
BY
Dr.Raied Alattar
Is defined as,
―The Study of
composition
and
properties
of dental materials
and the
manner in which they
interact with the environment
in
which they are placed‖.
2
Science Of Dental
Materials

Very demanding.
There can be
1.
Temperature variations.
2.
pH Variations.
3.
Variations in Masticatory forces.
3
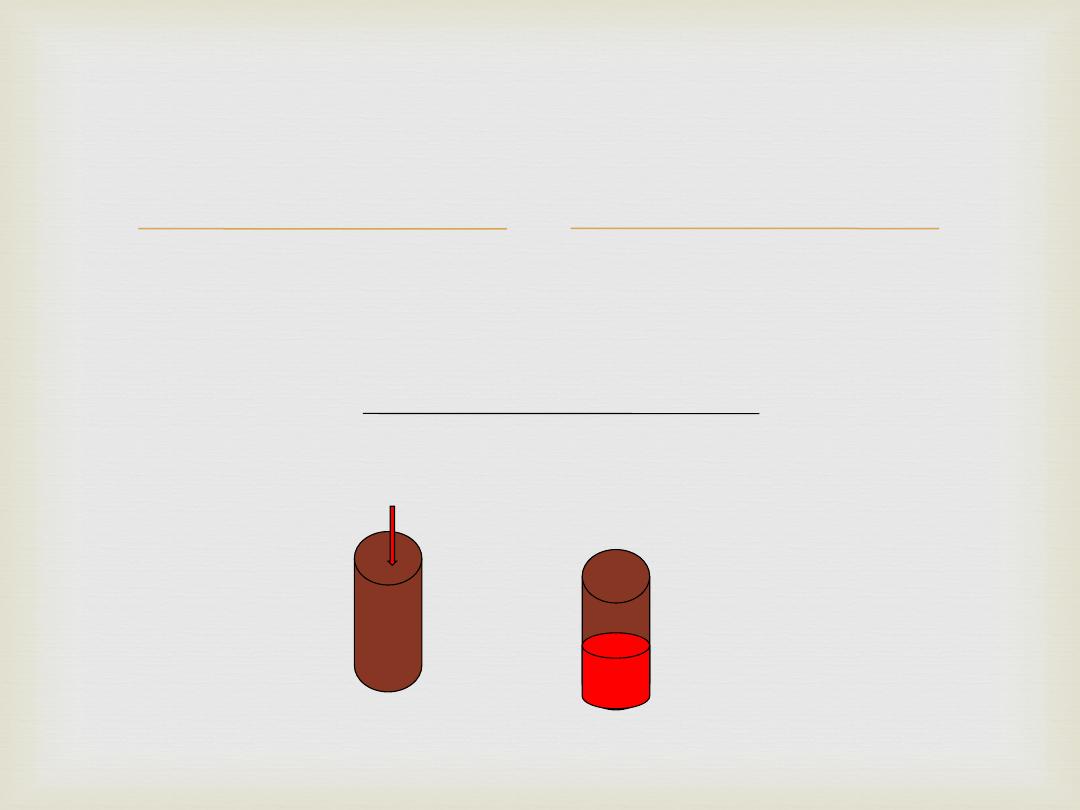
Oral Environment

Normal temperature of oral cavity (32℃ to
37℃).
On intake of a cold/hot food or drink
temperature range increases (0℃ to 70℃).
4
Temperature Variations

pH of saliva is neutral (7.0)
On intake of acidic fruit juices
or alkaline medicaments, pH
may vary from 2.0 to 11.0 .
5
pH Variations

1.
Analysis of the problem.
2.
Consideration of the
requirements.
3.
Consideration of the available
materials and their properties.
4.
Choice of a suitable material.
6
SELECTION OF DENTAL
MATERIALS

Basic and very important step.
Incorrect analysis may lead to
wrong treatment plan.
Poor prognosis and failure of
treatment.
e.g. Selection of a filling material.
7
1. Analysis of the
problem

