
Genetics
Lecture 4 -
Dec. 21
st
. 2015

Numerical abnormalities
•
Haploid; chromosome count is 23 i.e n
• Diploid; Normal chromosome count is 46 i.e 2n
• Polyploid; Chromosomal No. such as 3n & 4n
• Aneuploid; Any number which is not an exact
multiple of n (2n + 1, 2n + 3).
Trisomy (2n+1)
&
Monosomy (2n-1).

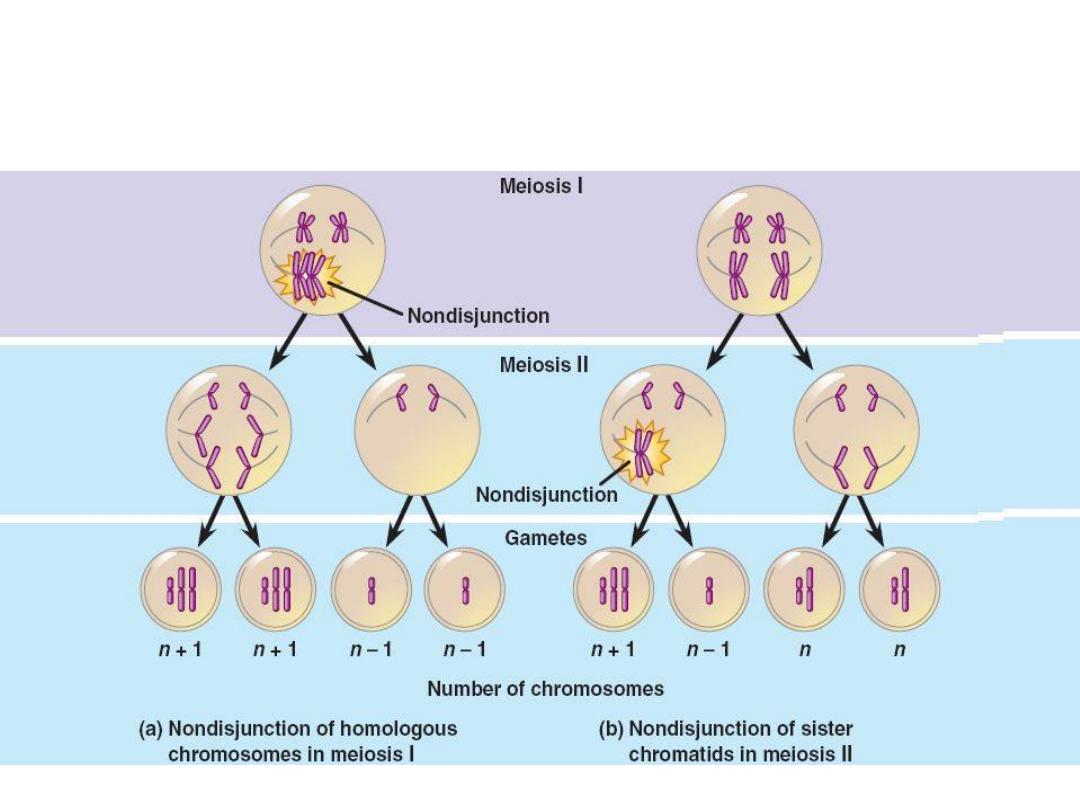
The chief cause of aneuploidy is non disjunction of a homologous
pair of chromosome at first meiotic division or failure of chromatids
to separate during the second meiosis.
When non disjunction occurs at time of meiosis, the gametes formed
have either extrachromosome (n+1) or 1 less chromosome (n-1),
then fertilization lead to either trisomy (2n+1) or monosomy (2n-1).
Monosomy involving an autosome is incompatible with life, while
monosomy involving sex chromosomes is compatible with life.
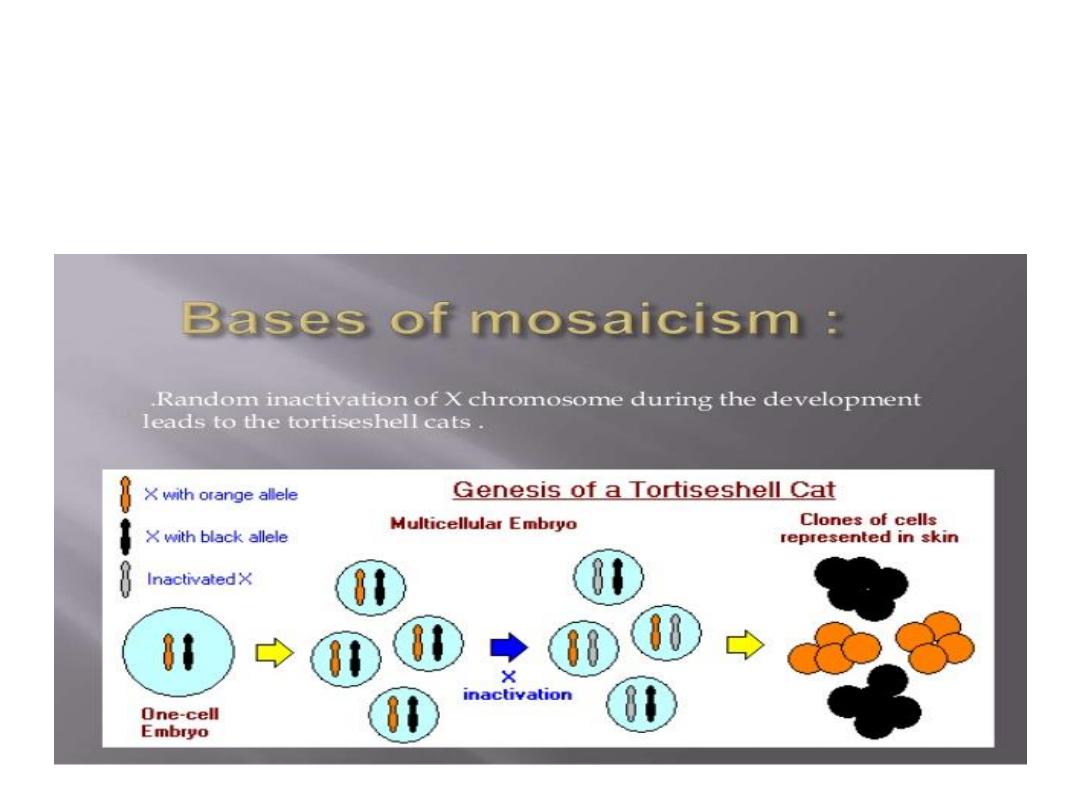
Mosaicism
:
the presence of 2 or more populations of
cells in the same individual, mosaicism affecting sex
chromosomes is common while autosomal is not.

Mosaicism
Mosaicism

Structural abnormalities:
• Involve breakage of the chromosome & then
rearrangement,
• patterns of rearrangement as follows:
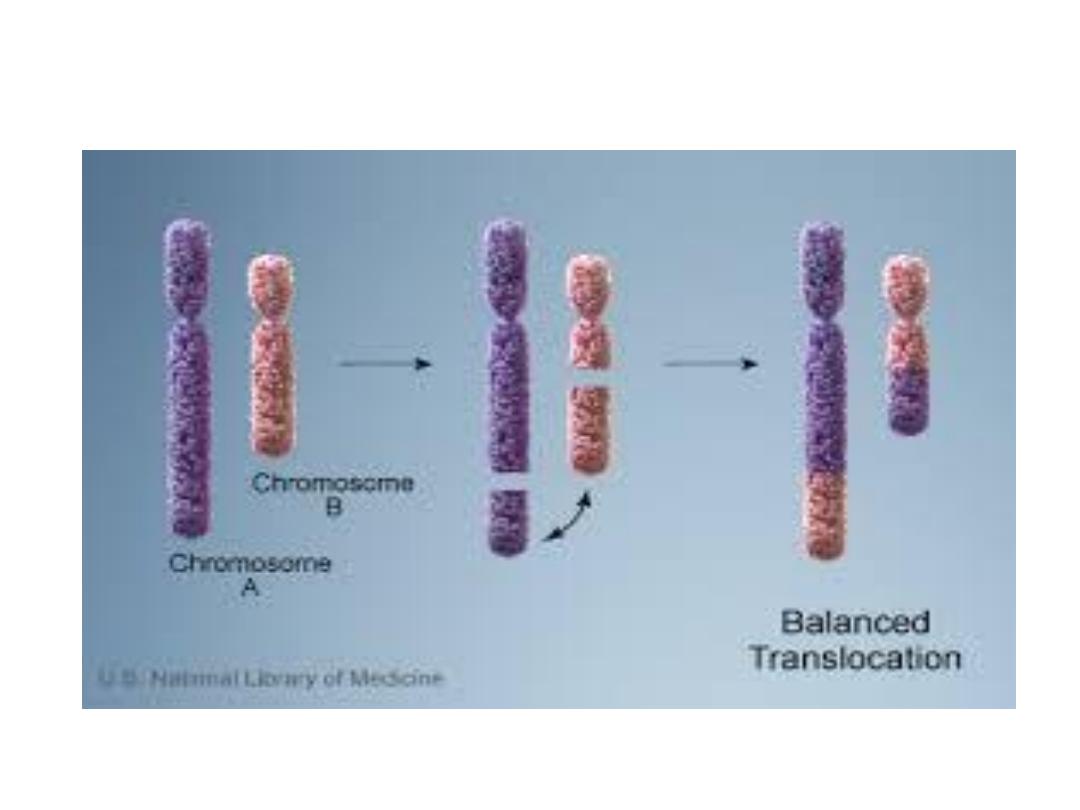
1. Translocation

