Immunity
Dr. Donya A Makki Antibodies
1
Immunoglobulins (Ig)
(also called gamma globulins
or antibodies), are
glycoprotein molecules that are produced by plasma cells in response to an
immunogen. They are found in blood and body fluids of humans and other
vertebrates. They are used by the immune system to identify and destroy
foreign substances such as bacteria and viruses.
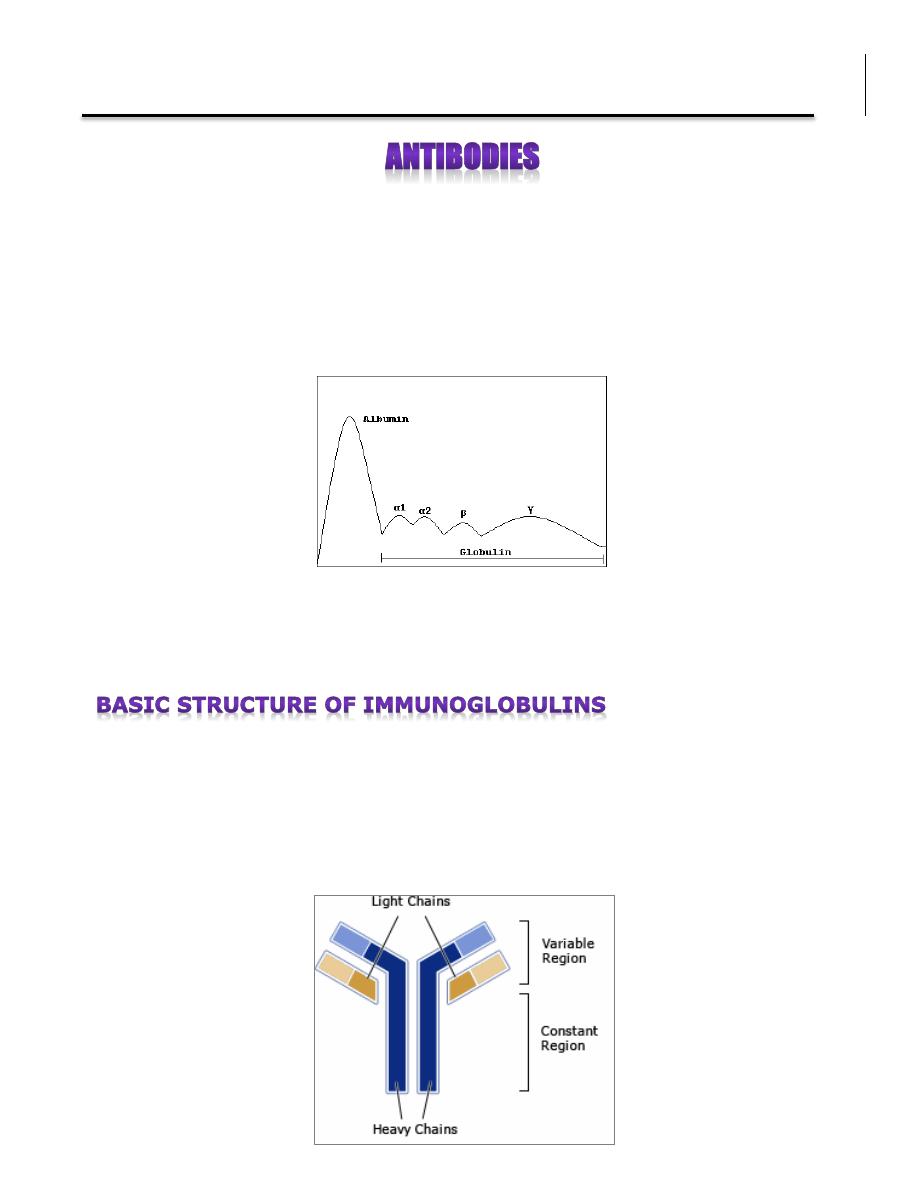
The name immunoglobulin was derived from the finding that antibodies migrate
with globulin proteins (namely Gamma globulins which contain antibodies: IgM,
IgG, and IgA) when serum is placed in an electrical field.
