.
Hemoglobin
Lec.3 Nov. 2016
Objectives
1
.
Hemoglobin
Structure
Variation in structure of HB
Reactions
Normal level
Synthesis
O2 capacity of blood
Iron Metabolism
2
.
functions of RBC
3
.
Life span and destruction of RBC
4
.
Jaundice (Icterus
:(
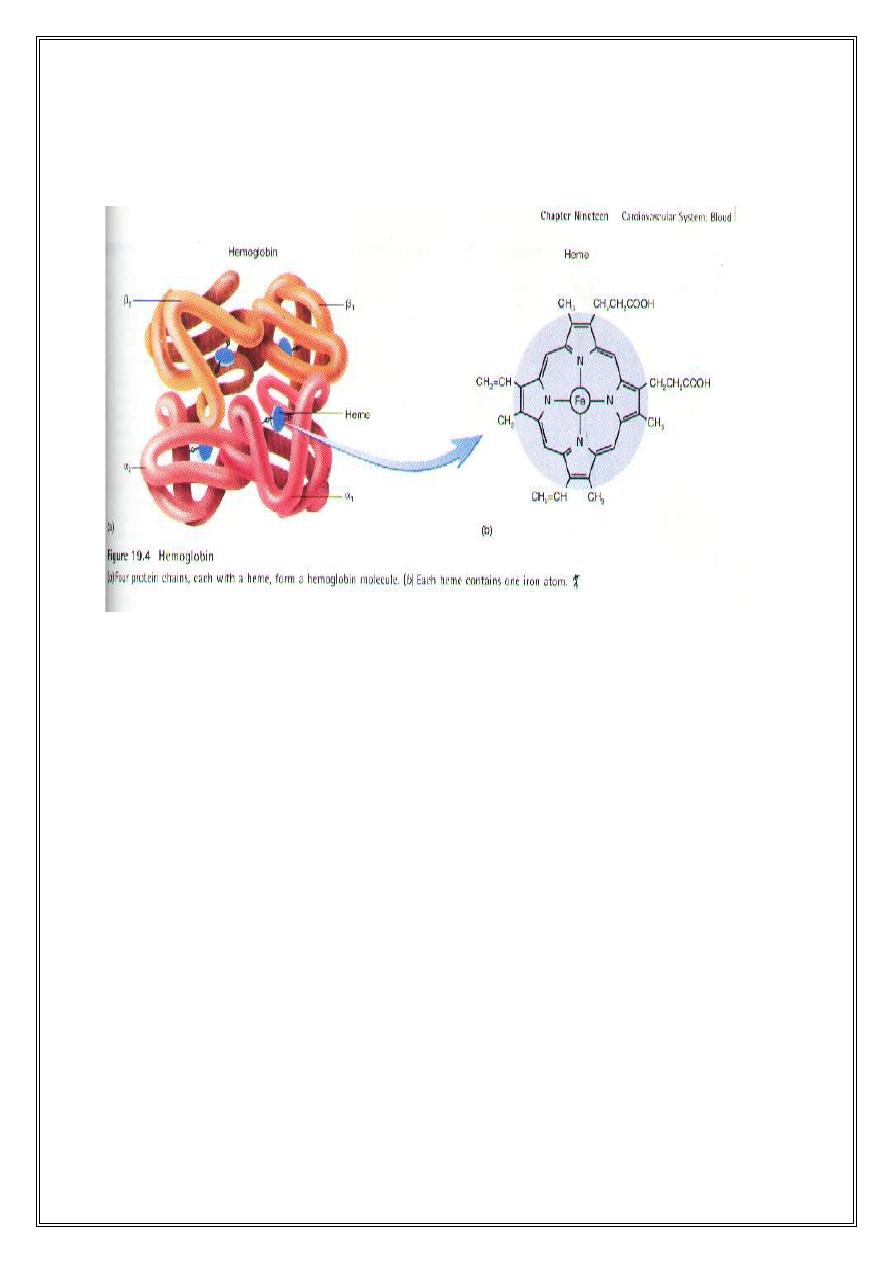
Structure of Hb
The red, oxygen-carrying pigment in the red blood cells is
hemoglobin, a protein with a molecular weight of 64,450.