Enlist the requirements that a
material must meet.
Requirements of a material are
dependent upon the situation.
8
2. Consideration of the
requirements

Clear concept about the properties.
In case of immediate problem,
Must choose from the materials in hand.
Dentist must be up to date with the
advancements.
Thorough comparison of Properties of the
available materials with requirements.
9
3. Consideration of the available
materials and their properties

Final step.
Narrowing the range of choice.
Factors:
1.
Availability.
2.
Ease of handling.
3.
Cost effectiveness.
10
4. Choice of a suitable
material
Biocompatible –
Non-toxic, non-irritating, non-allergenic
Mechanically stable & durable –
Strong, resistant to fracture
Resistant to Corrosion –
Does not deteriorate over time
Dimensionally Stable –
Little change by temperature & solvents
Characteristics of Ideal
Materials:
Minimal conduction –
Insulates against thermal/electrical change
Esthetic –
Looks like oral tissue
Easy to manipulate –
Minimal/reasonable effort & time needed
Adheres to tissues –
Retains onto, and seals, tooth structure
Characteristics of Ideal
Materials:
Tasteless and Odorless –
Not unpleasant to patient
Cleanable/Repairable –
Easily maintained or fixed
Cost-effective –
Affordability vs. benefits/disadvantages
Characteristics of Ideal
Materials:

Four Classes of Materials
Structure of Materials
Physical Characteristics
Mechanical Characteristics
Biologic Characteristics
Characteristics of
Materials:
METALS:
High thermal & electrical conductivity
High ductility
(bend without breakage)
High opacity
(do not transmit light)
High luster
(reflect light; appear shiny)
Crystalline arrangement of atoms
(solid)
Strong metallic bond
(high melting point - except
Mercury)
Classes of Materials:
METALS:
Strong, rigid, and stable materials
Can be ―cast‖ or formed into various shapes
Classes of Materials:
CERAMICS:
Compound of metal with non-metal
High melting points
Low thermal & electrical conductivity
Crystalline or Non-crystalline
Inert – biologically compatible
Used as fillers to reinforce composites
Esthetic - porcelains
Classes of Materials:
POLYMERS:
Man-made, long-chain, organic molecules
(carbon
atoms linked together)
Low thermal & electrical conductivity
Low strength and stability
Dental acrylics – dentures, sealants, temps
Impression materials
Adhesives – dental cements
Resin base for dental composites
Classes of Materials:
COMPOSITES:
Mixtures of two or more of the other classes (metals,
ceramics, polymers)
Example:
Dental Composite filling material
Resin matrix
(polymer)
+
glass filler
(ceramic)
Classes of Materials:
BONDS: forces holding atoms together
Primary Bonds – solids
Covalent: sharing electrons
Ionic: interaction of + and – charges
Metallic: share electrons of outer shell
Secondary Bonds – liquids
Less stable; weaker attractions
Bond liquid to liquid, or liquid to solid
Structure of Materials:
ATOMIC ARRANGEMENTS:
(when a material is
in a solid state)
Amorphous – irregular pattern of atoms;
―frozen liquids‖
i.e. glass, polymers
Crystalline – a regular pattern of columns
and rows, stacked upon each other; ―cubic‖
or other crystal patterns
i.e. metals
Structure of Materials:
THE END

The American National Standards Institute (ANSI), and
the American Dental Association (ADA), have
established more than 100 standard or specifications,
and helpful in the selection of materials for dental
practice

Thermal conductivity
Thermal conductivity: has been used as a
measure of the heat transferred and is
defined as a number of calories per second
flowing through an area of 1cm
2
In which the temperature drop along the length
of the specimen is 1
0
C/cm
Physical Characteristics:
Thermal Expansion
: temperature change causing a
material to expand or contract can create change in
dimension
Coefficient of Thermal Expansion:
the amount of dimensional change as a material
expands/contracts
Ideally, choose a restoration that expands &
contracts same as tooth.
Physical Characteristics:
Electrical & Thermal Conduction:
No need for materials to be conductive
Metals ARE good conductors
Galvanism
– a ―shock‖ created by 2 unlike metals in
contact + saliva so we need to Protect teeth from
stimulation; insulate!