Each antibody consists of four polypeptides: two heavy chains and two light
chains, they are joined by disulfide bridges to form a "Y" shaped molecule. The
two arms of the “Y” contain the variable region (V) at the tips, responsible for
the recognition of the antigen (e.g. bacteria or virus). The stem can take one of
only a limited number of forms and is known as constant region (C).
Immunity
Dr. Donya A Makki Antibodies
2
The constant region determines isotype and hence the functional properties of
the antibody. In humans, there are five heavy chain (H) isotypes :
α - IgA 1, 2
δ - IgD
γ - IgG 1, 2, 3, 4
ε - IgE
μ - IgM
and two light chain isotypes (L), Kappa and lambda :
κ
λ 1,2,3,4
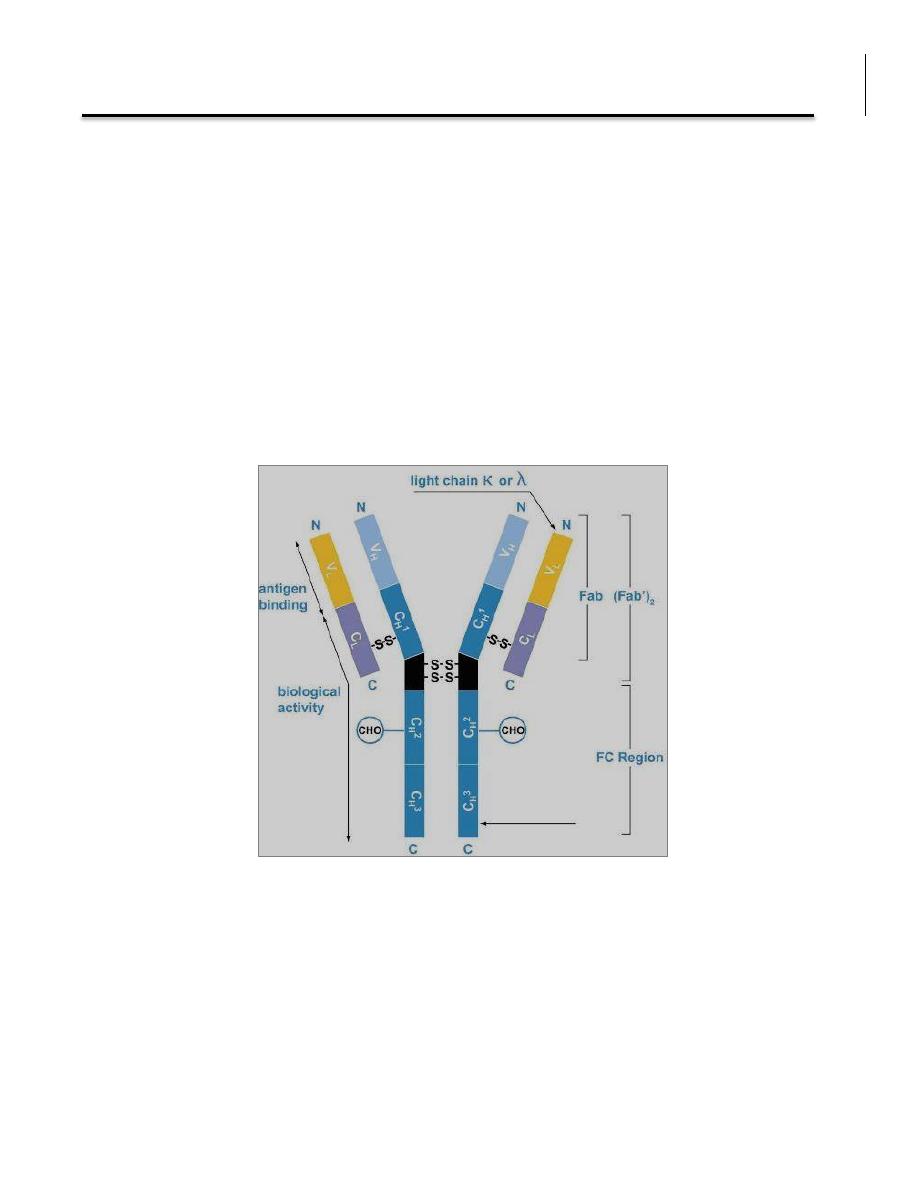
The fragment antigen-binding (Fab fragment) is the antigen binding site. It is
composed of one constant and one variable domain of each of the heavy and
the light chain. The fragment crystallizable region (Fc region) is the tail region
of an antibody that interacts with cell surface receptors called Fc receptors and
some proteins of the complement system. This property allows antibodies to
activate the immune system.