1- Translocation: transfer of a part of one
chromosome to another chromosome, the
process usually reciprocal ( i.e fragment
exchanged between chromosome)
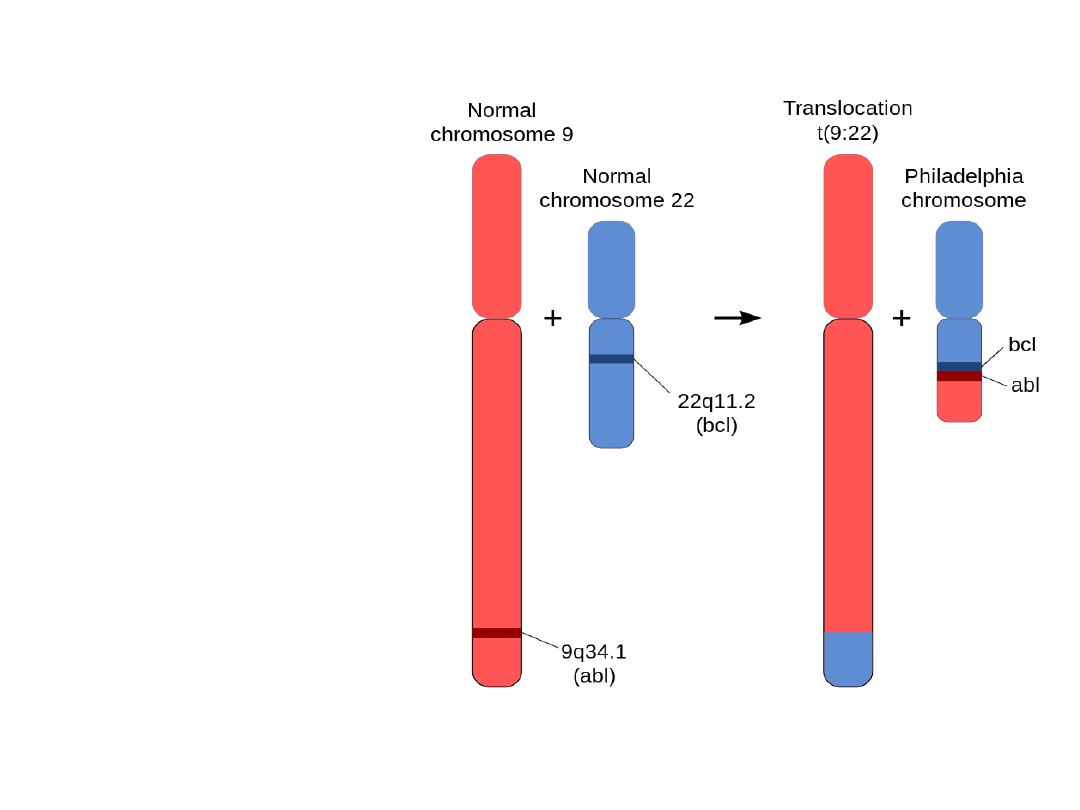
Translocation
• Philadelphia (Ph)
chromosome
• t (9,22)
• CML
• ABL & BCR
are normal genes
on ch 9 & 22,
respectively
.

• Philadelphia chromosome (Ph): The chromosome
abnormality that causes chronic myeloid leukemia
(CML). Abbreviated as the Ph chromosome
• The Ph chromosome is an abnormally short
chromosome 22 that is one of the two chromosomes
involved in a translocation (an exchange of material)
with chromosome 9. This translocation takes place in a
single bone marrow cell and, through the process of
clonal expansion (the production of many cells from
this one mutant cell), it gives rise to the leukemia.

Philadelphia (Ph) chromosome
• The ABL gene encodes a tyrosine kinase
enzyme.
• In the formation of the Ph translocation, two
fusion genes are generated: BCR-ABL on the
Ph chromosome.
• The BCR-ABL gene encodes a protein with
uncontrolled tyrosine kinase activity.
• The BCR-ABL oncoprotein is the unique cause
of CML.

• 2- Deletion:
• Involve loss of a portion of a chromosome,
single break may delete a terminal segment.
• 2 interstitial breaks may result in loss of
intermediate segment.
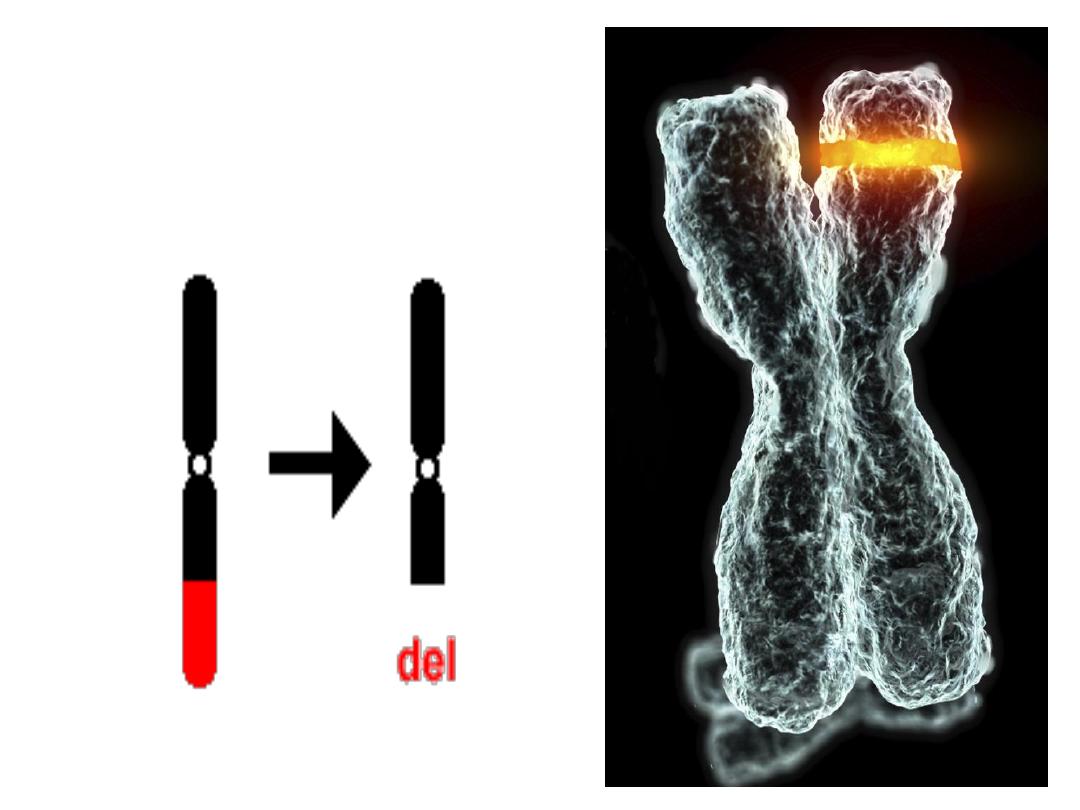
Deletion
loss of a portion of a chromosome
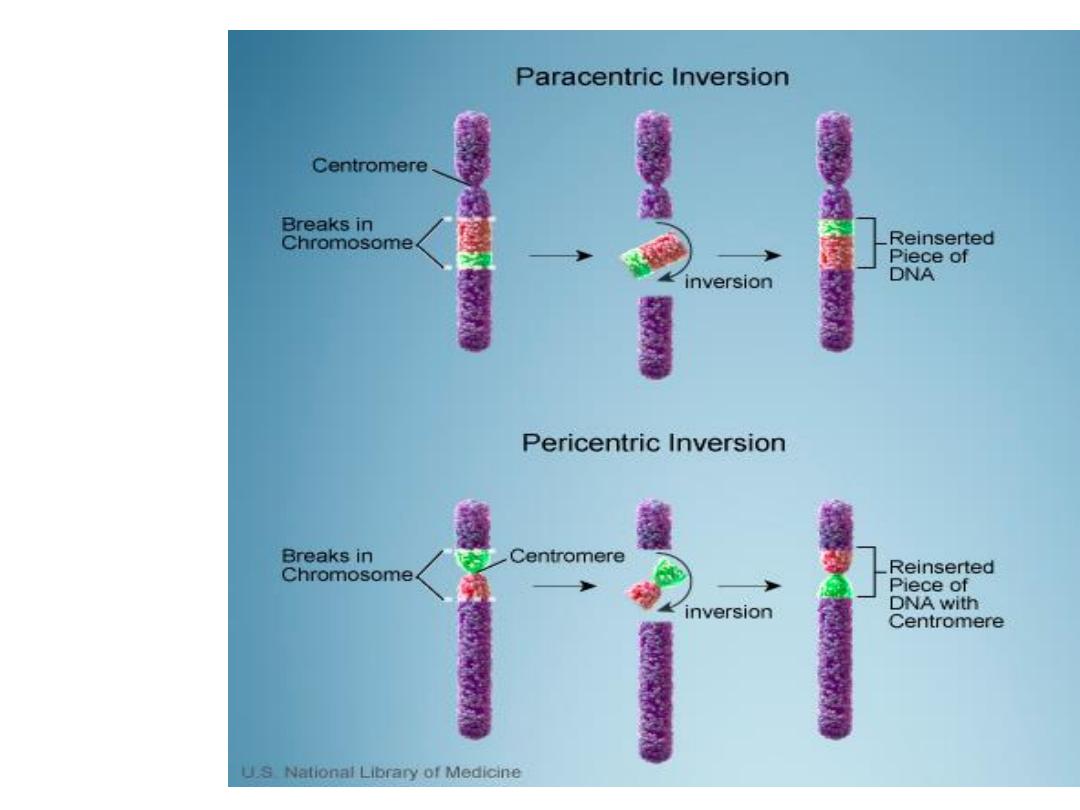
3. Inversion

