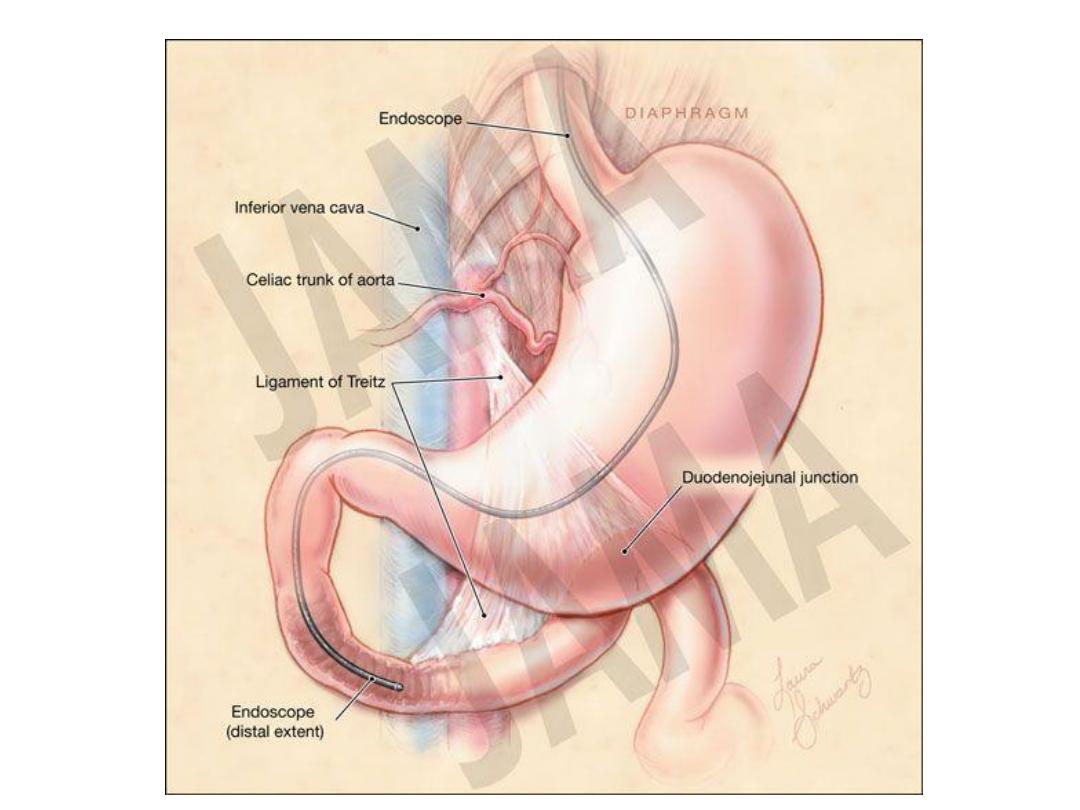
The majority of melena (black, tarry stool) originates
proximal to the ligament of Treitz (90 percent), though
it may also originate from the oropharynx, small bowel,
or right colon. Melena may be seen with variable
degrees of blood loss, being seen with as little as 50 mL
of blood

Hematochezia
Hematochezia (red or maroon blood in the
stool) is usually due to lower GI bleeding.
However, it can occur with massive upper GI
bleeding, which is typically associated with
orthostatic hypotension.

Factors that are predictive of a bleed coming from
an upper GI source included
A. A patient-reported history of melena
B. Melenic stool on examination
C. Blood or coffee grounds detected during nasogastric
lavage
D. A ratio of blood urea nitrogen to serum creatinine
greater than 30
On the other hand
, the
presence of blood clots in the
stool
made an upper GI source less likely
Factors
associated with severe bleeding included
1. red blood detected during nasogastric lavage.
2. Tachycardia, syncope, shock. presence of frankly
bloody emesis suggests moderate to severe
bleeding
3. or a hemoglobin level of less than 8 g/dL.


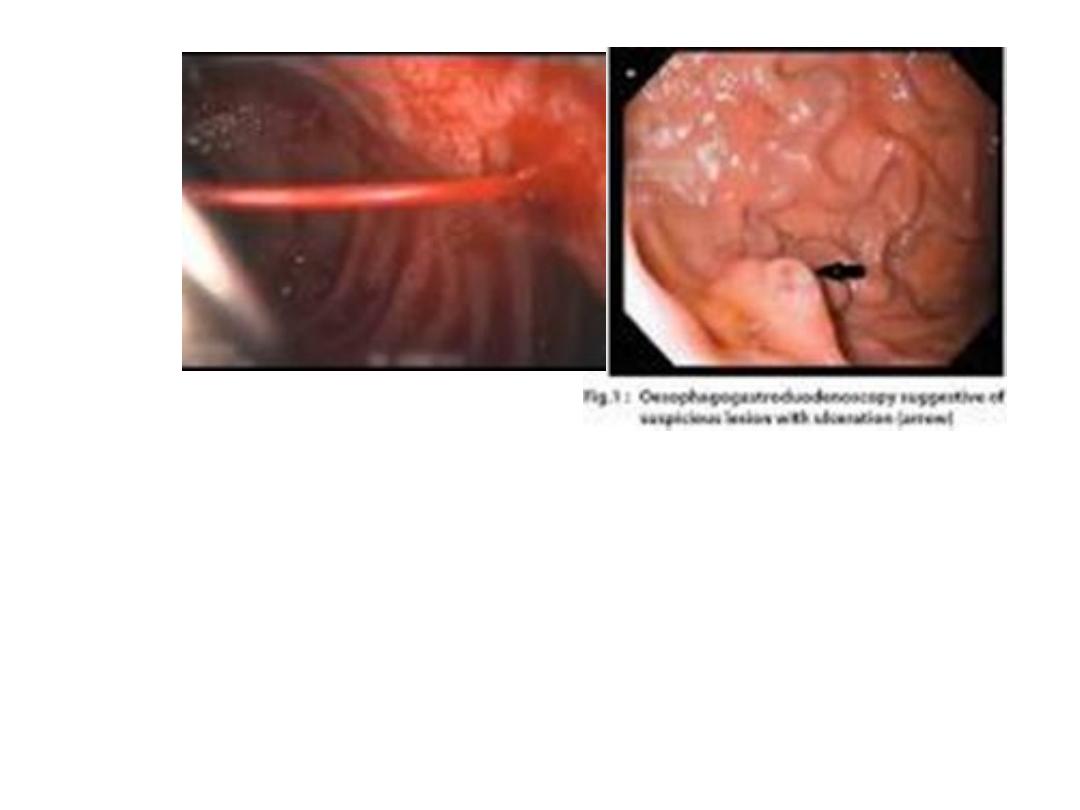

INITIAL EVALUATION
— The initial evaluation of a
patient with a suspected clinically significant acute
upper GI bleed includes a
history
,
physical
examination
,
laboratory tests
, and in some cases,
nasogastric lavage
. The goal of the evaluation is to
assess the severity of the bleed, identify potential
sources of the bleed, and determine if there are
conditions present that may affect subsequent
management. The information gathered as part of
the initial evaluation is used to guide decisions
regarding triage, resuscitation, empiric medical
therapy, and diagnostic testing.