Solubility and Sorption
The solubility of materials in the mouth and
sorption (adsorption plus absorption ) of oral fluids
by the material are important.) Least soluble =
porcelains & ceramics.)(Most soluble = polymers &
acrylics)
Solubility and Sorption are reported in two ways:
1.
In weight percent of soluble or sorption material.
2.
The weight of dissolved or sorption material per
unit of surface area (e.g. milligrams per square
centimetre)

Solubility and Sorption
Absorption : the uptake of liquid by the bulk solid.
E.g. the equilibrium absorption of water by acrylic
plastics is in the range of 2%.
Adsorption
:
concentration of molecules at the
surface of a solid or liquid, e.g. adsorption of
components of saliva at the surface of tooth
structure.
Physical Characteristics:
ADHESION:The force of attraction between
molecules or atoms of two different surfaces brought
into contact.
COHESION:The force of attraction between
molecules or atoms within a material.
Adhesive:
that which is being attached (“glue”)
Adherend:
the surface to which it will be attached

Surface tension
: a property of liquids in
which the exposed surface tends to contract
to the smallest possible area, as in the
spherical formation of drops; this is a
phenomenon attributed to the attractive
forces, or cohesion, between the molecules
of the liquid

Physical Characteristics:
Factors influencing ADHESION, (
adhesion):
Wettability
– the ability of the surface to become wet;
(
wettability
)
Surface Energy
– the available energy at the surface;
(
surface energy)
Surface Tension
– amount of attraction the molecules
have for one another;
(
surface tension)
Viscosity
– resistance to flow;
(
viscosity)
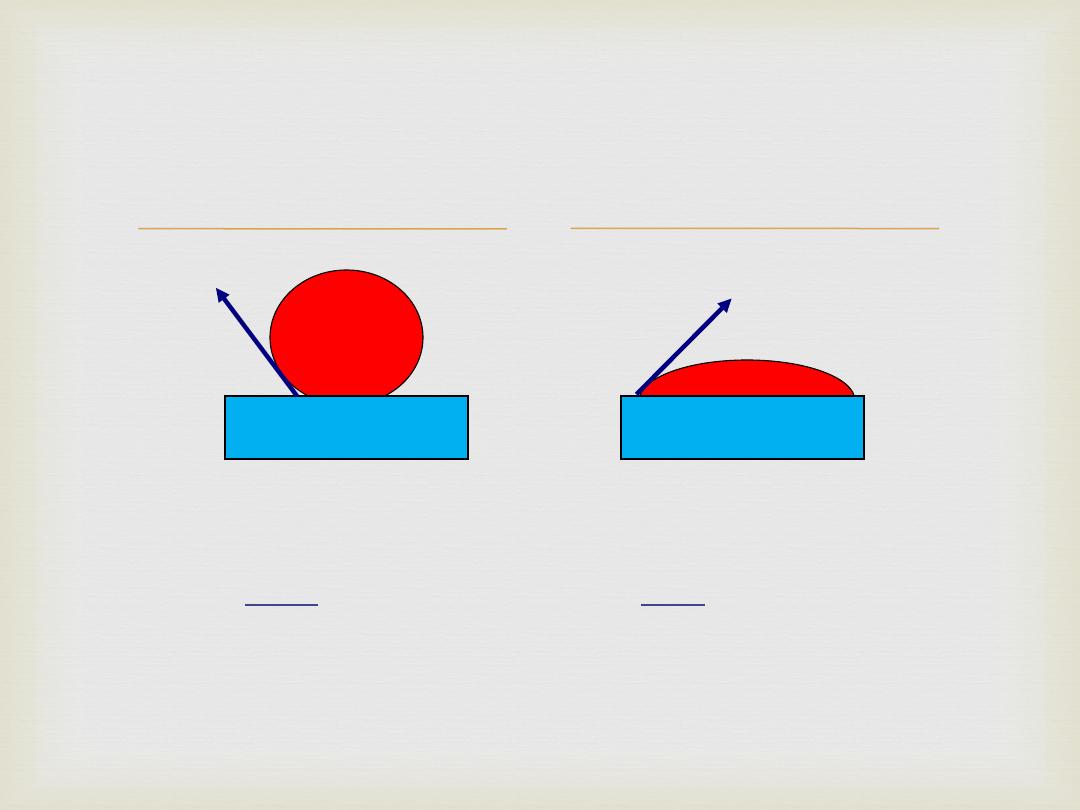
Wettability: Contact
Angle
adherend
adherend
Good Wetting:
Low contact angle;
Less than 90°
Poor Wetting:
High contact angle;
Over 90°

