COLORIMETRY & SPECTROPHOTOMETRY
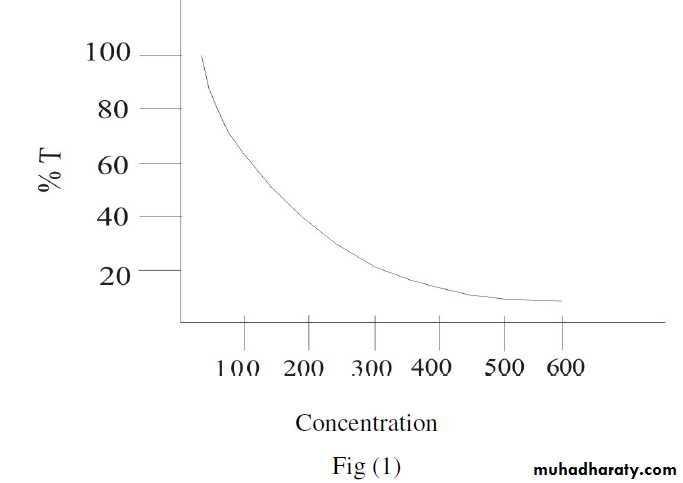
The measurement of concentration of coloured substances in solution forms the basis of colorimetric analysis, many substances of biological and medical interest are either coloured or form coloured derivatives when entered into chemical reactions.When a ray of monochromatic light of initial intensity (Io) passes through a coloured solution, some of the light is transmitted with intensity (I) and some is absorbed.
The intensity I/Io (usually expressed as percentage) is called
‘transmittance T’ of the solution. As the concentration (C) of the
compound is increased, the transmittance decreases inversely and
logarithmically, as in figure (1
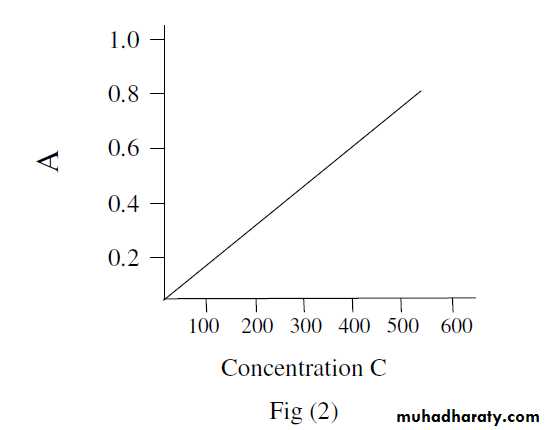
With modern photoelectric equipments , the colorimeters and spectrophotometers, another property of the coloured solution is
measured and is called absorbance ‘A’ which increases as the
concentration of the solution increases.
Transmittance and Absorbance are related to each other by the
following relationship:
A = log10 [100/T] = 2 – log10 T
Where T = percentage transmittance (%T)
A = absorbance
Beer’s Lambert law
Under suitable conditions, if a coloured solution is illuminated with monochromatic light, its absorbance (A) will be directly proportional to the concentration (C) of the coloured substance multiplied by the depth(I) of the solution in the light path thus:-
A C x I
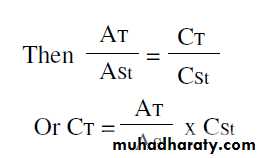
Or A = K x C x I where K is constantThis relation is known as the Beer’s-Lambert law which is used to compare the concentration of unknown test solution with a standard solution measured in the same way, then:
A test = K x C test x I
A St = K x C st x IIf at any step of the experiment, the test is treated not exactly the same as the standard (dilution, units … etc) the equation should be multiplied by a factor considering the variation.
Wavelength and Choice of Light Colour for Colorimetry
‘White’ light (coming from the sun or tungsten lamp) is a mixture of lights of various colors and wavelengths. A red solution absorbs the green component and reflects red so it appears red to the eye.Hence, for the quantitation of a colored compound in a solution, the sample has to be illuminated by a colored light supplied by the proper wavelength to get maximum absorbance of that light.
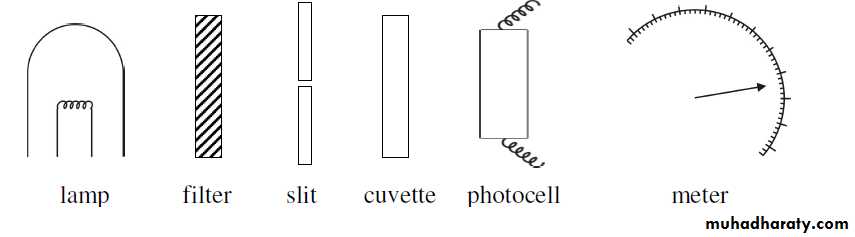
Measurement of absorbance ‘A’ (which is directly proportional to concentration ‘C’) is usually achieved using colorimeter or
spectrophotometer. A diagram of the basic arrangement of colorimeter is
given in fig. (3).
Solutions Required for Photometric Measurements
In general, it is necessary to prepare three solutions:
1- Test solution that is made from serum, plasma or blood or other specimen being analysed.
2- Standard solution that is made from a known quantity of the
substance to be measured.3- Blank solution containing all the reagents used except of the
substance to be measured. The blank solution compensates for nonspecific colour already present such as reagent colour. Its absorbance is usually deducted from that of the test and standard respectively.It is important to avoid cloudiness, turbidity or bubbles which absorb light and introduce error. The solutions should be optically clear.