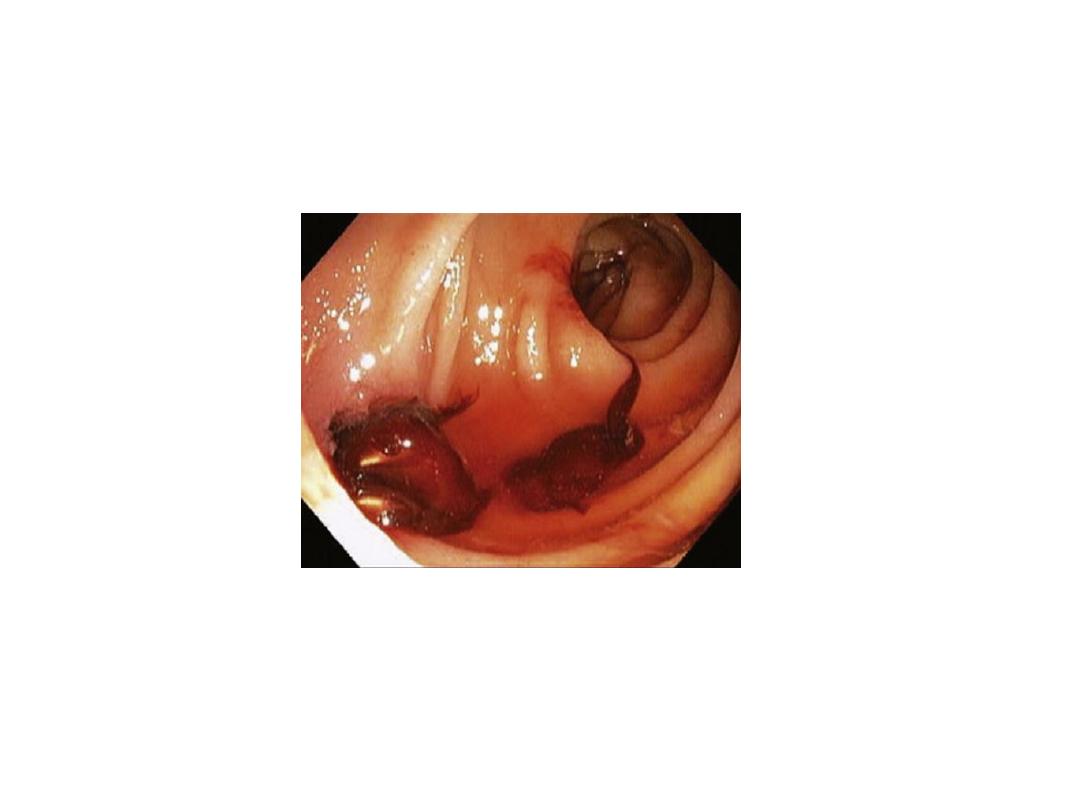
Past medical history
— Patients should be asked about prior episodes of
upper GI bleeding, since up to 60 percent of patients with a history of an
upper GI bleed are bleeding from the same lesion . In addition, the patient's
past medical history should be reviewed to identify important comorbid
conditions that may lead to upper GI bleeding or may influence the patient's
subsequent management.
Potential bleeding sources suggested by a patient's past medical history
include:
●Varices or portal hypertensive gastropathy in a patient with a history of liver
disease or alcohol abuse
●Aorto-enteric fistula in a patient with a history of an abdominal aortic
aneurysm or an aortic graft
●Angiodysplasia in a patient with renal disease, aortic stenosis, or hereditary
hemorrhagic telangiectasia
●Peptic ulcer disease in a patient with a history of
Helicobacter pylori
,
nonsteroidal anti-inflammatory drug (NSAIDs) use, or smoking
●Malignancy in a patient with a history of smoking, alcohol abuse, or
H.
pylori
infection
●Marginal ulcers (ulcers at an anastomotic site) in a patient with a
gastroenteric anastomosis

Medication history
— A thorough medication history
should be obtained, with particular attention paid to
drugs that:
●Predispose to peptic ulcer formation, such as
aspirin and other NSAIDs.
●Promote bleeding, such as antiplatelet agents (eg,
clopidogrel) and anticoagulants
●May alter the clinical presentation, such as bismuth
and iron, which can turn the stool black
.

Symptom assessment
— Patients should be asked about
symptoms as part of the assessment of the severity of the
bleed and as a part of the evaluation for potential bleeding
sources. Symptoms that suggest the bleeding is severe
include orthostatic dizziness, confusion, angina, severe
palpitations, and cold/clammy extremities.
Specific causes of upper GI bleeding may be suggested by
the patient's symptoms :
●Peptic ulcer: Epigastric or right upper quadrant pain
●Esophageal ulcer: Odynophagia, gastroesophageal reflux,
dysphagia
●Mallory-Weiss tear: Emesis, retching, or coughing prior to
hematemesis
●Variceal hemorrhage or portal hypertensive gastropathy:
Jaundice, weakness, fatigue, anorexia, abdominal distention
●Malignancy: Dysphagia, early satiety, involuntary weight
loss, cachexia

Physical examination
— The physical examination is
a key component of the assessment of hemodynamic
stability. Signs of hypovolemia include:
●
Mild to moderate hypovolemia
: Resting tachycardia.
●
Blood volume loss of at least 15 percent
: Orthostatic
hypotension (a decrease in the systolic blood pressure
of more than 20 mmHg and/or an increase in heart
rate of 20 beats per minute when moving from
recumbency to standing).
●
Blood volume loss of at least 40 percent:
Supine
hypotension.

Examination of the stool color may provide a clue to the
location of the bleeding, but it is not a reliable indicator.
In a series of 80 patients with severe hematochezia
(red or maroon blood in the stool), 74 percent had a
colonic lesion, 11 percent had an upper GI lesion, 9
percent had a presumed small bowel source, and no
site was identified in 6 percent . Nasogastric lavage
may be carried out if there is doubt as to whether a
bleed originates from the upper GI tract.

The presence of abdominal pain, especially if severe and
associated with rebound tenderness or involuntary
guarding, raises concern for perforation. If any signs of an
acute abdomen are present, further evaluation to exclude a
perforation is required prior to endoscopy.
Finally, as with the past medical history, the physical
examination should include a search for evidence of
significant comorbid illnesses
.

Laboratory data
— Laboratory tests that should be obtained
in patients with acute upper gastrointestinal bleeding include a
complete blood count, serum chemistries, liver tests, and
coagulation studies. In addition, serial electrocardiograms and
cardiac enzymes may be indicated in patients who are at risk
for a myocardial infarction, such as older adults, patients with a
history of coronary artery disease, or patients with symptoms
such as chest pain or dyspnea
.

The initial hemoglobin in patients with acute upper GI bleeding
will often be at the patient's baseline because the patient is
losing whole blood. With time (typically after 24 hours or more)
the hemoglobin will decline as the blood is diluted by the influx
of extravascular fluid into the vascular space and by fluid
administered during resuscitation. It should be kept in mind that
overhydration can lead to a falsely low hemoglobin value. The
initial hemoglobin level is monitored every two to eight hours,
depending upon the severity of the bleed.