Rheological properties
Rheological properties: is the study of the
flow or deformation of materials (solid and
liquid).
Rheological properties of liquids and pastes
normally involve the measurement of
viscosity.
viscosity= Shear stress/shear rate.
Material of low viscosity requires only a low
pressure to produce a high flow rate
Physical Characteristics:
COLOR & ESTHETICS:
Created by light’s interaction with material
Hue
– dominant color
Value
– lightness of a color
Chroma
– intensity of a color
Translucency - teeth permit light to transmit
through them
High demand today for materials to match
natural tooth
Physical Characteristics:
CORROSION:
The deterioration of a metal by a chemical or
electrochemical reaction; irreversible
Tarnish: surface deterioration; discoloration
Methods of Corrosion:
―Battery‖ created: 2 metals + saliva =
release of metallic ions & destruction of metal

STRESS
STRESS
is the force with which a structure resists an external
load placed on it. It is the internal reaction to an
externally applied load and is equal in magnitude
but opposite in direction to the external load;
although technically the internal force, this is
difficult to measure and so the accepted way of
measuring stress is to measure the external load
applied to the cross sectional area; measured in
force per area units such as kg/cm2, MPa
(MN/m2),
Stress
= Force/Area

STRESS
Stress may be tensile sT,
compressive sC,
or shearing st,
depending on the sense of the strain produced.
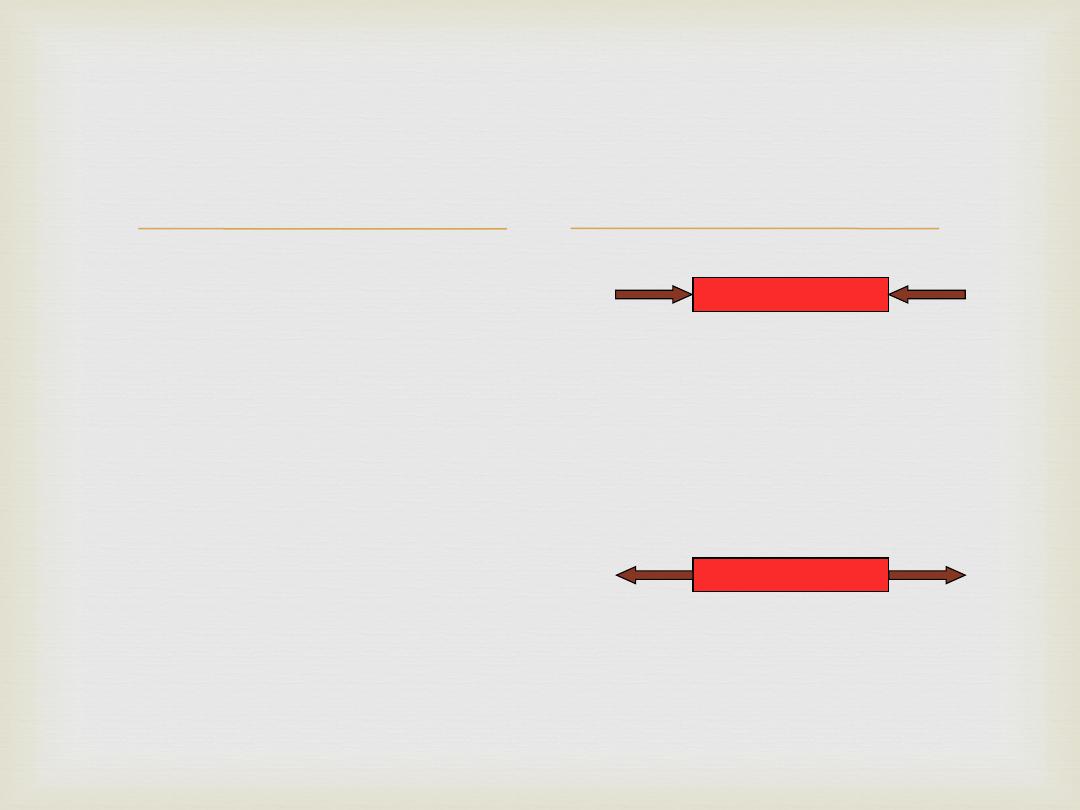
Types of stresses
Axial
Compressive
Tensile
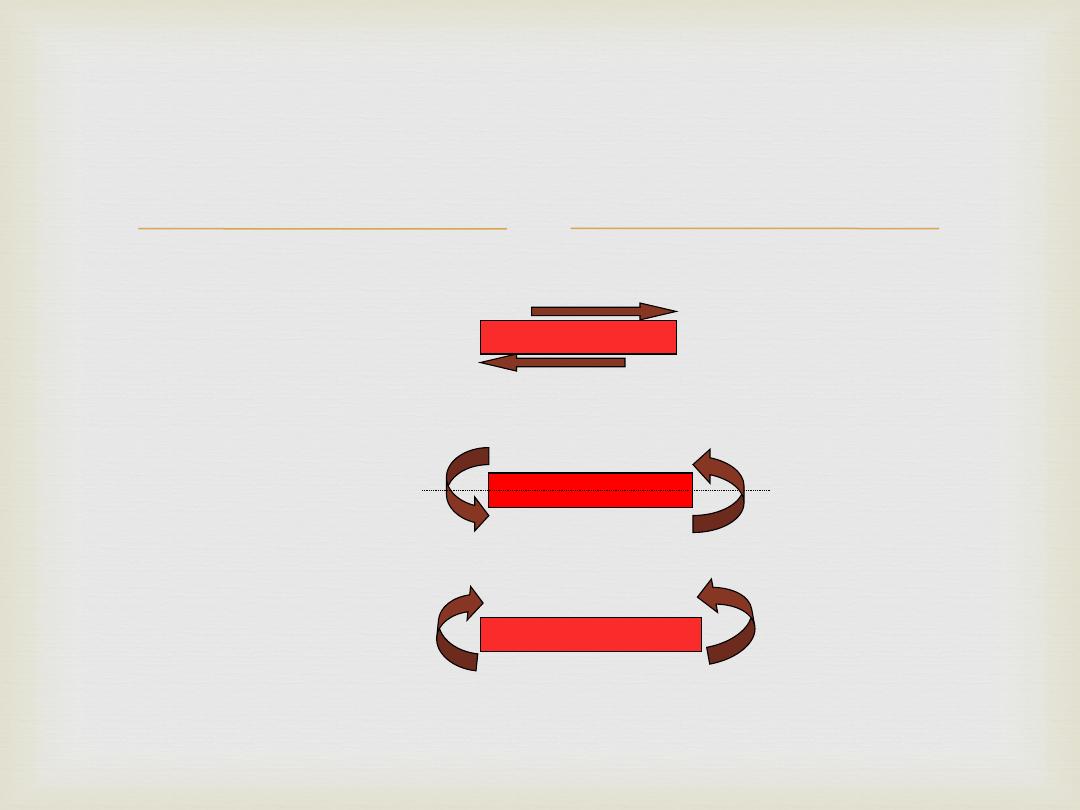
Types of stresses
Non Axial
Shear
Torsion
Bending

