Physiology of body fluids
Daily Intake of WaterWater is added to the body by two major sources:
(1) It is ingested in the form of liquids or water in the food, which together add about 2100 ml/day to the body fluids.
(2) It is synthesized in the body as a result of oxidation of carbohydrates, adding about 200 ml/day. This provides a total water intake of about 2300 ml/day. Intake of water is highly variable depending on climate, habits, and level of physical activity.
Daily Loss of Body Water
1-Insensible Water Loss. There is a continuous loss of water by evaporation from the respiratory tract and diffusion through the skin, which together account about 700ml/day of water loss under normal conditions.
2-Sweating:The volume of sweat normally is about 100 ml/day, but in very hot weather or during heavy exercise, water loss in sweat occasionally increases to 1 to 2 L/hour.
3-Water Loss in Feces. Only a small amount of water 100ml/day) normally is lost in the feces.
4-Water Loss by the Kidneys. The remaining water loss from the body occurs in the urine excreted by the kidneys average about 1400ml/day
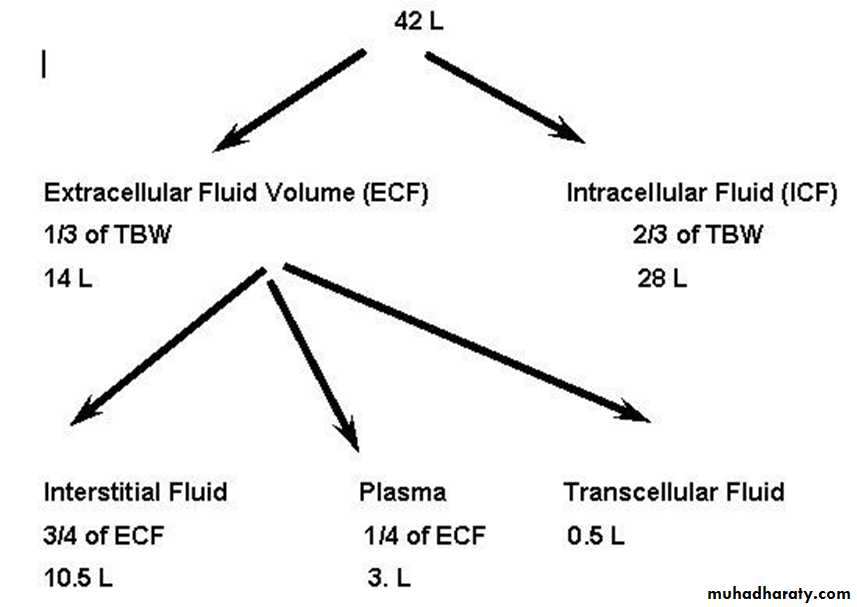
Body Fluid Compartments
The total body fluid is distributed mainly between two compartments: the extracellular fluid and the intracellular fluid. The extracellular fluid is divided into the interstitial fluid and intravascular fluid(the blood plasma) There is another small compartment of fluid that is referred to as transcellular fluid. This compartment includes fluid in the synovial, peritoneal, pericardial and intraocular spaces, as well as the cerebrospinal fluid; it is usually considered to be a specialized type of extracellular fluid. All the transcellular fluids together constitute about 1 liters.
In the average 70-kilogram adult human, the total body water is 60 % of the body weight, or about 42 liters. (This percentage can change, depending on age, gender, and degree of obesity). As a person grows older, the percentage of total body fluid gradually decreases. Women normally have more body fat than men; they contain slightly less water than men in proportion to their body weight.
Intracellular Fluid Compartment(ICF)
About 28 of the 42 liters of fluid in the body are inside the cells (40 % of the total body weight)
Extracellular Fluid Compartment(ECF)
All the fluids outside the cells these fluids account for about 14 liters (20% of the body weight). The two largest compartments of the extracellular fluid are the interstitial fluid, which makes up more than 3/4 of the extracellular fluid, and the plasma, which makes up almost 1/4 of the extracellular fluid, ( about 3 liters).