Patients with acute bleeding should have
normocytic
red blood cells
. Microcytic red blood cells or iron
deficiency anemia suggest chronic bleeding. Because
blood is absorbed as it passes through the small bowel
and patients may have decreased renal perfusion,
patients with acute upper GI bleeding typically have an
elevated blood urea nitrogen (BUN)-to-creatinine or
urea-to-creatinine ratio (>20:1 or >100:1, respectively)
[The higher the ratio, the more likely the bleeding is
from an upper GI source

Nasogastric lavage
— Whether all patients with suspected
acute upper GI bleeding require nasogastric tube (NGT)
placement is controversial, in part because studies have failed
to demonstrate a benefit with regard to clinical outcomes
More often, NGT lavage is used when it is unclear if a patient
has ongoing bleeding and thus might benefit from an early
endoscopy. In addition, nasogastric tube lavage can be used to
remove particulate matter, fresh blood, and clots from the
stomach to facilitate endoscopy.
he presence of red blood or coffee ground material in the
aspirate also confirms an upper GI source of bleeding and
predicts whether the bleeding is caused by a lesion at increased
risk for ongoing or recurrent bleeding However, lavage may not
be positive if bleeding has ceased or arises beyond a closed
pylorus. The presence of nonbloody bilious fluid suggests that
the pylorus is open and that there is no active upper GI bleeding
distal to the pylorus.

We suggest that patients only undergo NGT lavage
if particulate matter, fresh blood, or clots need to be
removed from the stomach to facilitate endoscopy
.

All patients with hemodynamic instability (shock, orthostatic
hypotension) or active bleeding (manifested by
hematemesis, bright red blood per nasogastric tube, or
hematochezia) should be admitted to an intensive care unit
for resuscitation and close observation with automated
blood pressure monitoring, electrocardiogram monitoring,
and pulse oximetry.
A table outlining the emergency management of acute
severe upper gastrointestinal bleeding is provided.
Other patients can be admitted to a regular medical ward,
though we suggest that all admitted patients with the
exception of low-risk patients receive electrocardiogram
monitoring. Outpatient management may be appropriate for
some low-risk patients.

General support
— Patients should receive
supplemental oxygen by nasal cannula and should
receive nothing per mouth. Two large caliber (16 gauge
or larger) peripheral intravenous catheters or a central
venous line should be inserted and placement of a
pulmonary artery catheter should be considered in
patients with hemodynamic instability or who need close
monitoring during resuscitation
.

Elective endotracheal intubation in patients with ongoing
hematemesis or altered respiratory or mental status may
facilitate endoscopy and decrease the risk of aspiration.

Fluid resuscitation
— Adequate resuscitation and
stabilization is essential prior to endoscopy to minimize
treatment-associated complications. Patients with active
bleeding should receive intravenous fluids (eg, 500 mL of
normal saline or lactated Ringer's solution over 30 minutes)
while being typed and cross-matched for blood transfusion.
Patients at risk of fluid overload may require intensive
monitoring with a pulmonary artery catheter.
If the blood pressure fails to respond to initial resuscitation
efforts, the rate of fluid administration should be increased.

Blood transfusions
— The decision to initiate blood transfusions
must be individualized.
The approach is to initiate blood
transfusions if the hemoglobin is <7 g/dL (70 g/L) for most
patients (including those with stable coronary artery
disease), with a goal of maintaining the hemoglobin at a level
≥7 g/dL (70 g/L).
However, our goal is to maintain the
hemoglobin at a level of ≥9 g/dL (90 g/L) for patients at increased
risk of suffering adverse events in the setting of significant
anemia, such as those with unstable coronary artery disease. We
do not have an age cutoff for determining which patients should
have a goal hemoglobin of ≥9 g/dL (90 g/L), and instead base the
decision on the patient's comorbid conditions. However, patients
with active bleeding and hypovolemia may require blood
transfusion despite an apparently normal hemoglobin.

It is particularly important to avoid overtransfusion in patients
with suspected variceal bleeding, as it can precipitate worsening
of the bleeding. Transfusing patients with suspected variceal
bleeding to a hemoglobin >10 g/dL (100 g/L) should be avoided.
A randomized trial suggests that using a lower hemoglobin
threshold for initiating transfusion improves outcomes. In the
trial, 921 adults with acute upper GI bleeding were assigned to
either a restrictive transfusion strategy (transfusion only when
the hemoglobin fell to <7 g/dL [70 g/L]) or a liberal transfusion
strategy (transfusion when the hemoglobin fell to <9 g/dL
Patients with active bleeding and a low platelet
count (<50,000/microL) should be transfused with platelets.
Patients with a coagulopathy that is not due to cirrhosis
(prolonged prothrombin time with INR >1.5) should be transfused
with fresh frozen plasma (FFP)
.

Acid suppression
— Patients admitted to the hospital with
acute upper GI bleeding are typically treated with a proton
pump inhibitor (PPI). Some opinions suggest that patients
with acute upper GI bleeding be started empirically on an
intravenous (IV) PPI (eg,omeprazole 40 mg IV twice daily
but not H2 blockers). It can be started at presentation and
continued until confirmation of the cause of bleeding
.
But other opinions regard the use of PPI only if nonvariceal bleeding is
confirmed endoscopically.
So both opinions are correct
PPIs may also promote hemostasis in patients with
lesions other than ulcers. This likely occurs because
neutralization of gastric acid leads to the stabilization of
blood clots

Prokinetics
—
Both
have been
studied in patients with acute upper GI bleeding. The
goal of using a prokinetic agent is to improve gastric
visualization at the time of endoscopy by clearing the
stomach of blood, clots, and food residue. We suggest
that erythromycin be considered in patients who are
likely to have a large amount of blood in their stomach,
such as those with severe bleeding. A reasonable dose is
3 mg/kg intravenously over 20 to 30 minutes, 30 to 90
minutes prior to endoscopy
.

Somatostatin and its analogs
— Somatostatin, or its
analog octreotide, is used in the treatment of variceal
bleeding and may also reduce the risk of bleeding due to
nonvariceal causes . In patients with suspected variceal
bleeding, octreotide is given as an intravenous bolus of
20 to 50 mcg, followed by a continuous infusion at a rate
of 25 to 50 mcg per hour.
Octreotide is not recommended for routine use in
patients with acute nonvariceal upper GI bleeding, but it
can be used as adjunctive therapy in some cases. Its
role is generally limited to settings in which endoscopy
is unavailable or as a means to help stabilize patients
before definitive therapy can be performed
.
Antibiotics for patients with cirrhosis
— Bacterial
infections are present in up to 20 percent of patients with
cirrhosis who are hospitalized with gastrointestinal bleeding;
up to an additional 50 percent develop an infection while
hospitalized. Such patients have increased mortality.
Multiple trials evaluating the effectiveness of prophylactic
antibiotics in cirrhotic patients hospitalized for bleeding
suggest an overall reduction in infectious complications and
possibly decreased mortality. Antibiotics may also reduce the
risk of recurrent bleeding in hospitalized patients who bled
A reasonable conclusion from these
.
varices
from esophageal
data is that patients with cirrhosis who present with acute
or other causes) should be
varices
upper GI bleeding (from
given prophylactic antibiotics, preferably before endoscopy
There is no role for tranexamic acid in the treatment of upper
GI bleeding
, since the current standard of care is to treat
patients with proton pump inhibitors and endoscopic therapy
(if indicated).
Anticoagulants and antiplatelet agents
— When
possible, anticoagulants and antiplatelet agents
should be held in patients with upper GI bleeding.
However, the thrombotic risk of reversing
anticoagulation should be weighed against the risk of
continued bleeding without revers

As a general rule, we obtain surgical and interventional
radiology consultation if endoscopic therapy is unlikely to be
successful, if the patient is deemed to be at high risk for
rebleeding or complications associated with endoscopy, or if
there is concern that the patient may have an aorto-enteric
fistula. In addition, a surgeon and an interventional radiologist
should be promptly notified of all patients with severe acute
upper GI bleeding