Hemoglobin is a globular molecule made up of 4 subunits. Each
subunit contains a heme conjugated to a polypeptide (globin) i.e. Hb
consists of 4 protein chains and 4 heme groups. Each protein, called
globin, is bound to one heme
.
Structure of Heme:Heme is a chemical structure made up of a
porphyrin ring with an iron atom inserted in the center. Each heme is a
red-pigment molecule containing one iron atom in ferrous state (Fe
+2
).
The porphyrin ring is made up of 4 pyrrole units Synthesis of heme
takes place in the mitochondria
.
Structure of globin:The globin protein of the Hb is a protein (simple
polypeptide chains made up of amino acids). There are two Paris of
polypeptides in each Hb molecule, two of the subunits containing one
1
type of polypeptide and two containing another. Synthesis of globin
takes place in the ribosomes
.
Variation in the structure of Hb
More than 300 different variation of the Hb molecule have been
described
.
1
-
Normal variations
.
2
-
Abnormal variations
.
Normal variations: There are several slight variations in different
subunit Hb chains, depending on the amino acid composition of the
polypeptide protein. The different types of chains are designated
alpha(a-chains) chains beta (β) chains, gamma( ) chains, and delta( )
chains.4 types of normal hemoglobin
;
1
-
Embryonic Hb: this Hb occur early in gestation
:
a
-
Gower 1
.
b
-
Gower 2
.
c
-
Portland
.
2
Embryonic Hb is replaced by
:
2
-
Fetal Hb (HbF): which consist of two alpha(α )– chains and two
gamma( ) chains
.
Before birth, fetal Hb is gradually replaced by
3
-
Adult Hb (HbA): there are two types
:
1
.
HbA1: made up of two: alpha(α )– chain – two and beta(β) –
chains
.
HbA1 is the important which accounts for about 97.5% of the
totalHbA
.
2
.
HbA2: which consists of two alpha(α) – chains & two delta
–
chains.About 2.5% of adult Hb is HbA2