Immunity
Dr. Donya A Makki Antibodies
3
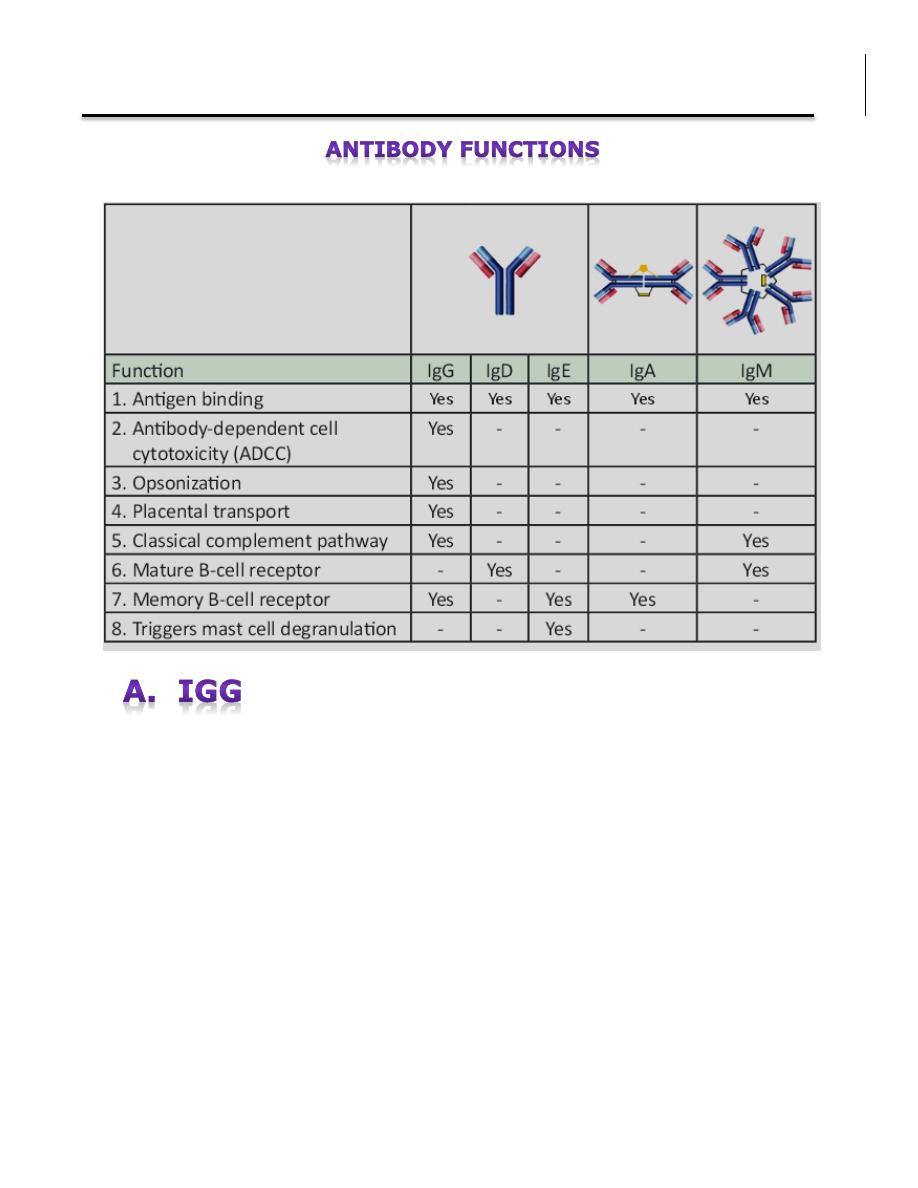
1. Antigen binding Immunoglobulins bind specifically to one or a few closely
related antigens. Antigen binding by antibodies is the primary function of
antibodies and can result in protection of the host through neutralization,
opsonization and complement activation. All types of Igs have the ability to bind
to antigens.