SUMMARY OF INITIAL APPROACH
TO A PATIENT WITH AUGIB
in the next 2 slides
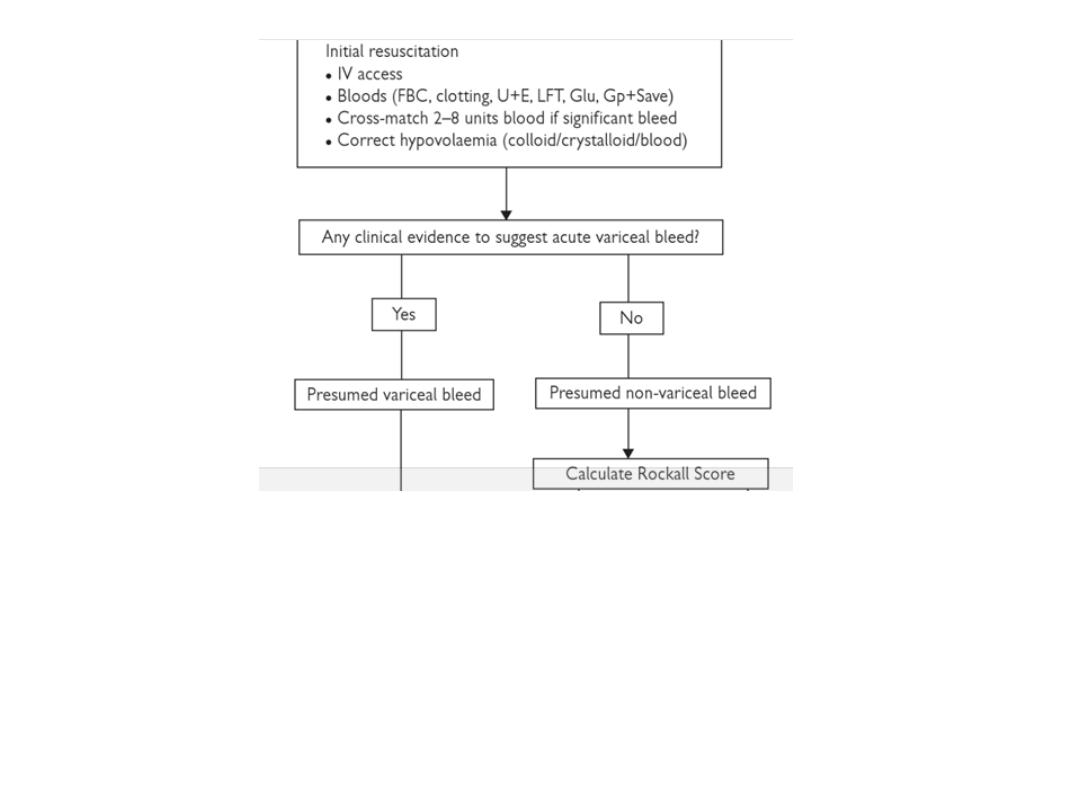
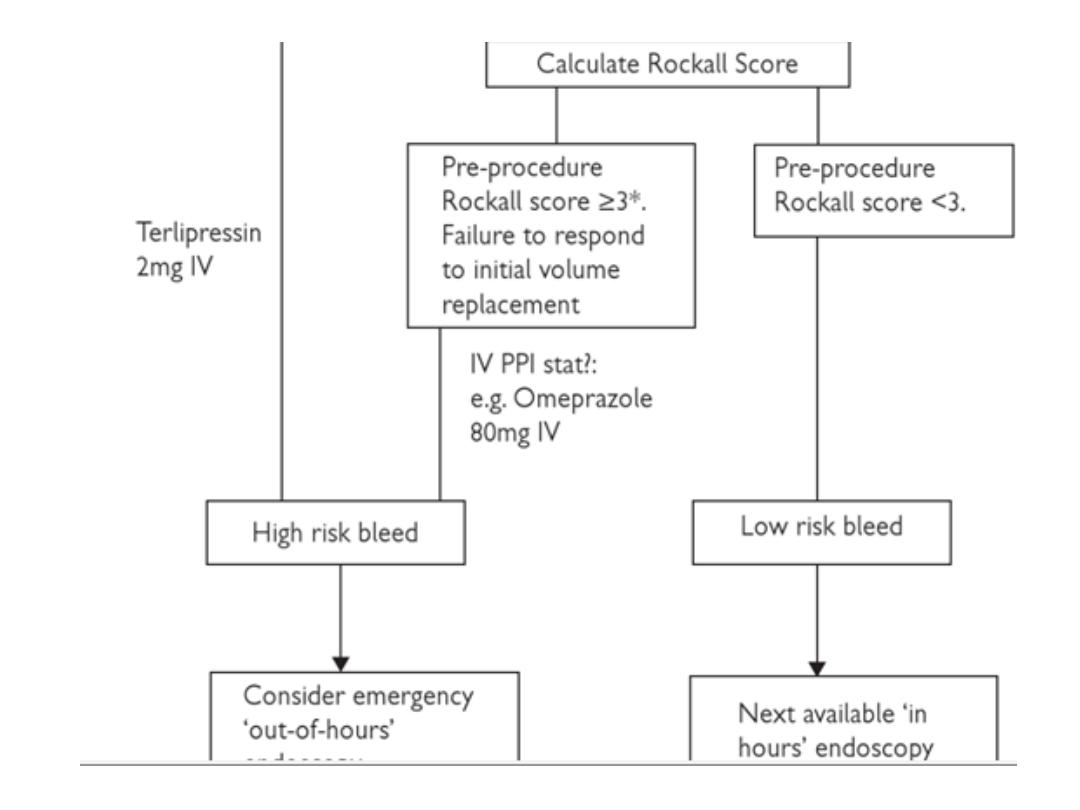