Inversion: occur when there are 2 interstitial
breaks in a chromosome & the segment reunites
after a complete turnaround.
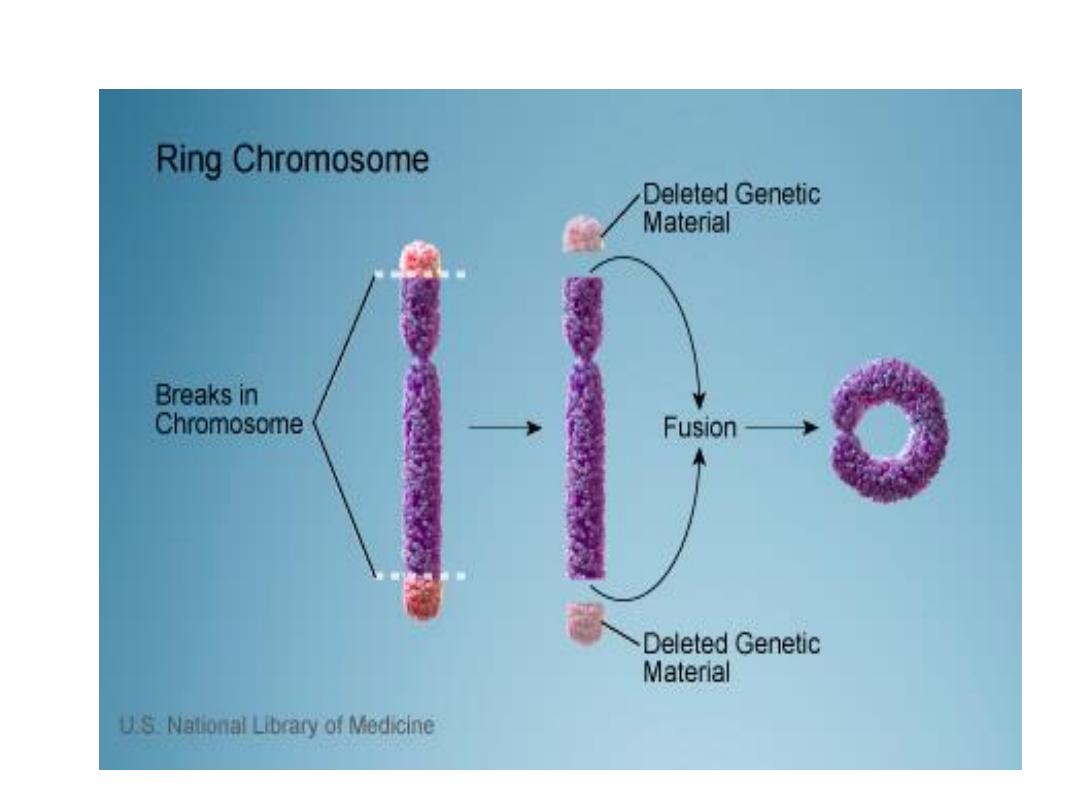
4. Ring chromosome

• Ring chromosome: is a variant of deletion,
after loss of segments from each end of the
chromosome, the arms uniting to form ring.
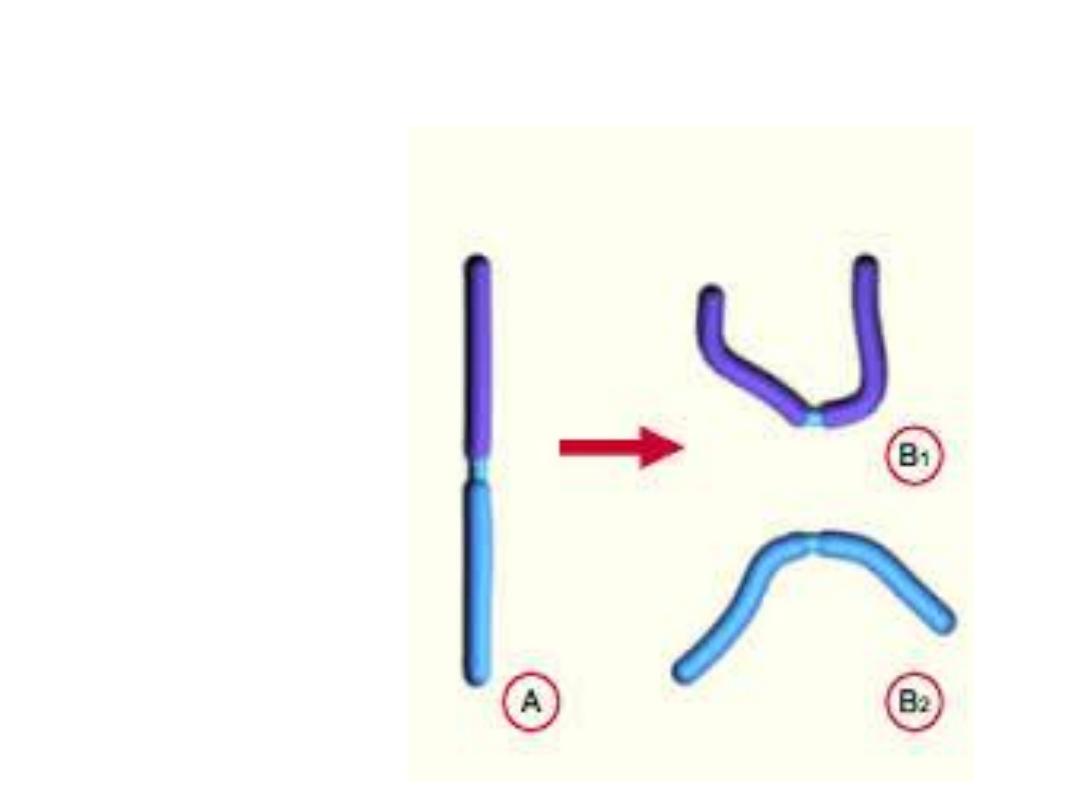
Isochromosomes
centromere divides horizontally

*
Chromosomal disorders may be associated with
absence (deletion or monosomy), excess (trisomy),
or abnormal rearrangement (translocation).
* In general loss of chromosomal material produces
more severe defects than does gain of
chromosomal material.