2. Antibody-Dependent Cell Cytotoxicity (ADCC) Only IgG has the function
of ADCC. Lymphocytes will attack and destroy a target cell that has been bound
by IgG.
3. Opsonization Another function solely of IgG where it coats particles for the
engulfment by a phagocyte.
4. Placental Transport Only one isotype, IgG, is capable of transporting across
the placenta from mother to fetus.
5. Classical Complement Pathway IgG and IgM bind to an antigen and
initiates a biochemical cascade consisting of a number of blood proteins and
leads to the killing of pathogens
6. Mature B-cell receptor IgD or an IgM monomer serve as antigen receptors
on a mature B-cell surface.
7. Memory B-cell receptor IgA, IgE, and IgG serve as antigen receptors on a
memory B-cell surface.
8. Mast cell degranulation An allergen binds to two or more IgE antibodies
on a mast cell and activates degranulation, which is the release granules and
hormonal mediators that create allergic reactions.
Immunity
Dr. Donya A Makki Antibodies
4
All IgG's are monomers. IgG is the most versatile immunoglobulin because it is
capable of carrying out all of the functions of immunoglobulin molecules.
a) IgG is the major Ig in serum - 75% of serum Ig is IgG
b) IgG is the major Ig in extra vascular spaces
c) Placental transfer. Its the only class that crosses the placenta (except IgG2).
d) Fixes complement (except IgG4)
e) Binding to cells - Macrophages, monocytes, PMNs and some lymphocytes
have Fc receptors for the Fc region of IgG (except IgG2 and IgG4) resulting in
opsonization.

Immunity
Dr. Donya A Makki Antibodies
5
IgM normally exists as a pentamer, but it can also exist as a monomer. IgM has
another protein covalently bound via a S-S bond called the J chain. This chain
functions in polymerization of the molecule into a pentamer.
a) IgM is the third most common serum Ig.
b) IgM is the first Ig to be made by the fetus and the first Ig to be made by a
virgin B cells when it is stimulated by antigen.
c) As a consequence of its pentameric structure, IgM is a good complement
fixing Ig. Thus, IgM antibodies are very efficient in leading to the lysis of
microorganisms.
d) As a consequence of its structure, IgM is also a good agglutinating Ig . Thus,
IgM antibodies are very good in clumping microorganisms for eventual
elimination from the body.
e) IgM binds to some cells via Fc receptors.
f) B cell surface Ig : Surface IgM exists as a monomer functions as a receptor
for antigen on B cells, leading to B cell activation.