20%
20%
40%%
40%%
3L
3L
10 L
10 L
1L
1L
Blood Volume
Blood contains both extracellular fluid (the fluid in plasma) and intracellular fluid (the fluid in the red blood cells).The average blood volume of adults is about 7% of body weight, or about 5 liters. About 60% of the blood is plasma(3 liters) and 40 % is red blood cells, but these percentages can vary considerably in different people, depending on gender, weight, and other factors. 70 Kg adult man
Blood 7% of body weight
5 L
Blood 7% of body weight
5 L
2 L RBC
(40%)2 L RBC
(40%)
3L plasma
(60%)
3L plasma
(60%)
Constituents of Extracellular
extracellular fluid, including the plasma and interstitial fluidIonic Composition of Plasma and Interstitial Fluid Is Similar Because the plasma and interstitial fluid are separated only by highly permeable capillary membranes, their ionic composition is similar. The difference between these two compartments is the higher concentration of protein in the plasma; because the capillaries have a low permeability to the plasma proteins only small amounts of proteins are leaked into the interstitial spaces.
The extracellular fluid, including the plasma and the interstitial fluid, contains large amounts of sodium and chloride ions, reasonably large amounts of bicarbonate ions, but only small quantities of potassium calcium, magnesium, phosphate, and organic acid ions.
Important Constituents of the Intracellular Fluid
the intracellular fluid contains only small quantities of sodium and chloride ions and almost no calcium ions. Instead, it contains large amounts of potassium and phosphate ions plus moderate quantities of magnesium and sulfate ions. Also, cells contain large amounts of protein, almost four times as much as in the plasma
Composition of Body Fluid Compartments
Ion Plasma (mmol/L) ICF (mmol/L
Na+ 142 14
K+ 4.2 140
Ca2+ 1.3 0
Mg2+ 0.8 20
Cl- 108 4
HCO3- 24 10
Sulfate- 0.4 19
Proteinate- 1.2 4
Measurement of FluidVolumes in the Different BodyFluid Compartments
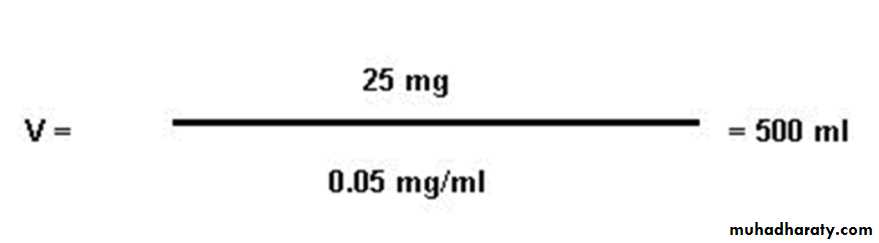
Dilution PrincipleThe volume of a fluid compartment in the body can be measured by placing an indicator substance in the compartment, allowing it to disperse evenly throughout the compartment’s fluid .Then a sample of fluid containing the dispersed substance is taken and the concentration is analyzed chemically,
Example: if 25 mg of glucose are added to an unknown volume of distilled water and the final concentration of glucose after mixing is 0.05 mg/ml, then the volume of solvent is
Indicators used for measurement must share following qualities:
1-They are measurable2-They remain in the compartment being measured
3-They are not toxic
-total body water(TBW) is measured by triated water (tritium oxide)
-ECF volume is measured by inulin that is distributed between plasma volume and interstitial volume
-Plasma volume can be measured by radioactive albumin. These substances neither leave the vascular system nor penetrate the erythrocytes
-Interstitial volume cannot be measured directly, because no substance is distributed exclusively within this compartment. interstitial fluid volume=ECF - plasma
-ICF volume cannot be measured directly by dilution because no substance is confined exclusively to this compartment.
The ICF volume=TBW - ECF volume
Relation Between Moles and Osmoles.
The total number of particles in a solution is measured in osmoles.(One osmole (osm) is equal to 1 mole (mol)of solute particles). Therefore, a solution containing 1 mole of glucose in each liter has a concentration of 1 osm/L. If a molecule dissociates into two ions (giving two particles), such as sodium chloride ionizing to give chloride and sodium ions, then a solution containing 1 mol/L will have an osmolar concentration of 2 osm/L. Likewise, a solution that contains 1 mole of a molecule that dissociates into three ions, such as sodium sulfate (Na2SO4), will contain 3 osm/L
Thus, the term osmole refers to the number of osmotically active particles in a solution rather than to the molar concentration
In general, the osmole is too large a unit so we use milliosmole (mOsm), which equals 1/1000 osmole,
Osmolality and Osmolarity.