*
Imbalances of sex chromosome (excess or
loss) are tolerated much better than are similar
imbalance of autosomes.

Cytogenic disorders involving
autosomes:
• Trisomy 21 (Down
syndrome):
• chromosomal count is 47,
• meiotic non disjunction
in the ovum,
• the parents are normal
but maternal age is
important, in women
more than
45---1:25 birth.
Mother’s age
Chances of having
child with Down
syndrome
20
1 in 1,600
25
1 in 1,300
30
1 in 1,000
35
1 in 365
40
1 in 90
45
1 in 30

• t was first described in 1866 and is named
after John Langdon Down
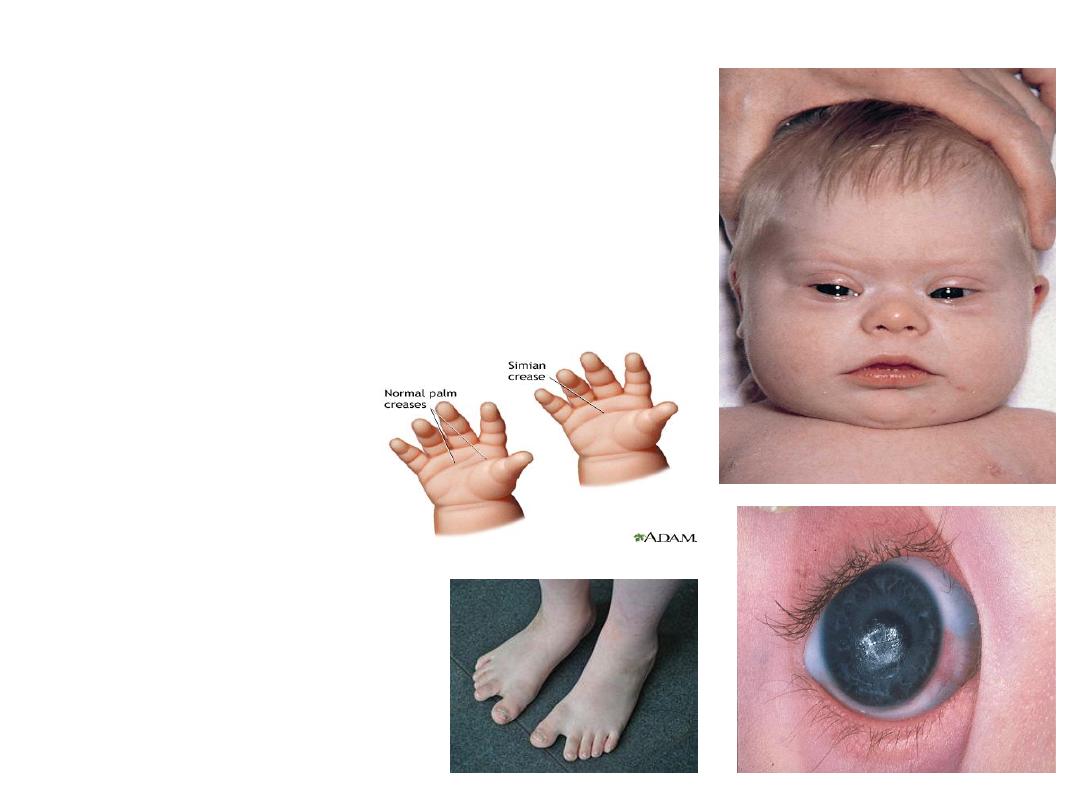
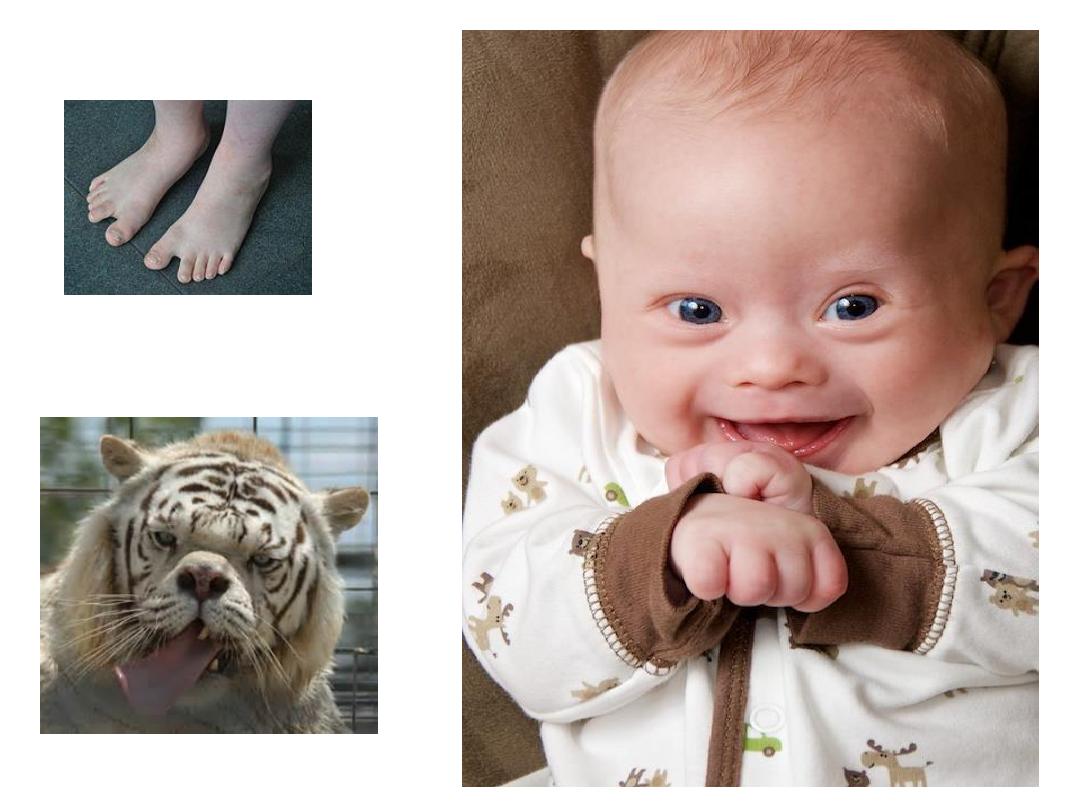
Clinical features:
•
1-
Mental retardation.
•
2- Epicanthic folds &flat facial profile.
•
3- Abundant neck skin.
•
4- Simian creases.
•
5- Congenital heart defects
It is the principle cause of death
in addition to serious infection.
•
6- Umbilical hernia.
•
7- Intestinal stenosis.
•
8- Hypotonia.
•
9- Gab between first &second toe.
•
10- Predisposition to leukemia.
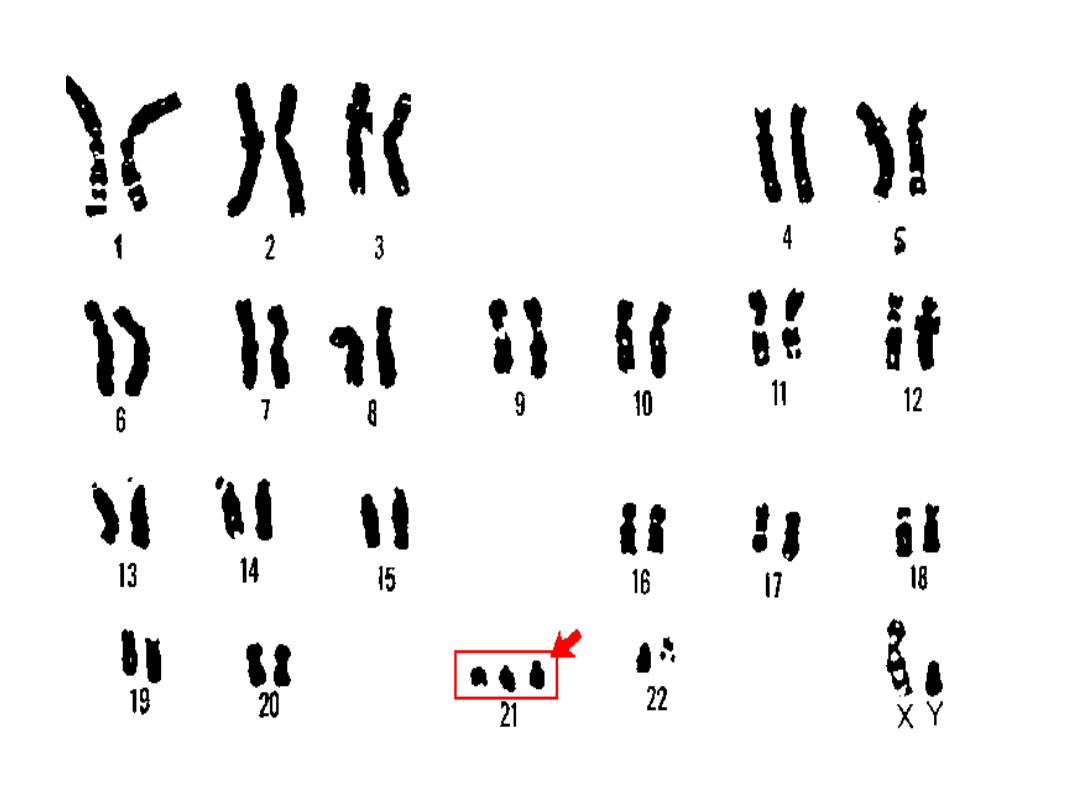