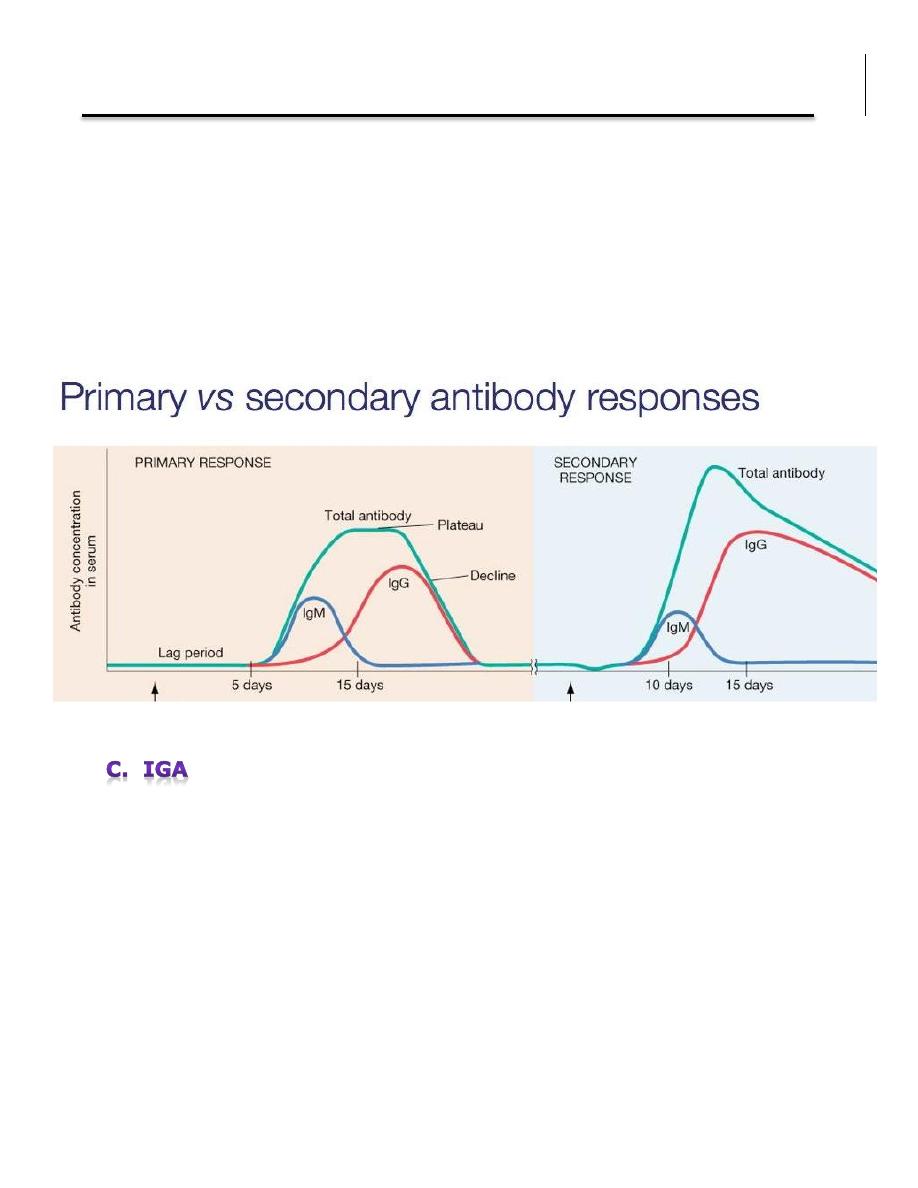
A different immune response between first contact with the antigen and the
second interaction, is summarized as follows:
1. In primary antibody response , the lag phase (the time that separates the
contact with the antigen and the appearance of specific antibodies) is
approximately 5-7 days for primary responses. It is shortened to 2-3 days
for secondary and later responses.
2. The titre of the antibody response is higher by several log factors in
secondary or later responses.
Immunity
Dr. Donya A Makki Antibodies
6
3. The primary response is dominated by IgM type antibody whereas the
seondary and later response are typically dominated by IgG type
antibodies. Thus between the primary and the secondary response a
phenomenon of isotype switch takes place.
4. The affinity (ie the strength) of the binding of the antibody to its antigen
is higher in the secondary response.