.
4
.
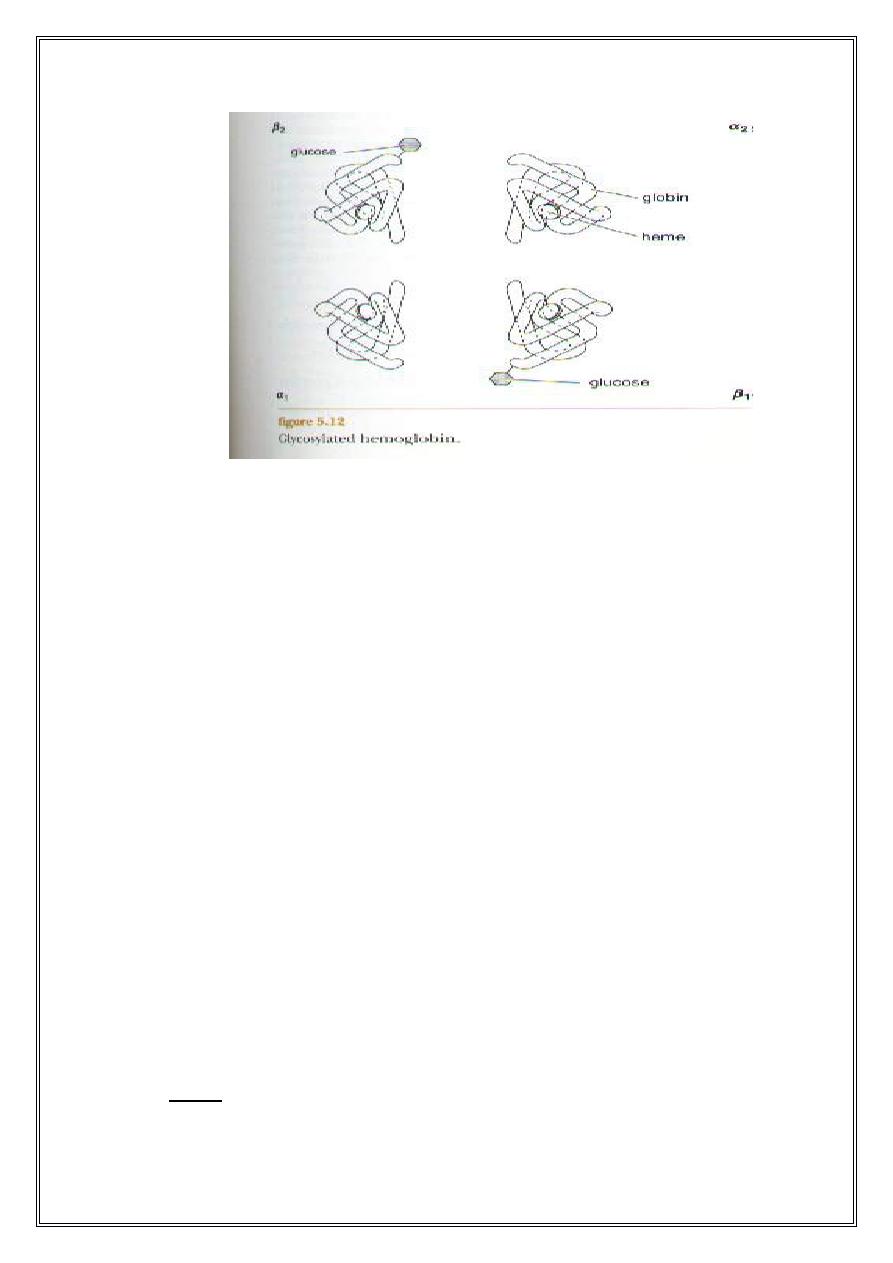
Glycosylated Hb (HbA1c): its normal consists of a minor variation
of HbA1. It differs from normal HbA1 in that if has a molecule of
glucose attached to the N-terminal valine of the β - chains. This test is
performed on patients suspected of having diabetes mellitus. The
normal level of HbA1c in adults is 5% of Hb (HbA1).In diabetics this
level is elevated
.
3
Reactions of Hb
1
-
Reaction of Hb with O2: the most important feature of the Hb
molecule is its ability to combine loosely and reversibly with O2. Hb
binds O2 to form oxyhemoglobin, O2 attaching to Fe
2+
(ferrous iron) in
the heme. There are 4 iron atoms in each Hbmolecule, each of these
can bind with one molecule of O2, making a total of 4 molecules of O2
(or 8 O2 atoms) that can be transported by each molecule. This
oxyhemoglobin form of Hb is called oxyhemoglobin
.
Hb containing no 02 is called deoxyhemoglobin
.
Hb4O6 + O2
Hb4O8
The affinity of Hb for O2 is affected by PH, temperature & the
concentration of 2, 3 diphosphoglycerate (2,3 – DPG). Embryonic
and HbF are more effective at binding O2 than in adult Hb (HbA
.(
Abnormal Hb is less effective at attracting O2 than is normal Hb and
can results in anemia
.
Reaction of Hb with CO2: CO2 is combine amino group of the
globin molecule & not with iron atom and form carbamino
hemoglobin (carbaminoHb
.(
4

2
-
CO reachs with Hb to form carboxyhemoglobin (carbon
monoxyhemoglobin). The affinity of Hb for O2 is much lower than its
affinity for CO, which consequently displaces O2 on Hb, reducing the
O2 – carrying capacity of blood e.g. incomplete combustion of
gasoline produces carbon monoxide (CO) which combine with iron
and death occur. Cigarette smoke also produces CO and the blood of
heavy smokers can contain 5% - 15% carboxyHb. Carbon monoxide is
a poisonous substance
.