STRAIN
is the change in length per unit length that a material
undergoes when a force is applied to it; it is dimensionless because
it has length per length units of measurement; is often expressed as
a percentage.
Strain = Change in Length/Original Length
Strain can either be
elastic or plastic.
Elastic strain
is strain that totally disappears
once the external load that caused it is
removed. Elastic strain is based upon the
fact that a net force of zero exists between
two atoms when they are at equilibrium. If a
compressive or tensile force is exerted on
the atoms, an opposite force will attempt to
move them back to their equilibrium
position. When the applied force is released,
the atoms return to their original position;
therefore, the material is not permanently
deformed.

Strain
Plastic strain:
is strain that permanently remains once
the external load that caused it is removed. It occurs
when the force applied to the atoms moves
them so far from their equilibrium position that they do
not
return to it once the force is removed.
Change in length
Strain =
Original length
Compression

Strain may be recoverable or the material may
remain deformed (non-recoverable), or partially
recoverable. The extent to which the strain is
recovered is a function of the elastic properties of
materials.
Elastic Limit
– maximum stress level
tolerated by a material without
deformation
(a material will be deformed if any
more stress is put upon it)
Ultimate Strength
– the highest stress
tolerated before failure
(any more stress will
result in breakage of the material)
Yield strength :
the strength at which a small
amount of permanent (plastic) strain occurs,
usually 0.1% or 0.2%, and most frequently
measured in Mpa
Modulus of elasticity
: in metallurgy,
the coefficient found by dividing the unit stress, at
any point up to the proportional limit, by its
corresponding unit of elongation
(tension) or strain. A ratio of stress to strain. As the
modulus of elasticity rises, the material becomes
more rigid

Proportional Limit
- The point at which the
most metallic materials the elastic limit and
proportional limit are essentially the same.
Toughness
is the resistance to
. It is defined as the amount of
that a
material can absorb before
, and can be
found by finding the area
Toughness is measured in units of
per
3
)