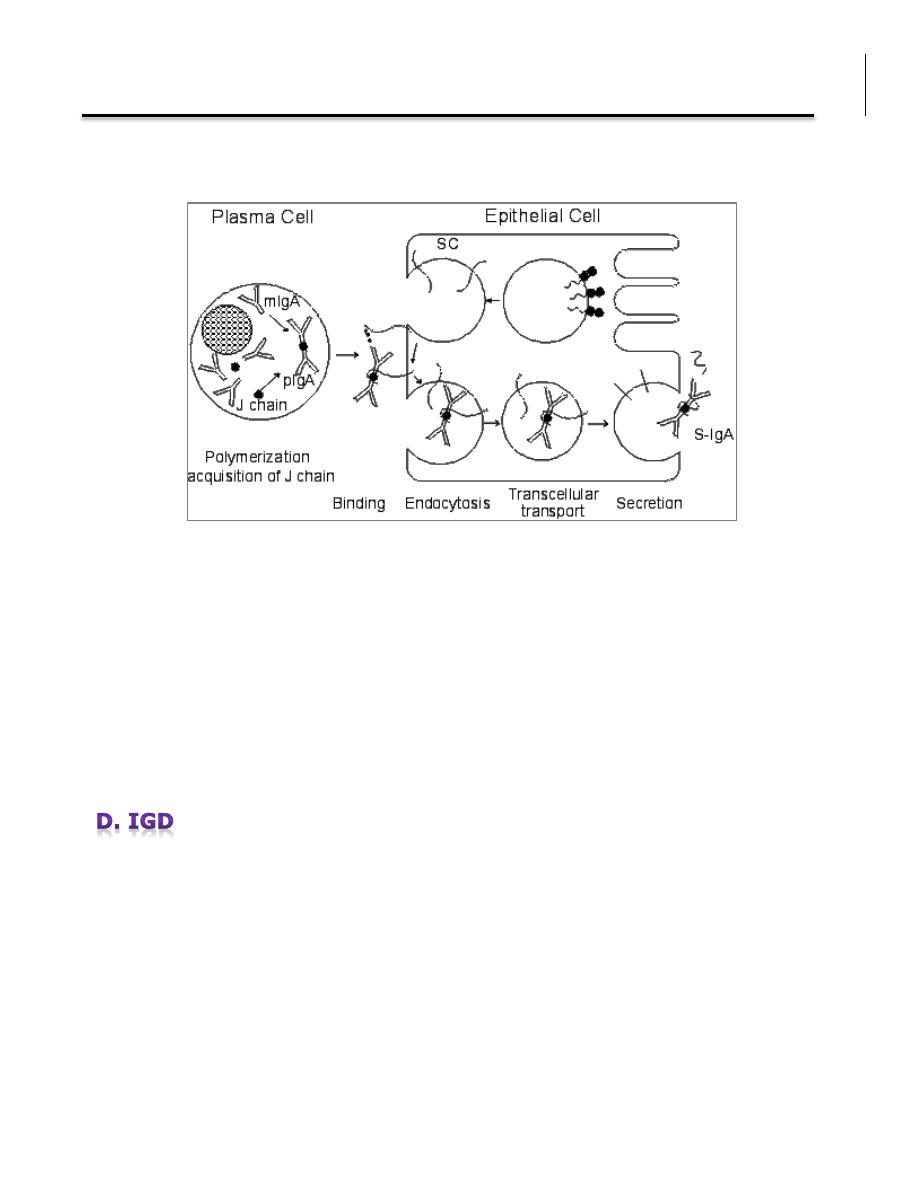
Serum IgA is a monomer but IgA found in secretions is a dimer. When IgA exits
as a dimer, a J chain is associated with it. When IgA is found in secretions is
also has another protein associated with it called the secretory piece or T piece;
sIgA is sometimes referred to as 11S immunoglobulin.
Unlike the remainder of the IgA which is made in the plasma cell, the secretory
piece is made in epithelial cells and is added to the IgA as it passes into the
secretions. The secretory piece helps IgA to be transported across mucosa and
also protects it from degradation in the secretions.
Immunity
Dr. Donya A Makki Antibodies
7
a) IgA is the 2nd most common serum Ig.
b) IgA is the major class of Ig in secretions - tears, saliva, colostrum, mucus.
Since it is found in secretions secretory IgA is important in local (mucosal)
immunity.
c) Normally IgA does not fix complement, unless aggregated.
d) IgA can bind to some cells - PMN's and some lymphocytes.
IgD exists only as a monomer.
a) IgD is found in low levels in serum; its role in serum uncertain.
b) IgD is primarily found on B cell surfaces where it functions as a receptor for
antigen.
c) IgD does not bind complement.