• In 4%, the extrachromosomal material is translocation of
long arm of chromosome 21 to 22 or 14.
• 1% is mosaicism with mixture of 46 &47 chromosome.
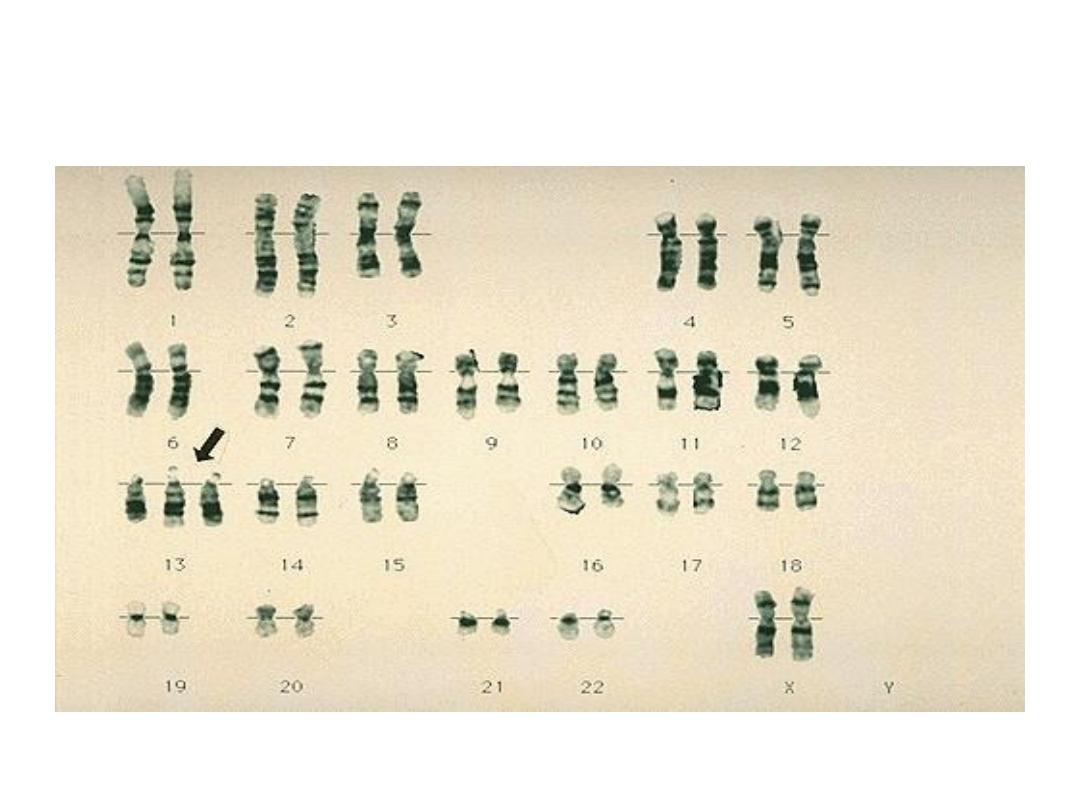
Trisomy 13 (Patau syndrome):
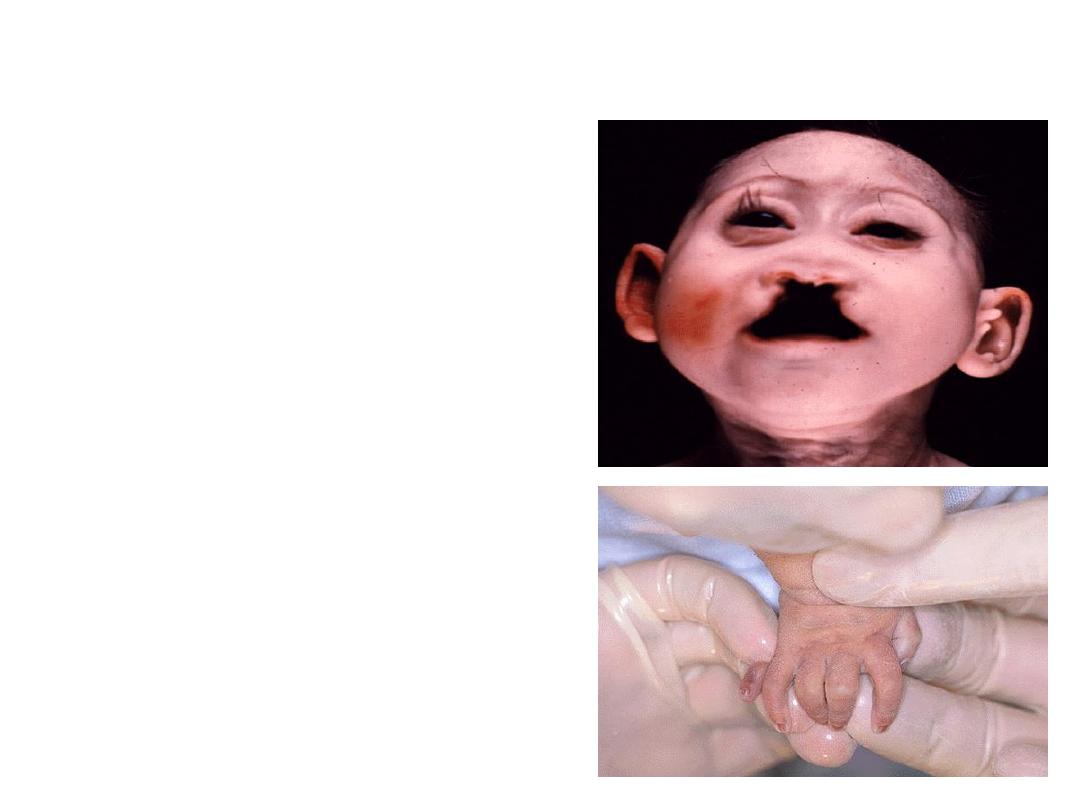
Trisomy 13 (Patau syndrome):
•1- Microcephaly &mental retardation.
•2- Microphthalmia.
•3- Cleft lips &palate.
•4- Cardiac defects.
•5- Umbilical hernia.
•6- Renal defects.
•7- Polydactyly.
•8- Rocker-bottom feet.