Upper endoscopy
— Upper endoscopy is the diagnostic
modality of choice for acute upper GI bleeding. Endoscopy has
a high sensitivity and specificity for locating and identifying
bleeding lesions in the upper GI tract. In addition, once a
bleeding lesion has been identified, therapeutic endoscopy can
achieve acute hemostasis and prevent recurrent bleeding in
most patients. Early endoscopy (within 24 hours) is
recommended for most patients with acute upper GI bleeding,
though whether early endoscopy affects outcomes and
resource utilization is unsettled.
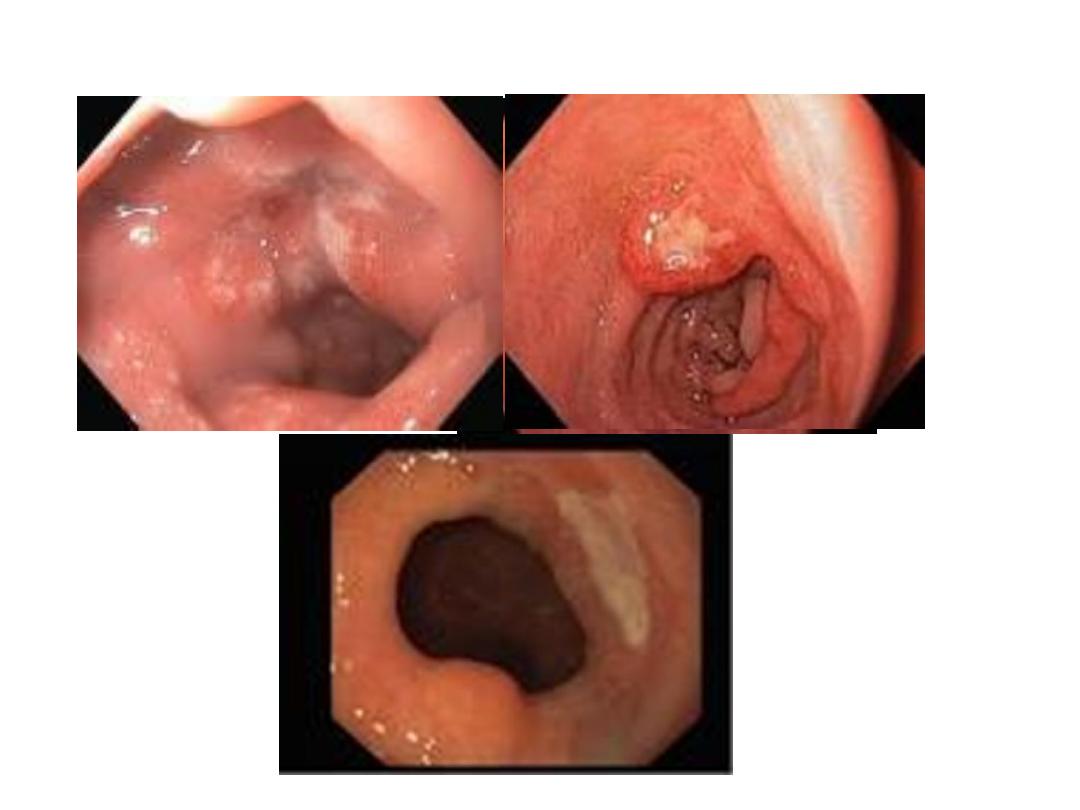
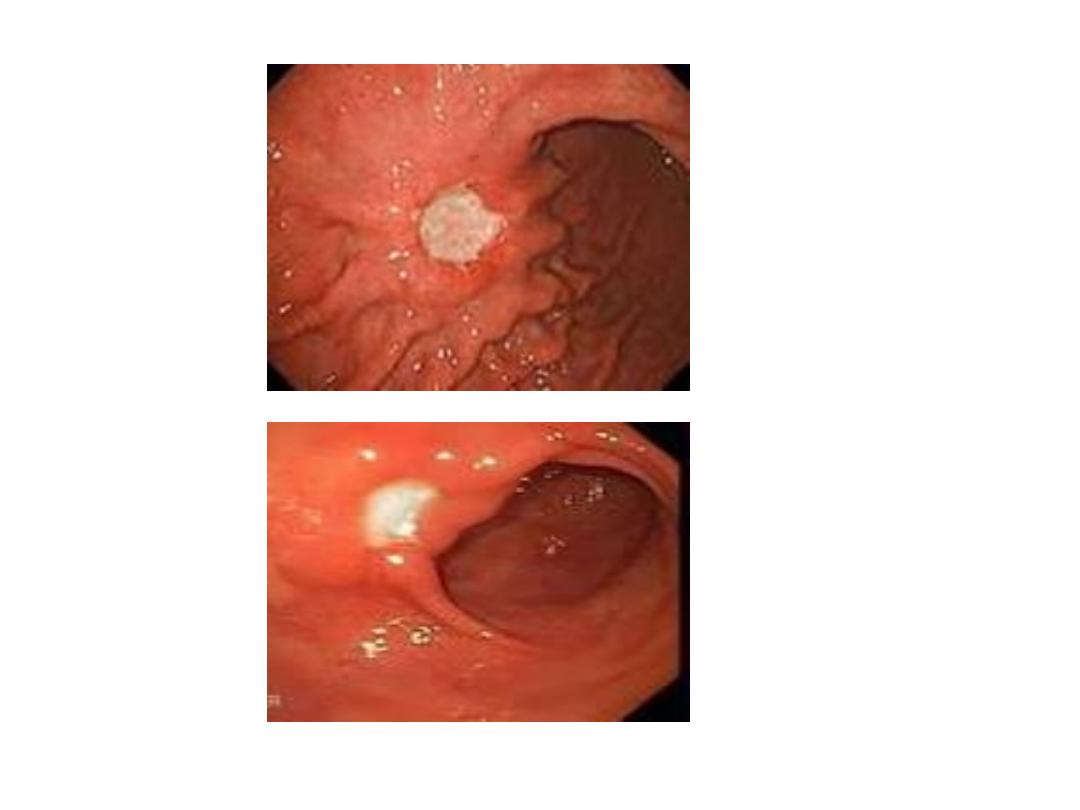
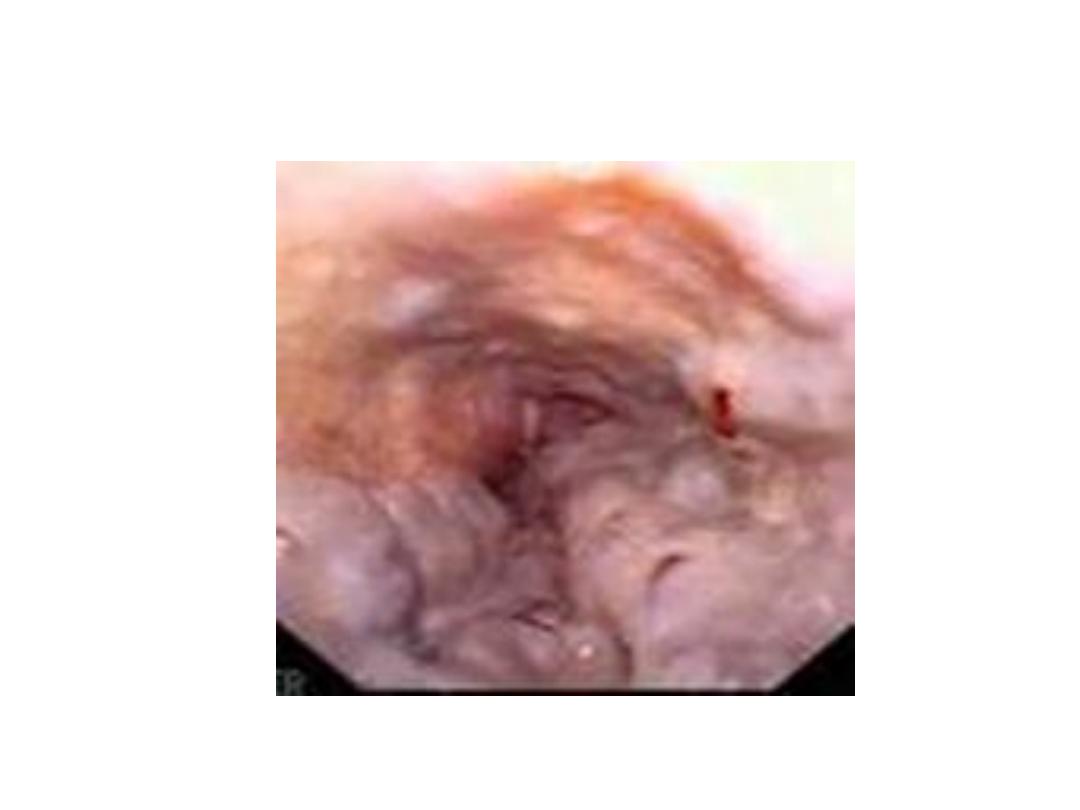
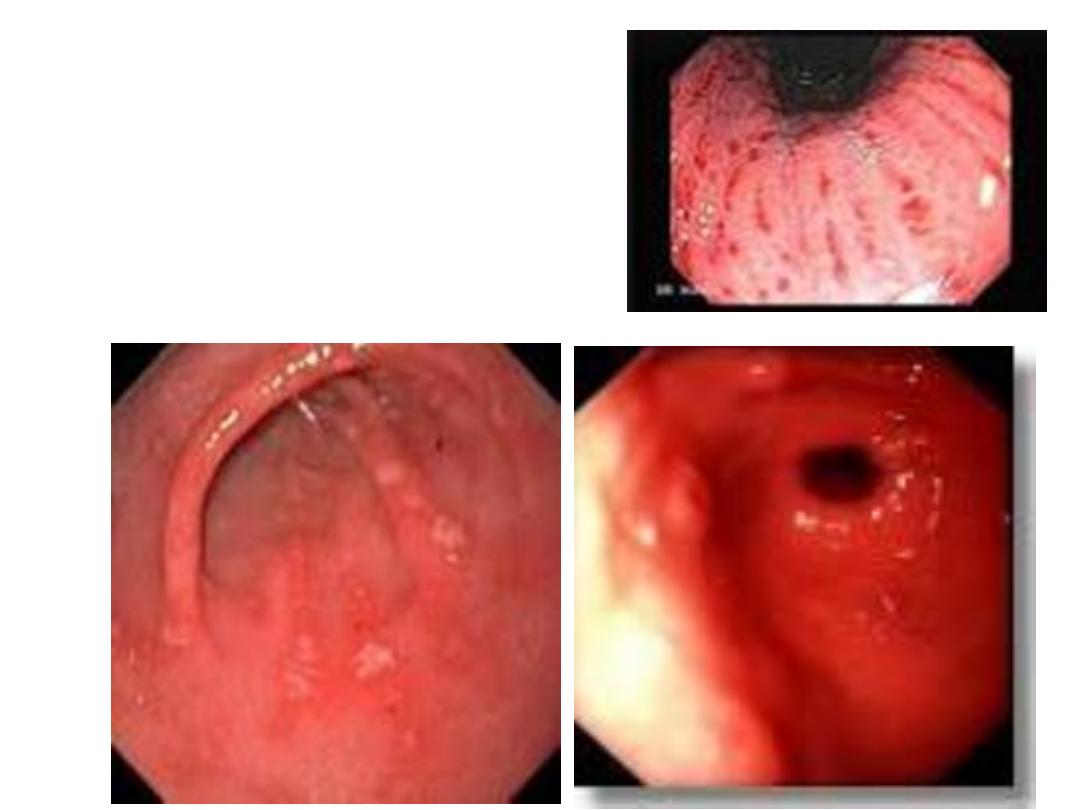
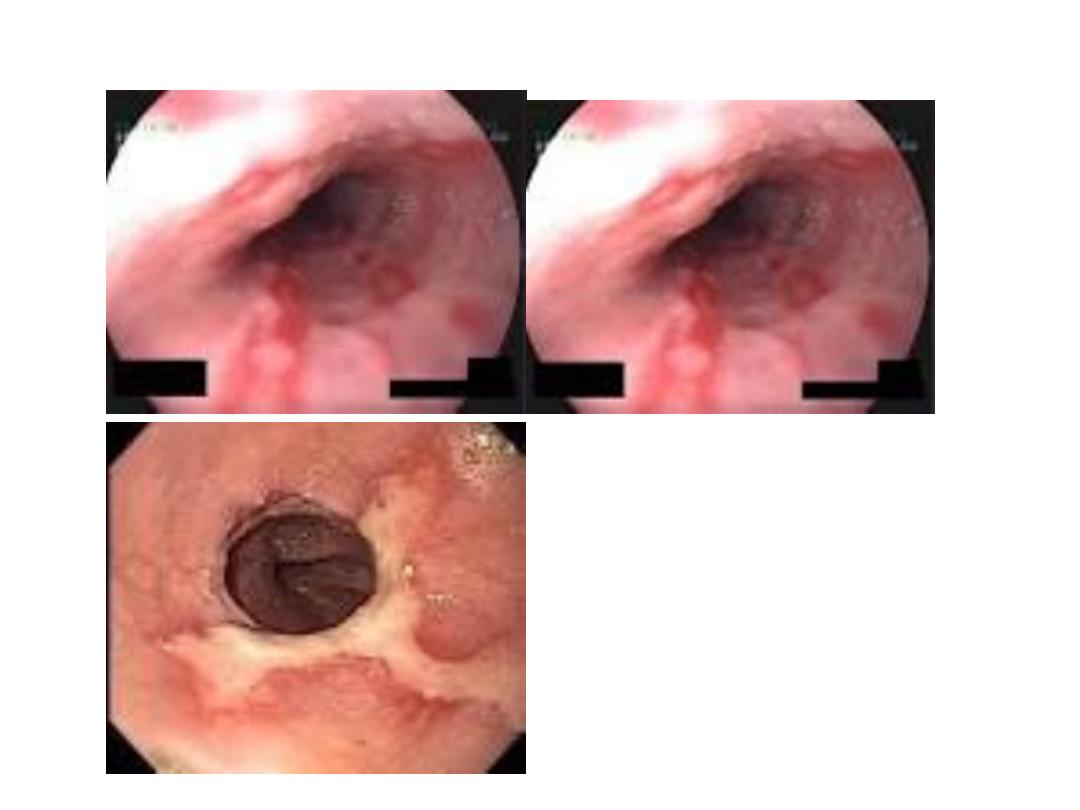
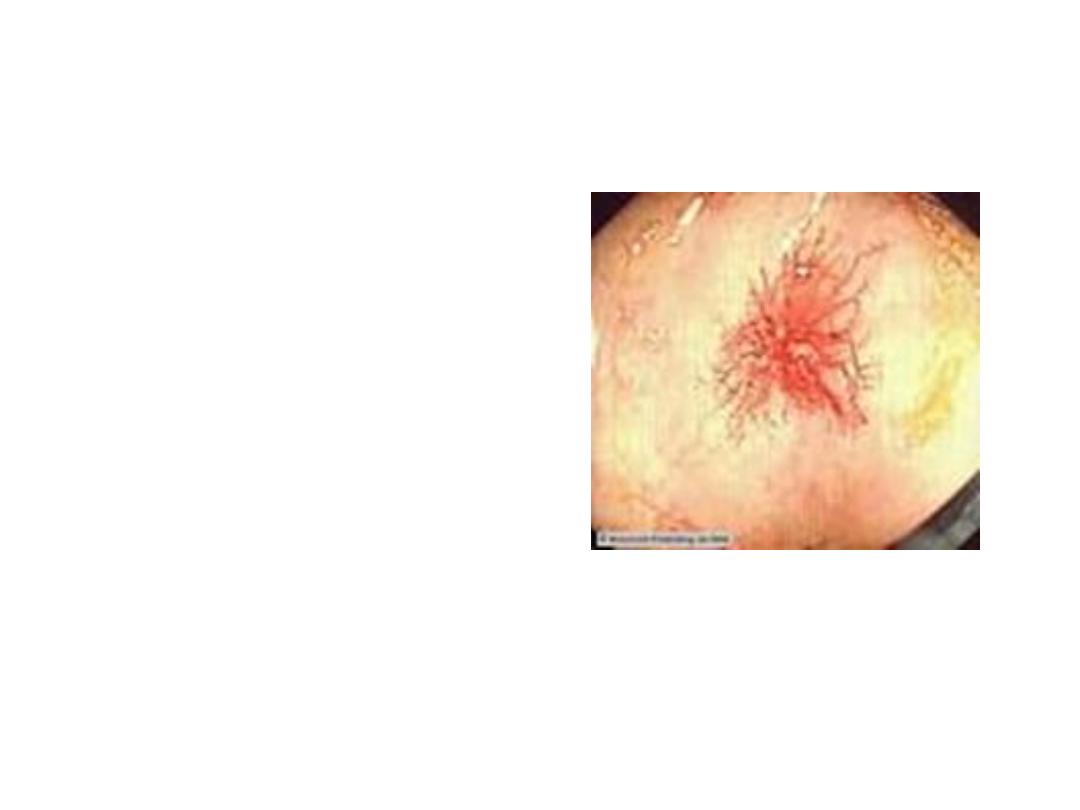
Endoscopic findings in patients with peptic ulcers may be
described using the Forrest classification . Findings include
spurting hemorrhage (class Ia) , oozing hemorrhage (class
Ib), a nonbleeding visible vessel (class IIa) , an adherent clot
(class IIb) , a flat pigmented spot (class IIc), and a clean ulcer
base (class III). The endoscopic appearance helps determine
which lesions require endoscopic therapy