3
-
When the blood is exposed to various drug and other oxidizing agents
in vitro or in vivo, the ferrous iron (Fe
2+
) in the Hb molecule is
converted to ferric iron (Fe
3+
) forming methemoglobin (metHb).
MetHb is dark – colored and when it is present in large quantities in
the circulation, it causes a dusky discoloration of the skin resembling
cyanosis
.
Level of Hb in the blood
The average normal Hb content of blood is 16g/dL in men and 14g/dL
in women
.
O2 carrying power of the blood i.e. O2 capacity of blood
:
O2 capacity of blood defined as the maximum amount of O2 that can
combine with Hb of the blood .One gram of Hb can bind with 1.34 mL
of O2. Whole blood in health adults contains 15g of Hb per dl of blood
thus
.
O2 capacity of blood = 1.34 mL O2 x gHb/vol. of blood
.
=
1.34
mL O2/gHb x 15gHb/dL blood
.
=
20.1
mL O2 combine with Hb/dl.blood
Synthesis of Hb
Synthesis of Hb begins in the proerythroblasts and continue
slightly even into the reticulocyte stage because when the
reticulocytes leaves the bone marrow and pass into the blood stream
.,
5

The heme portion of Hb molecule is synthesized inmitochondria and
globin in ribosomes
.
Iron Metabolism
:
Iron is necessary for the normal function of Hb because each O2
molecule that is transported in associated with an iron atom. Iron is
also important for formation of myoglobin, cytochromes. If blood is
lost from the body and the iron deficiency is not corrected, iron
deficiency anemia results
.
Iron metabolism include: dietary allowance; sources; absorption;
transport; storage; daily loss or excretion
.
1
.
Dietary allowances: the total iron content of the adult is
approximately 55 mg/kg body weight or about 4g in 70 kg man. About
65% of which is in the form of Hb, 4% is in the form of myoglobin,
1% is in the form of the various heme compounds, 15 – 30% is stored
in the liver cells in the form of ferritin, and 0.1% is combined blood
plasma. Recommended daily intake of iron is 10-20 mg for females,
because female lose additional iron as a result of menstrual bleeding
and therefore require more dietary iron than do males. Average adult
male needs1-1.5 mg of dietary iron each day
.
2
.
Sources:
The main sources are vegetables, cereals, fruits, molasses,
iron – fortified food and eggs. Organ meats, red meats and myoglobin
from animal muscle tissues, are essential sources of dietary heme iron
.
Milk and milk products are poor sources. In the dietary food iron is in
the form of ferric (Fe
3+
) form
.
3
.
Absorption: The primary absorption site for iron is in the duodenum
and jejunum of the small intestine. Iron can cross the intestinal cell
membrane only in the ferrous (Fe
2+
) form
.
factors that enhance iron absorption include:1.Vitamin C
.
2
.
Ascorbic acid. 3.HCl in gastric juice.( Note: Gastric secretion
dissolve the iron and permit it to form soluble complexes with ascorbic
6
acid and other substances that aid its reduction to the Fe
2+
(ferrous) form.
The importance of this function in humans is indicated by the fact that
iron deficiency anemia is troublesome and frequent complication of
partial gastroectomy). 4. Fish. 5. red meat
.
factors that inhibit iron absorption include: a.Calcium (an individual
consuming a high calcium diet could develop iron deficiency anemia
.(
Milk. c. Drugs such as tetracycline and antacid. d. Tannins in tea and
polyphenol in coffee. E.geophagia. f..Phytic acid in
cereals.g.Pancreatic secretion
.
a
.
Regulation of total body iron by controlling rate of absorption:
when the body has become saturated with iron the rate of absorption of
iron from the intestinal tract becomes greatly decreased. On the other
hand, when the iron stores have been depleted of iron, the rate of iron
can becomes accelerated to five times as great as when iron stores are
saturated e.g. iron deficiency anemia
.