Disorders involving sex chromosomes
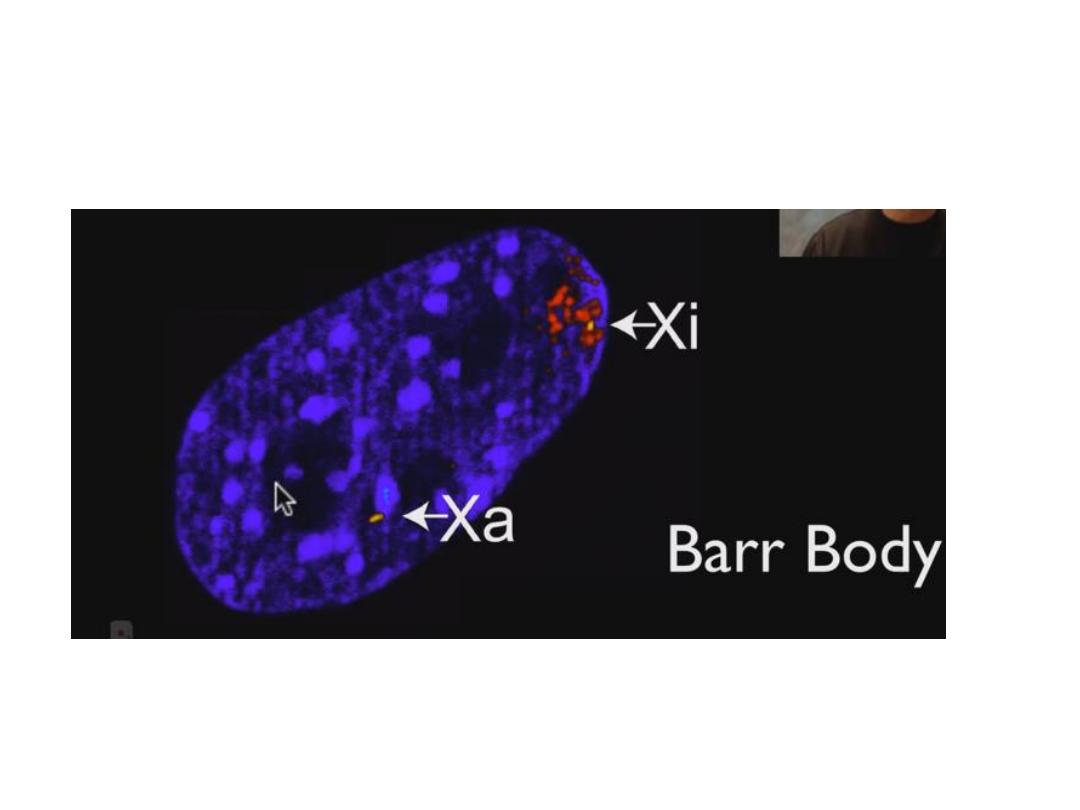
• 1- inactivation of one of the two X chromosome early
in fetal life & called Bar body.
• 2- Scant amount of genetic information carried by Y
chromosome.
• Extra Y chromosome readily tolerated because the
only information carried by it is related to male
differentiation.

Disorders involving sex chromosomes
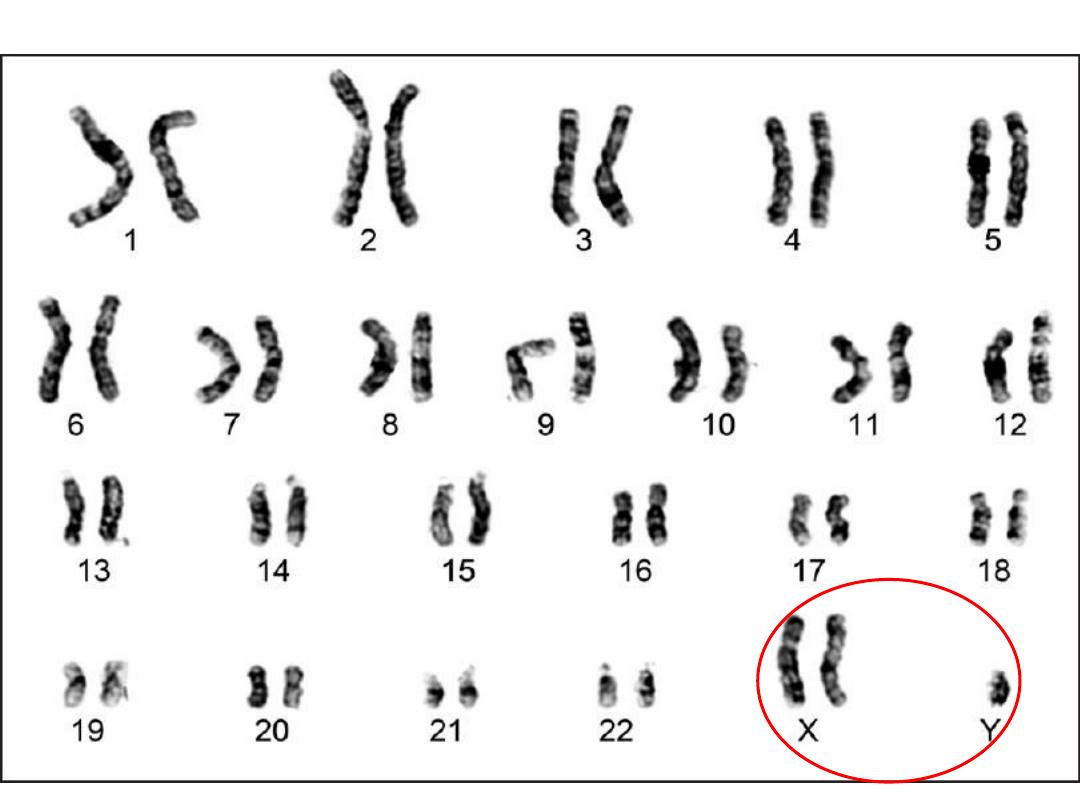
Klinefilter syndrome (47,XXY)
• Karyotype: 47,XXY
• Causes:
• * Advanced maternal age.
• * History of irradiation of either parent.
• #1 cause of male infertility
• L----O----N----G legs, hypogonadism.
• Serum testosterone decrease
• failure of male 2ry sexual characteristics development
(Reduced facial &body hair )
• Gynecomastia
• NO retardation unless more X’s
Klinefilter syndrome

• Histologically:
• Hyalinization of tubules (appear ghost like), in
contrast lyedig cells are prominent.
Klinefilter syndrome

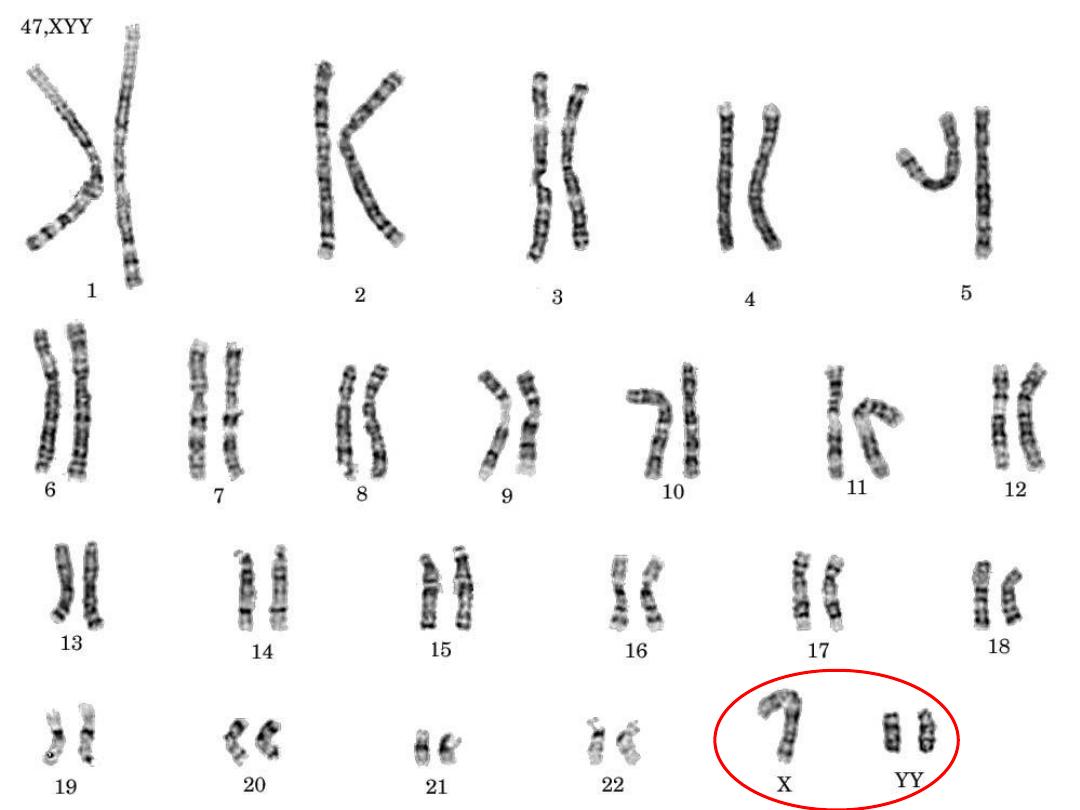
XYY males:
• Due to non disjunction at the second meiotic
division, most are phenotypically normal, but
taller than usual also with antisocial behavior.
XYY males