Endoscopy
Offer endoscopy to
unstable
patients with severe acute
upper gastrointestinal bleeding immediately after
resuscitation.
Offer endoscopy within 24 hours of
admission to all other patients with upper
gastrointestinal bleeding
.
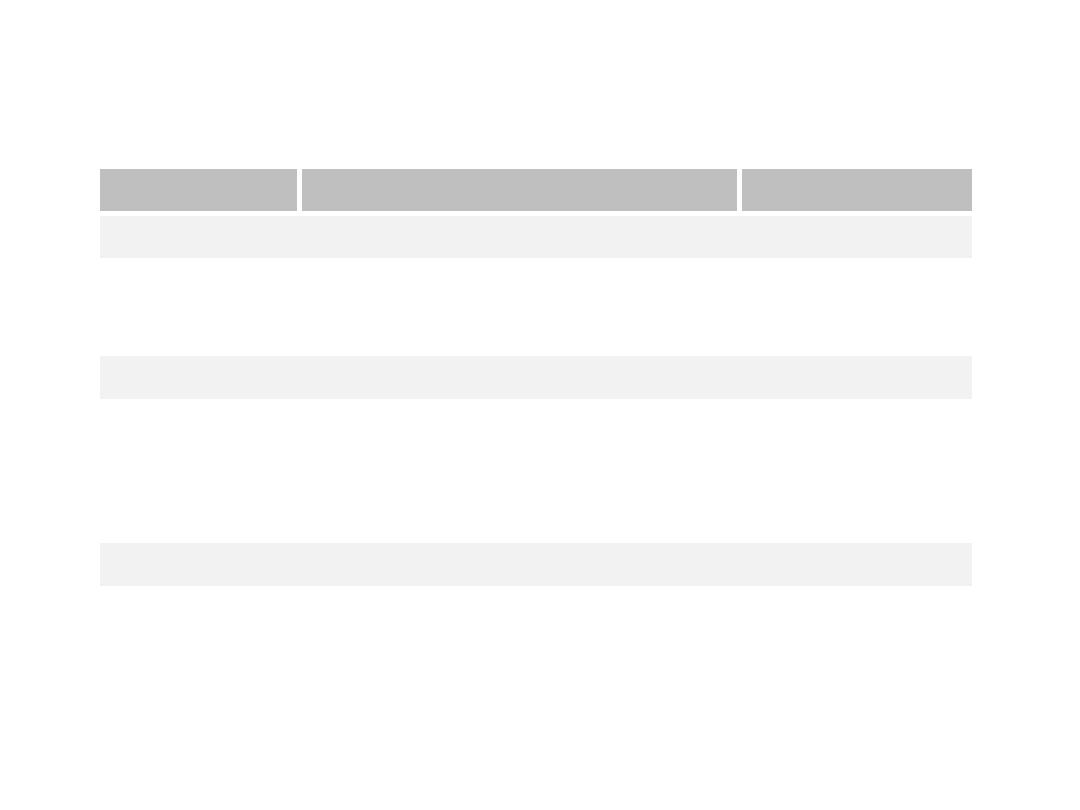
Types of lesion
Description
Rebleeding rate
Type I: Active bleeding
Ia
Spurting artery
55-100%
Ib
Oozing
Type II: Recent bleed
IIa
Non bleeding vessel
40-50%
IIb
Adherent clot
20-30%
IIc
Haemetinic spots (red or black spot)
10%
Type III: Lesions without bleeding
III
Clean base
<5%
FORREST CLASSIFICATION
مهم