4
.
Transport: When the iron is absorbed from the small intestine it
immediately combines in the blood plasma with β– globulin
transferrin. The major role of transferrin is to transport iron from
reticulendothlelial system (R-E-S) & the small intestine to the bone
marrow for fetal need; to all cells for iron – containing enzymes
.
5
.
Storage:
Iron in excess of need is stored intracellularly as
ferritin & hemosiderin. Ferritin can store about 4500 atoms of
iron per molecule. Small amount of iron are stored in theform of
hemosiderin
.
6
.
Daily loss of iron i.e. excretion of iron: Under normal conditions
the amounts excreted in the urine is small. Excretion of iron in urine
ranges from o.2 – 0.3 mg / day. About 5 – 25 mg or more is excreted in
feces / day. Iron also through the skin. In the women the mean monthly
menstrual loss of iron is about 16mg / month and the average is 0.5
mg/day, it can be higher in those with menorrhagia who may become
iron deficient
.
7
Function of red blood cells
The major function of erythrocytes (RBC’s) is to carry blood
gases. The primary functions of erythrocyte are to transport O2 from
the lungs to the various tissues of the body and also to transport
carbon dioxide (CO2) from the tissue to the lungs. These functions
are accomplished mainly by the protein hemoglobin which is ideally
suited to carry O2 and CO2 i.e. hemoglobin makes RBC’s such
efficient carrier of blood gases
.
1
.
Approximately 98.5% of the O2 transported in the blood from the
lungs to the tissue is transported in combination with Hb in the
erythrocytes
.
2
.
About 23% of CO2 is transported in combination with blood
protein (mostly Hb
.(
3
.
The erythrocytes have other functions besides transport of Hb. For
instance, they contain a large quantity of carbonic anhydrase which
catalyzes the reaction between carbon dioxide (CO2) and water,
increasing the rate of this reversible reaction several thousand fold.
The rapidity of this reaction makes it possible for the water in blood