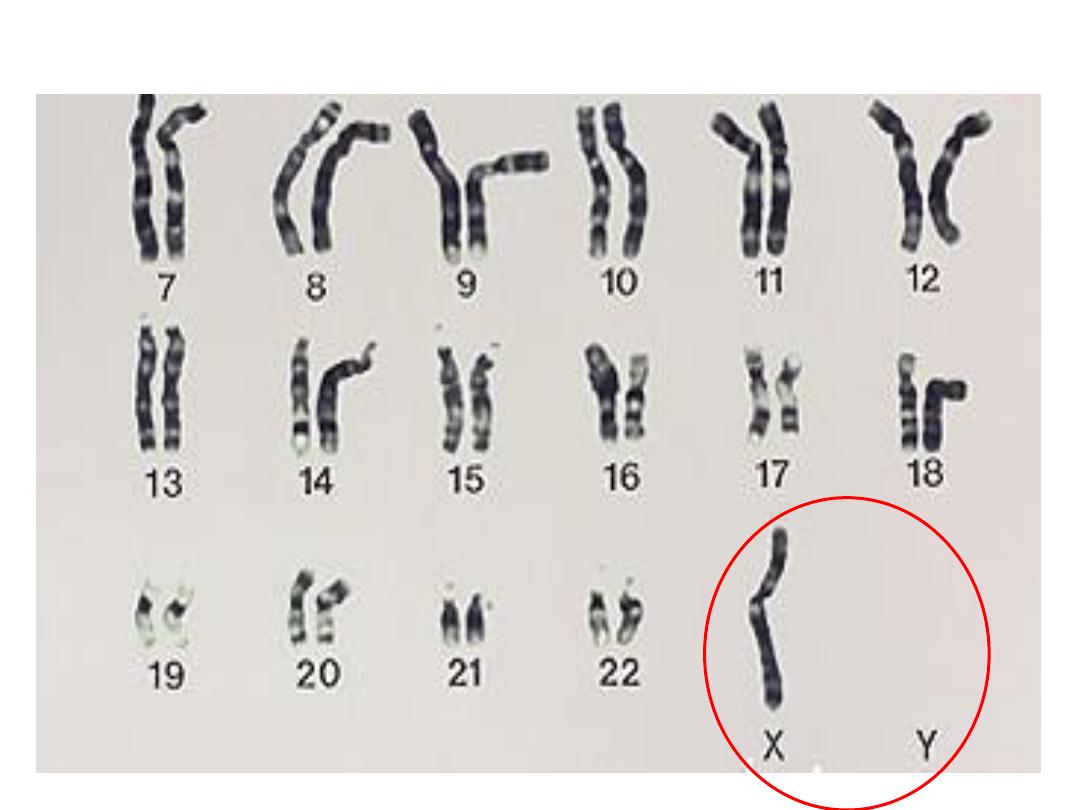
TURNER (XO)
• 45, X is the “proper” designation
• Mosaics common
• Often, the WHOLE chromosome is not missing, but just
part
• NECK “WEBBING”
• EDEMA of HAND DORSUM
• CONGENITAL HEART DEFECTS
Turner syndrome

Turner syndrome
• Monosomy of X chromosome. 45 XO
• hypogonadism in phenotypic female
• Short stature.
• Failure of development of 2ry sexual characteristics.
• Low posterior hair line.
• Cubitus vulgus (increase in carrying angle of the
arms).
• Shield like chest with widely spaced nipples
• Variaty of congenital malformation e.g horseshoe
kidney, coarctation of aorta, CONGENITAL HEART
DEFECTS
• NECK “WEBBING”

• Genitalia remain infantile (little pubic hair,
primary amenorrhea.
• Ovaries fibrosed which is devoid of follicles.
• Low estrogen decrease
• increase pituitary gonadotrophins
.
Turner syndrome

Genomic instability in cancer

Normal Regulatory Genes
• Four classes of normal regulatory genes—
• The growth-promoting
protooncogenes
,
• The growth-inhibiting
tumor suppressor genes
,
• Genes that regulate programmed cell death (
apoptosis
),
• Genes involved in
DNA repair
—are the principal targets
of genetic damage.

Normal regulatory genes
• Mutant alleles of protooncogenes are considered dominant.
• Tumor suppressor genes, referred to as recessive genes.
• There are exceptions to this rule.

Familial cancer
• An inherited genetic mutation predispose individuals
to cancers and may cause early age onset cancers.
• Development of multiple primary tumors.
• Many are caused by mutations in Tumor suppressor
genes
• Other genes affected are DNA repair genes,
Oncogenes.
• Common examples of inherited cancer syndromes are
breast - ovarian cancer, colon ca.
• 5 to 10% of all cancers.
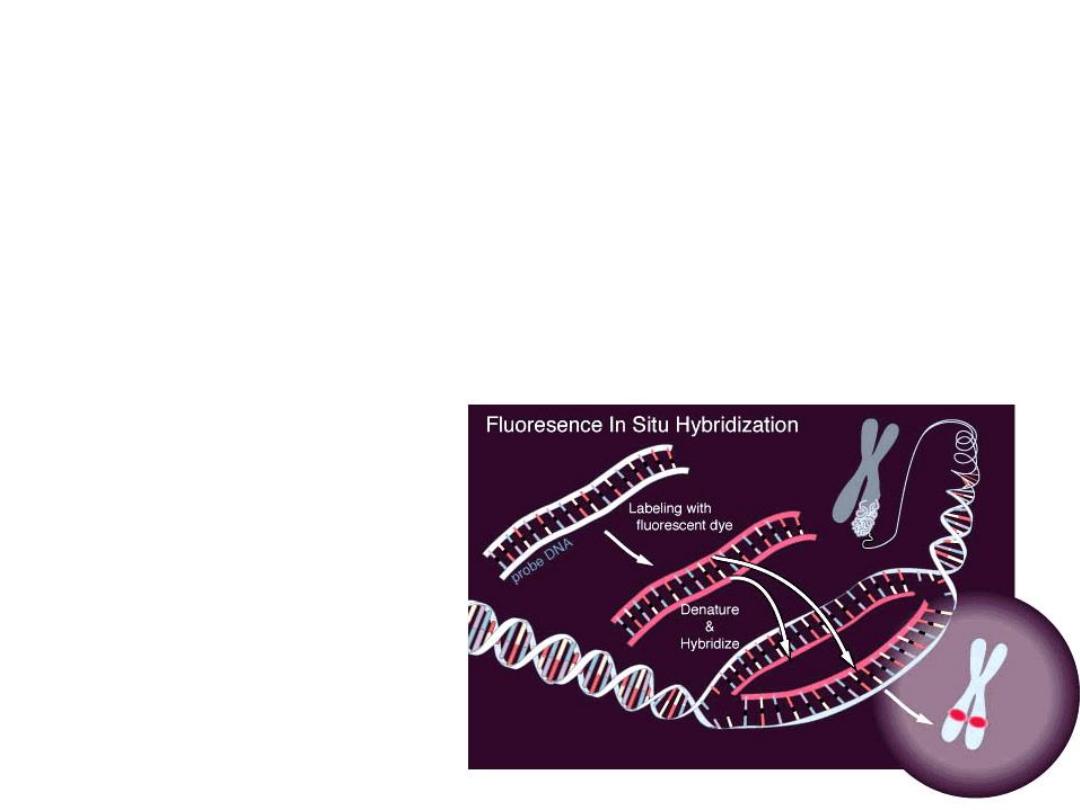
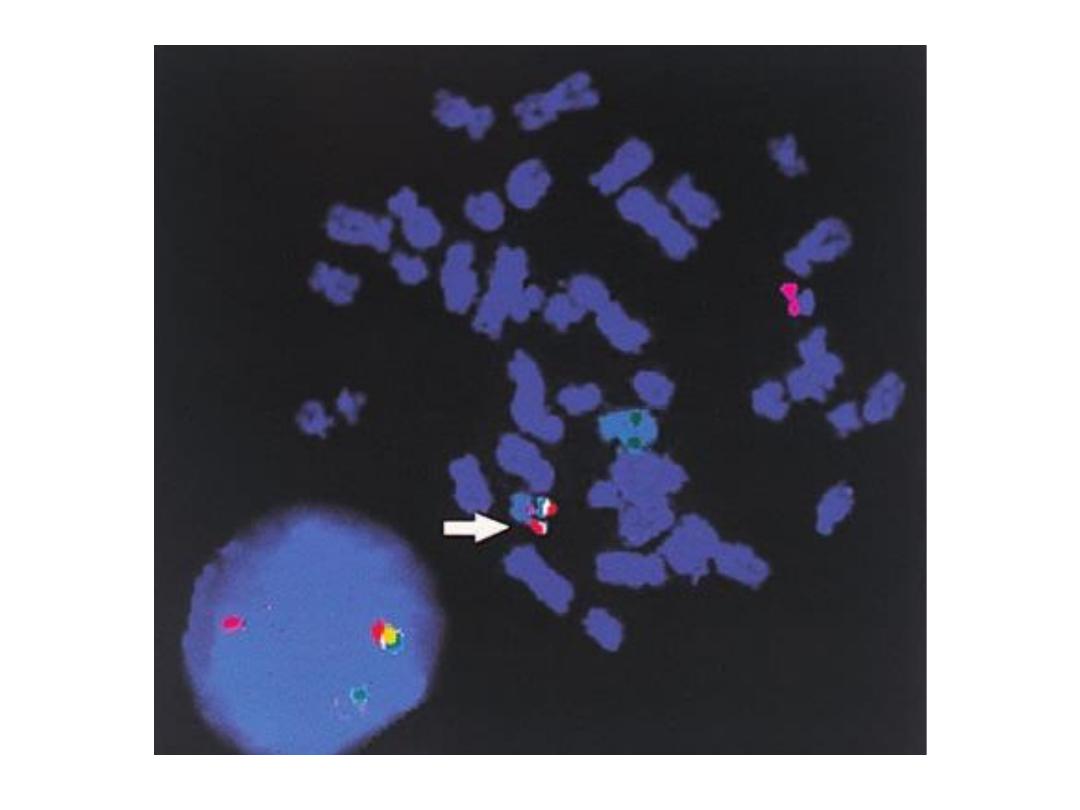
F.I.S.H.
greatly enhances G-banding
• F
luorescent
I
n-
S
itu
H
ybridization
• Uses fluorescent labelled
DNA fragments, ~10,000
base pairs, to bind (or not
bind) to its complement
• Awesome research technique, used rarely in
everyday pathology too, fluorescently “labels”
pieces of DNA which connect to the
corresponding strand during DNA replication.
In situ hybridization (ISH) is a type
of
labeled
or
(i.e.,
) to localize a specific DNA or RNA
sequence in a portion or section of
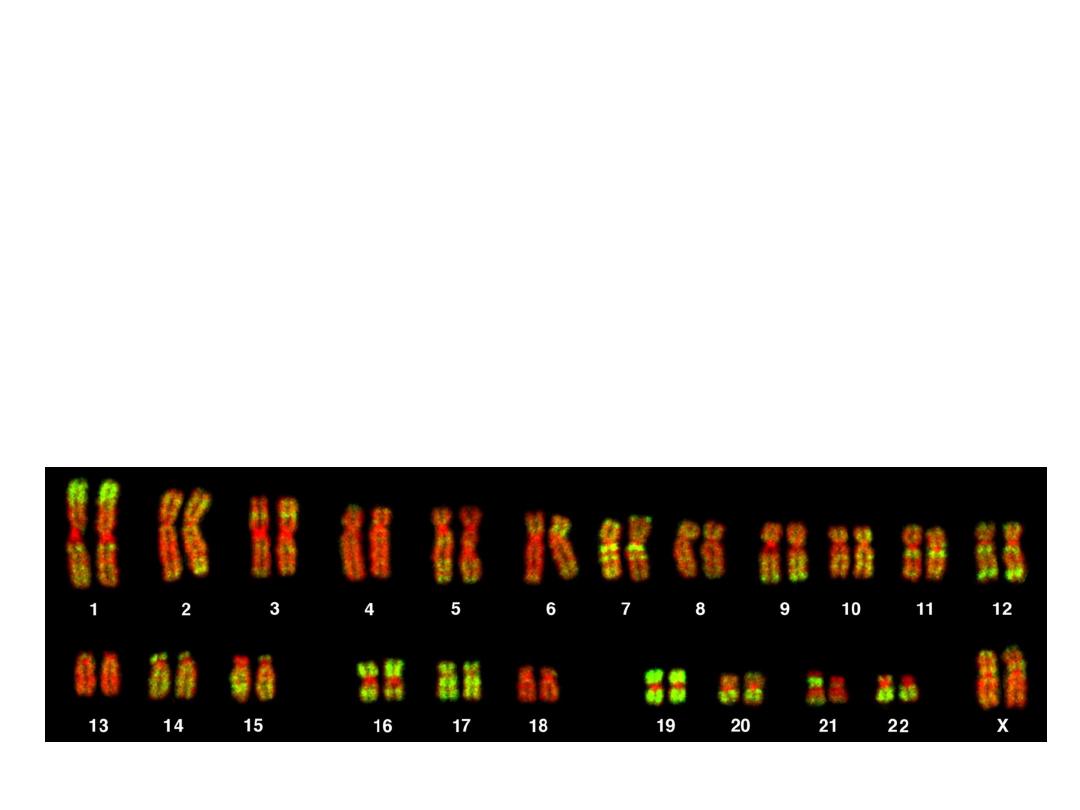
FISH
• SUBTLE MICRODELETIONS
• COMPLEX TRANSLOCATIONS
• AND TELOMERE ALTERATIONS