Risks of endoscopy
— Risks of upper endoscopy include
aspiration, adverse reactions to conscious sedation,
perforation, and increasing bleeding while attempting
therapeutic intervention. Patients need to be hemodynamically
stable prior to undergoing endoscopy
.
Other diagnostic tests
— Other diagnostic tests for acute
upper GI bleeding include angiography, which can detect active
bleeding, deep small bowel enteroscopy, and rarely,
intraoperative enteroscopy .
Upper GI barium studies
are contraindicated in the setting of acute upper GI bleeding
because they will interfere with subsequent endoscopy
,
angiography, or surgery. There is also interest in using wireless
capsule endoscopy for patients who have presented to the
emergency department with suspected upper GI bleeding. An
esophageal capsule (which has a recording time of 20 minutes)
can be given in the emergency department and reviewed
immediately for evidence of bleeding
.
مهم جدا
Factors associated with rebleeding identified in a meta-
analysis included:
●Hemodynamic instability (systolic blood pressure less than
100 mmHg, heart rate greater than 100 beats per minute)
●Hemoglobin less than 10 g/L
●Active bleeding at the time of endoscopy
●Large ulcer size (greater than 1 to 3 cm in various studies)
●Ulcer location (posterior duodenal bulb or high lesser gastric
curvature).