Residual stress
are stresses that remain after
the original cause of the stresses has been
removed. Residual stresses occur for a
variety of reasons, including inelastic
deformations and heat treatment. Heat from
welding may cause localized expansion.
When the finished weldment cools, some
areas cool and contract more than others,
leaving residual stresses. Castings may also
have large residual stresses due to uneven
cooling.
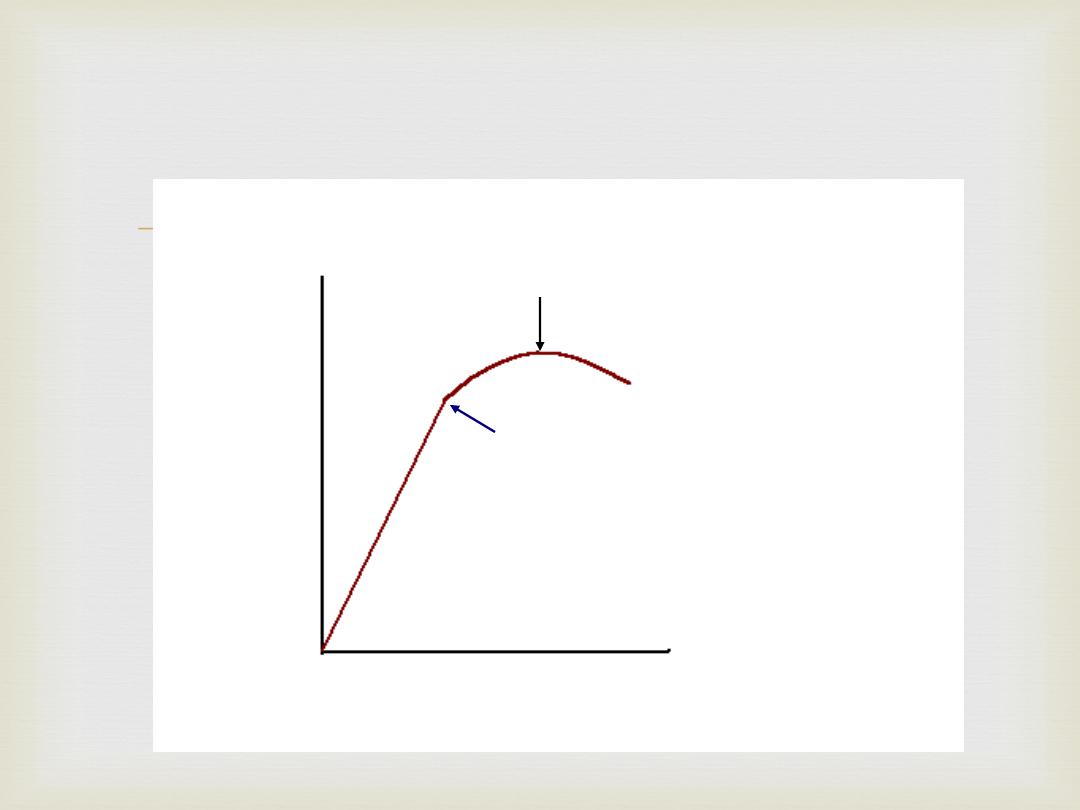
Stress-Strain Curve:
Strain
Stress
Elastic Limit
Proportional Limit
Modulus of
Elasticity
Ultimate Strength
Failure

Hardness:
an indication of the resistance to
penetration when indented by a hard
asperity. Low values of hardness number
indicate a soft material.
Common methods used for hardness test
include Vickers, Knoop, Brinell and Rockwell

Creep:
is simply defined as
1-
time-dependent plastic strain. The term
creep is usually applied to metals at
elevated temperatures, and is the low or
deformation of the material under a yield
strength.
2-
Creep: the slow change in dimensions of
an object due to prolonged exposure to high
temperature or stress

Fracture
This type of deformation is also not
reversible. A break occurs after the material
has reached the end of the elastic, and then
plastic, deformation ranges. At this point
forces accumulate until they are sufficient to
cause a fracture. All materials will eventually
fracture, if sufficient forces are applied.

DUCTILE MATERIALS
In ductile materials, such as metals, failure is
accompanied by plastic (permanent)
deformation. This
plastic deformation involves considerable
spending of energy - thus, the analysis
relating to
energy/unit volume = 0.5sTSe (Jm-3)
where sTS is the tensile strength and e the
strain at failure.

Abrasion 1
:
the wearing away of a substance or
structure (such as the skin or the teeth) through
some unusual or abnormal mechanical process
2
:
an abnormal wearing away of the tooth
substance by causes other than mastication—
attrition,erosion
Abrasive
:
a substance used for
abrading, smoothing, or polishing
Abrasive
1: tending to abrade 2: causing irritation
Abrasively
:
the property of one material to wear
away another material by means of frictional
contact

Setting expansion
: the dimensional increase
that occurs concurrent with the hardening of
various materials,
such as plaster of Paris, dental stone, die stone,
and dental casting investment
Biologic Characteristics:
The most prevalent concern:
Sealing
the interface
between restoration
and tooth!
Prevent leakage of bacteria, saliva, by-products
Prevent seepage of fluids into & out of the tooth
Dentinal tubules carry fluids, sealed by enamel
Hydrodynamic Theory – tooth pain is a result of the
fluid flow around odontoblastic processes, stimulating
nerve fibers
Temperature change can expand/contract these fluids