to react with large quantities of CO2 and there by transport it from
the tissue to the lungs in the form of HCO3
-
carbonic anhydrase
(C.A) is located primarily within erythrocytes
.
CA
CO2 + H2O
H
2
CO3
H
+
+ HCO3
Carbonic Acid
4
.
Hb in the erythrocytes is an excellent acid-base buffer so that
the RBC’s are responsible for most of the buffering power of
whole blood
.
Hb
-
+ H
+
HHb
8
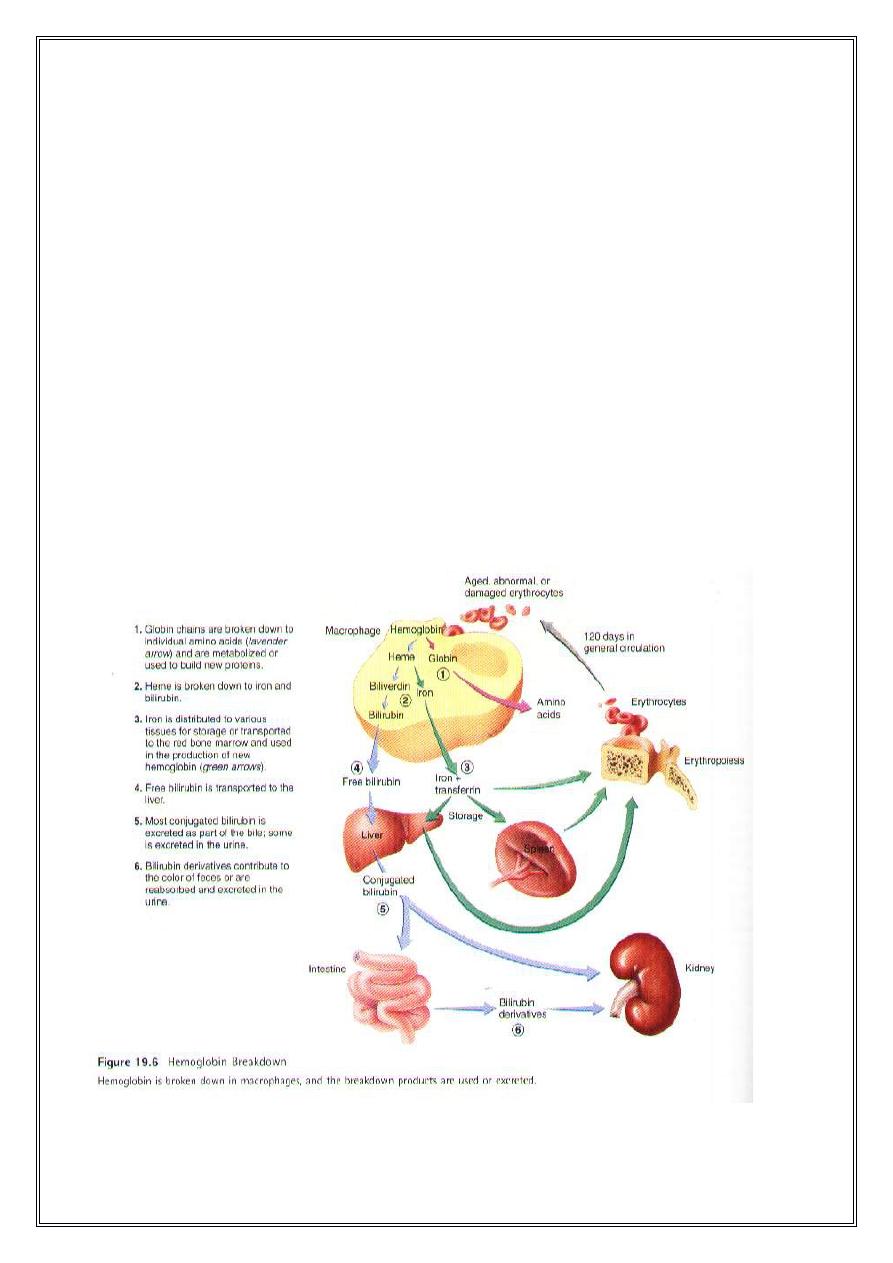
Life span and destruction of RBC :Erythrocytes normal stay in the
circulation for about 120 days in males and 110 days in females. As their
proteins, enzymes, cell membrane components, and other structures
degenerate, the erythrocytes become old and abnormal in form and
function. Erythrocytes can also be damaged in various ways while
passing through the circulation. Old damaged erythrocytes are removed
from the blood by macrophages located in the spleen, liver and other
lymphatic tissue. Within the macrophages, lysosomal enzymes break
open erythrocytes and being to digest Hb. Globin is broken down into its
components amino acids, most of which are reused in the production of
other proteins. In humans most of the biliverdin is converted to bilirubin
and excreted in the bile. The iron from the heme is reused for the
hemoglobin synthesis
.
9
Jaundice (Icterus
:(
Jaundice means a yellowish staining of the skin & sclera by bile
pigments, associated with a buildup of bilirubin in the circulation and
interstitial spaces. The normal total plasma bilirubin concentration is 0.5
mg/dL and the jaundice is usually detectable when the total plasma
bilirubin is greater than 2mg/dL (hyperbilirubinamia). Types
1
.
Hemolytic jaundice: for example sickle cell anemia; hereditary
spherocytosis; erythroblastosisfetalis
.
2
.
Obstructive jaundice: e.g. hepatitis (damage to hepatic cells
.(
Treatment of jaundice
:
Phototherapy (exposure to light) is of value in treating infants with
jaundice due to hemolysis. Exposure of the skin to white light
converts bilirubin to lumirubin, which has a shorter half-life than
bilirubin
.
BY : Ody Drd
10