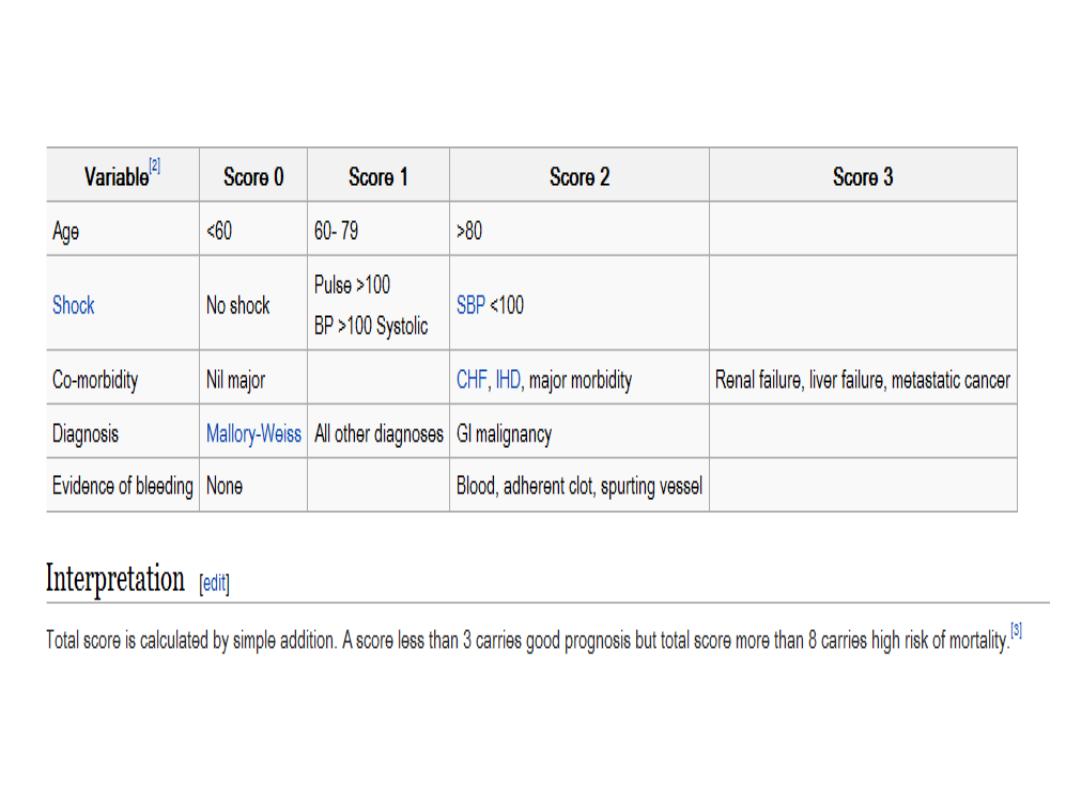
Risk scores

Many
risk factors
are known to influence the
outcome in UGIB setting.
Age.
Comorbidities.
Presence of shock.
Endoscopic diagnosis.
Haemoglobin values at the time.
Ulcers’ size.
Stigmata of recent haemorrhage.
And need for a blood transfusion have all been
described as significant risk factors for
rebleeding and death

In order to stratify the risk of complications, rebleeding,
need of clinical intervention or death, several clinical
scores are in use.
The two most important scoring system for such
assessment are :
The Glasgow Blatchford score:
(Urea, Hb, SBP,
others): is a screening tool to assess the likelihood
that a patient with an acute upper gastrointestinal
bleeding (UGIB) will need to have medical
intervention such as a blood transfusion or
endoscopic intervention.
The full Rockall score:
incorporates clinical and
endoscopic variables, has been validated to predict
mortality.Consider early discharge for patients with a
pre-endoscopy Blatchford score of 0
.
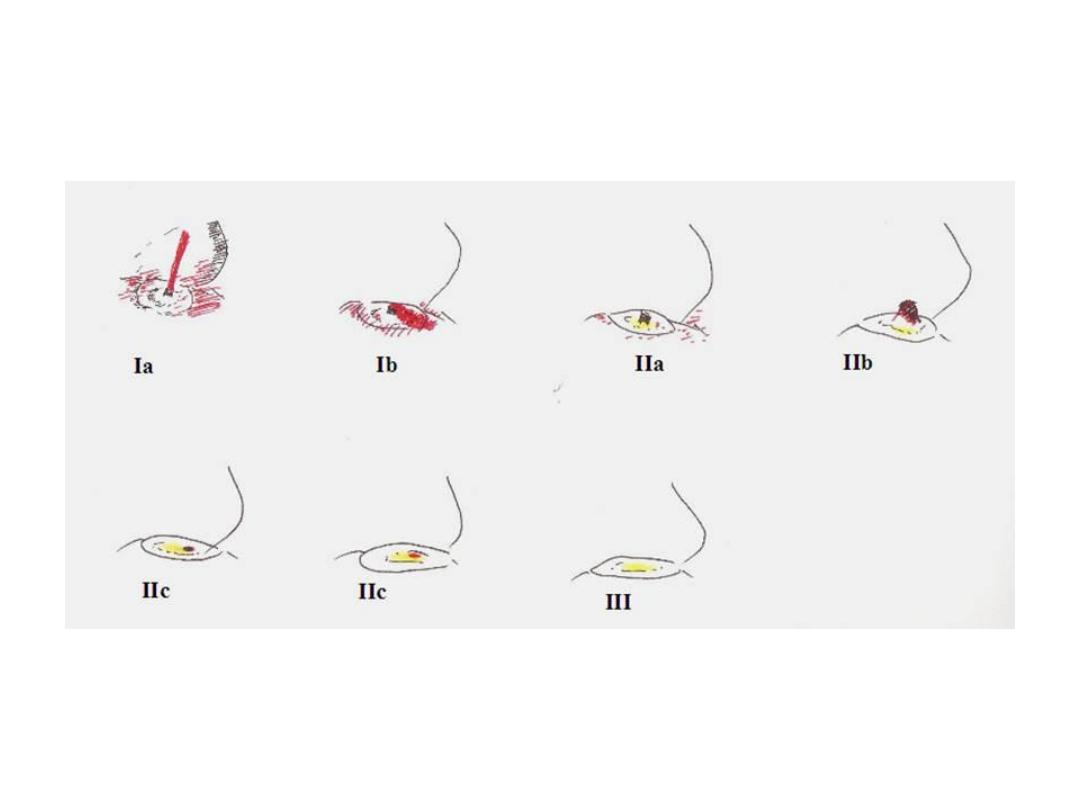
Diagrammatic representation of the
various type of Forrest lesions

Managing non-variceal bleeding
Endoscopic treatment
Do not use adrenaline as monotherapy for the
endoscopic treatment of non-variceal upper
gastrointestinal bleeding.
For the endoscopic treatment of non-variceal
upper gastrointestinal bleeding, use one of the
following:
1. a mechanical method (for example, clips) with
or without adrenaline
2. thermal coagulation with adrenaline
3. fibrin or thrombin with adrenaline.
Proton pump inhibitors
Do not offer acid-suppression drugs (proton
pump inhibitors or H2-receptor antagonists)
before endoscopy
to patients with suspected
non-variceal upper gastrointestinal bleeding.
Offer proton pump inhibitors to patients with
non-variceal upper gastrointestinal bleeding and
stigmata of recent haemorrhage shown at
endoscopy
.

Managing variceal bleeding مهم
Offer
terlipressin
to patients with suspected variceal
bleeding at presentation. Stop treatment
after definitive haemostasis has been achieved, or after 5
days, unless there is another indication for its use.
Offer
prophylactic antibiotic therapy
at presentation to
patients with suspected or confirmed variceal bleeding.

Gastric varices
1. Endoscopic injection of N-butyl-2-cyanoacrylate to
patients with upper gastrointestinal bleeding from gastric
varices.
2. TIPS if bleeding from gastric varices is not controlled by
endoscopic injection of Nbutyl-
2-cyanoacrylate
.

Oesophageal varices(NICE GUIDELINE)
1. band ligation in patients with upper gastrointestinal
bleeding from oesophageal varices.
2. Consider transjugular intrahepatic portosystemic
shunts (TIPS) if bleeding from oesophageal varices is not
controlled by band ligation
.


Other criteria used for predicating risk of upper
gastrointestinal bleeding and mortality are the
Rockall score
(utilises clinical and endoscopic
criteria) and
Glasgow Blatchford
(utilises clinical
and laboratory parameters) score. The Rockall
Score is particular useful for predicting mortality
whereas the Glasgow Blatchford score is good for
predicting early discharge or requiring for hospital
admission.