Immunity
Dr. Donya A Makki Antibodies
8
IgE exists as a monomer
a) IgE is the least common serum Ig since it binds very tightly to Fc receptors
on basophils and mast cells even before interacting with antigen.
b) Involved in allergic reactions - As a consequence of its binding to basophils
an mast cells, IgE is involved in allergic reactions. Binding of the allergen to the
IgE on the cells results in the release of various pharmacological mediators that
result in allergic symptoms.
c) IgE also plays a role in parasitic helminth diseases. Since serum IgE levels
rise in parasitic diseases, measuring IgE levels is helpful in diagnosing parasitic
infections. Eosinophils have Fc receptors for IgE and binding of eosinophils to
IgE-coated helminths results in killing of the parasite.
d) IgE does not fix complement.
The gamma globulin band as seen in conventional serum protein electrophoresis
consists of 5 immunoglobulins. In normal serum, about 80% is immunoglobulin
G (IgG), 15% is immunoglobulin A (IgA), 5% is immunoglobulin M (IgM), 0.2%
is immunoglobulin D (IgD), and a trace is immunoglobulin E (IgE).
Elevations of IgG, IgA, and IgM may be due to polyclonal immunoglobulin
production.
Monoclonal gammopathies of all types may lead to a spike in the gamma
globulin zone seen on serum protein electrophoresis. Monoclonal elevations of
IgG, IgA, IgD, and IgE characterize multiple myeloma. Monoclonal elevations of
IgM occur in macroglobulinemia.
Decreased immunoglobulin levels are found in patients with congenital
deficiencies.
Immunity
Dr. Donya A Makki Antibodies
9
Normal serum Ig values
IgG
1. Increases in:
a) Chronic granulomatous infections
b) Infections of all types
c) Hyperimmunization
d) Liver disease
e) Malnutrition (severe)
f) Dysproteinemia
g) Disease associated with hypersensitivity granulomas, dermatologic
disorders, and IgG myeloma
h) Rheumatoid arthritis
IgG
0-<5 months: 100-334 mg/dL
5-<9 months: 164-588 mg/dL
9-<15 months: 246-904 mg/dL
15-<24 months: 313-1,170 mg/dL
2-<4 years: 295-1,156 mg/dL
4-<7 years: 386-1,470 mg/dL
7-<10 years: 462-1,682 mg/dL
10-<13 years: 503-1,719 mg/dL
13-<16 years: 509-1,580 mg/dL
16-<18 years: 487-1,327 mg/dL
> or =18 years: 767-1,590 mg/dL
IgM
0-<5 months: 26-122 mg/dL
5-<9 months: 32-132 mg/dL
9-<15 months: 40-143 mg/dL
15-<24 months: 46-152 mg/dL
2-<4 years: 37-184 mg/dL
4-<7 years: 37-224 mg/dL
7-<10 years: 38-251 mg/dL
10-<13 years: 41-255 mg/dL
13-<16 years: 45-244 mg/dL
16-<18 years: 49-201 mg/dL
> or =18 years: 37-286 mg/dL
IgA
0-<5 months: 7-37 mg/dL
5-<9 months: 16-50 mg/dL
9-<15 months: 27-66 mg/dL
15-<24 months: 36-79 mg/dL
2-<4 years: 27-246 mg/dL
4-<7 years: 29-256 mg/dL
7-<10 years: 34-274 mg/dL
10-<13 years: 42-295 mg/dL
13-<16 years: 52-319 mg/dL
16-<18 years: 60-337 mg/dL
> or =18 years: 61-356 mg/dL

Immunity
Dr. Donya A Makki Antibodies
10
2. IgG Decreases in:
a) Agammaglobulinemia
b) Lymphoid aplasia
c) Selective IgG, IgA deficiency
d) IgA myeloma
e) Bence Jones proteinemia
f) Chronic lymphoblastic leukemia
IgM
1. Increases (in adults) in:
a) Waldenström's macroglobulinemia
b) Trypanosomiasis
c) Actinomycosis
d) Carrión's disease (bartonellosis)
e) Malaria
f) Infectious mononucleosis
g) Lupus erythematosus