Osmolarity – refers to the number of solute particles per liter of solution
Osmolality – refers to the number of solute particles per kg of water
In most cases, it is easier to express body fluid quantities in liters of fluid rather than in kilograms of water. Therefore,
most of the calculations used are based on osmolarities
The normal osmolality of the extracellular and intracellular fluids is about 300 milliosmoles per kilogram of water
Osmotic Pressure:
Pulling pressure because it pulls water.The higher the osmotic pressure of a solution means the higher the solute concentration of the solution.( lower water content(.The osmotic pressure of a solution is directly proportional to the osmolarity
According to van’t Hoff’s law, osmotic pressure (p) can be calculated as p = CRT
where C is the concentration of solutes in osmoles per liter, R is the ideal gas constant, and T is the absolute temperature in degrees kelvin (273° + centigrade°).p is expressed in millimeters of mercury (mm Hg)
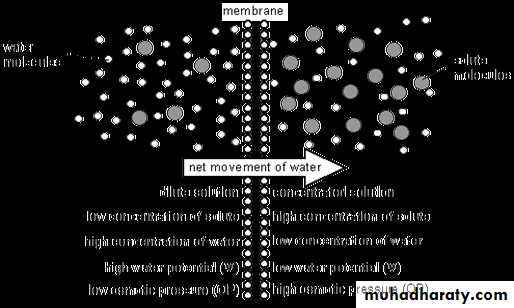
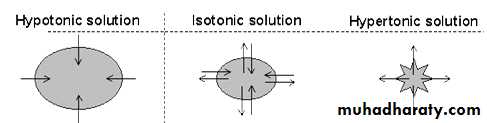
Isotonic, Hypotonic, and Hypertonic Fluids.
Tonicity of a solution is related to the effect of the solution on the volume of a cell (e.g. erythrocytes(Isotonic – it means that the solution does not change the volume of the cell, because the water concentration in the intracellular and extracellular fluids is equal .Examples of isotonic solutions include a 0.9 % solution of sodium chloride.
Hypotonic – it means that the solution causes a cell to swell. It has a lower concentration of solutes water will diffuse into the cell. E.x Solutions of sodium chloride with a concentration of less than 0.9 %
Hypertonic – it means that the solution causes a cell to shrink. This solution having a higher concentration of solutes, water will flow out of the cell into the extracellular fluid. E.X Sodium chloride solutions of greater than 0.9%
Edema:
Excess Fluid in the Tissues ,edema occurs mainly in the extracellular fluid compartment, but it can involve intracellular fluid as well.Intracellular Edema
condition cause intracellular swelling: lack of adequate nutrition to the cells, the cell membrane ionic pumps become depressed. When this occurs sodium ions can no longer be pumped out of the cells, and the excess sodium ions inside the cells cause osmosis of water into the cells. Intracellular edema can also occur in inflamed tissues.
Extracellular Edema
excess fluid accumulation in the extracellular spaces. There are two general causes of extracellular edema:
(1)abnormal leakage of fluid from the plasma to the interstitial spaces due to Increase Capillary Filtration
(2) failure of the lymphatics to return fluid from the interstitium back into the blood.
When lymphatic blockage occurs, edema can become severe because plasma proteins that leak into the interstitium have no other way to be removed.The rise in protein concentration raises the colloid osmotic pressure of the interstitial fluid, which draws even more fluid out of the capillaries
Fluids in the “Potential Spaces” of the Body
examples: pleural cavity, pericardial cavity, peritoneal cavity, and synovial cavities,all these potential spaces have surfaces that almost touch each other, with only a thin layer of fluid in between. The fluids, electrolytes, or even proteins, move back and forth between the space and the interstitial fluid.
Edema Fluid in the Potential Spaces Is Called “Effusion due to lymphatic blockage
-The general clinical terms for volume abnormalities are dehydration and overhydration. Both conditions are associated with a change in ECF volume
Dehydration: Excess loss of body water
Over hydration: excess water intake or water intoxication