h) Rheumatoid arthritis
I) Dysgammaglobulinemia (certain cases)
2. Decreases in:
a) Agammaglobulinemia
b) Lymphoproliferative disorders (certain cases)
c) Lymphoid aplasia
d) IgG and IgA myeloma
e) Dysgammaglobulinemia
f) Chronic lymphoblastic leukemia

Immunity
Dr. Donya A Makki Antibodies
11
IgA
1. Increases in:
a) Wiskott-Aldrich syndrome
b) Cirrhosis of the liver (most cases)
c) Certain stages of collagen and other autoimmune disorders such as
rheumatoid arthritis and lupus erythematosus
d) Chronic infections not based on immunologic deficiencies
e) IgA myeloma
2. Decreases in:
a) Hereditary ataxia telangiectasia
b) Immunologic deficiency states (e.g., dysgammaglobulinemia, congenital and
acquired agammaglobulinemia, and hypogammaglobulinemia)
c) Malabsorption syndromes
d) Lymphoid aplasia
e) IgG myeloma
f) Acute lymphoblastic leukemia
g) Chronic lymphoblastic leukemia
IgD
1. Increases in:
a) Chronic infections
b) IgD myelomas
IgE
1. Increases in:
a) Atopic skin diseases such as eczema
b) Hay fever
c) Asthma
Immunity
Dr. Donya A Makki Antibodies
12
d) Anaphylactic shock
e) IgE-myeloma
2. Decreases in:
a) Congenital agammaglobulinemia
b) Hypogammaglobulinemia due to faulty metabolism or synthesis of
immunoglobulins
References
Mayer G. IMMUNOGLOBULINS. STRUCTURE AND FUNCTION IMMUNOLOGY
– CH4.
http://pathmicro.med.sc.edu/mayer/igstruct2000.htm
Pier GB, Lyczak JB, Wetzler LM (2004). Immunology, Infection, and Immunity. ASM Press. ISBN 1-55581-246-5.
Antibody Structure (2000)The Biology Project . The University of Arizona
http://www.biology.arizona.edu/immunology/tutorials/antibody/structure.html
Gosnell et al (2008) Immunology: Antibody Basic. John A. Burns School of Medicine
http://jabsom.hawaii.edu/JABSOM/admissions/doc/Antibodies%20Instructional%20Module.pdf
Immunoglobulin - Overview
http://www.webmd.com/cancer/tc/immune-globulin-overview
Serum globulin electrophoresis
http://www.nlm.nih.gov/medlineplus/ency/article/003544.htm
Gamma globulin (2014) The Columbia Encyclopedia, 6th ed.
http://www.encyclopedia.com/topic/gamma_globulin.aspx
Gamma globulin
http://en.wikipedia.org/wiki/Gamma_globulin
Structure of Antibodies
Immunity
Dr. Donya A Makki Antibodies
13
Antibody structure and isotypes. An explanation and description of the different structural elements of an antibody
http://www.abcam.com/index.html?pageconfig=resource&rid=11258&pid=11287
Isotype (immunology)
http://en.wikipedia.org/wiki/Isotype_%28immunology%29
Fragment antigen-binding
http://en.wikipedia.org/wiki/Fragment_antigen-binding
Fragment crystallizable region
http://en.wikipedia.org/wiki/Fragment_crystallizable_region
Nieminen T (1999) Circulating Antibody-Secreting Cells and Salivary Antibodies Induced by the Capsular
Polysaccharide of Streptococcus Pneumoniae after Parenteral Immunisation and in Acute Otitis Media. Helsingin yliopiston
verkkojulkaisut, Helsinki. Thesis.
http://ethesis.helsinki.fi/julkaisut/laa/haart/vk/nieminen/review.html
Somatic hypermutation of antibody genes
http://nfs.unipv.it/nfs/minf/dispense/immunology/lectures/files/somatic_hypermutation.html#section1
Immunoglobulins (IgG, IgA, and IgM), Serum
http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8156