• FISH is POWERFULLY more sensitive, accurate,
and specific, than G-banding.
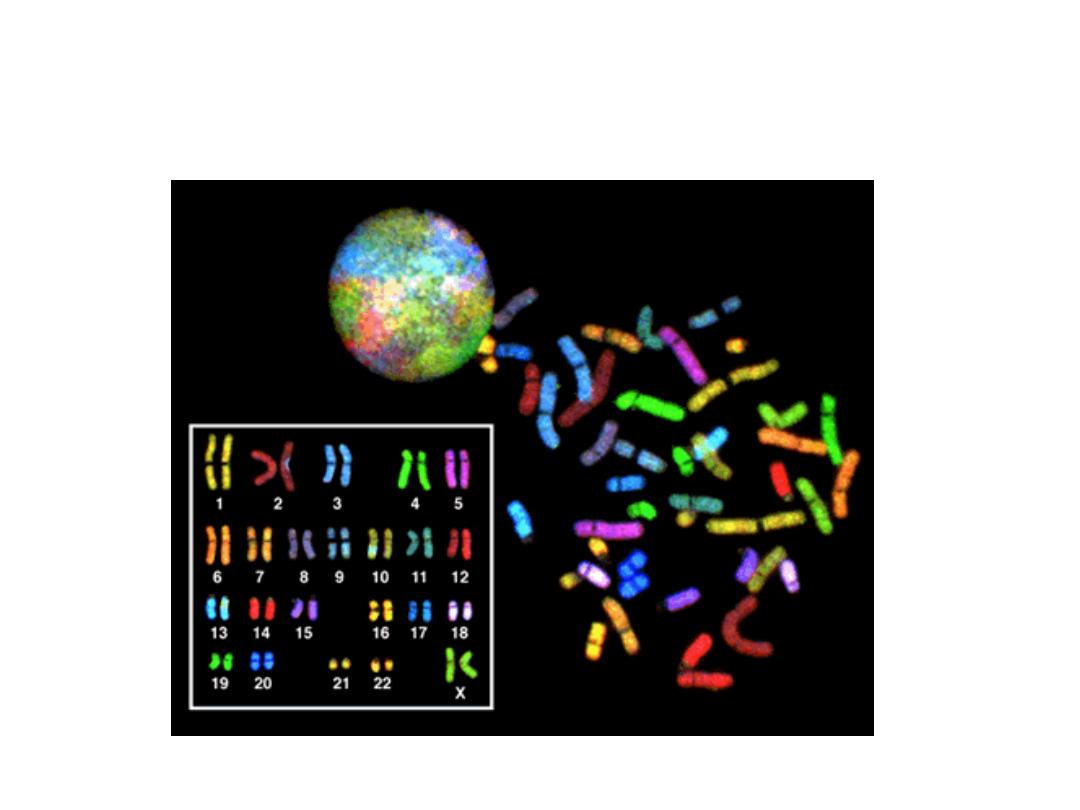
SPECTRAL KARYOTYPING

• This technique is used to identify structural
chromosome aberrations in cancer cells and
other disease conditions when Giemsa
banding or other techniques are not accurate
enough. Each chromosome has a different
color, sort of, although some of this id digital
false color techniques, much in the same way,
electron microscopy can generate “false”
colors.

Diseases caused by mutation in mitochondrial
genes
• Mitochondria contain several genes encodes for enzymes of
oxidative phosphorylation, usually the ovum contain the large
part of mitochondria, so the inheritance of mitochondrial
gene is maternal.
• Disease caused by mitochondrial genes are rare

Diseases associated with genomic imprinting
:
• All humans inherit two copies of each gene, carried on
homologous maternal & paternal chromosomes & there is no
difference between normal homologous genes.
• But now functional difference exists between maternal
&paternal genes is called genomic imprinting.

Chromosome 22q11.2
Deletion Syndrome
• Because of a DELETION, this cannot be detected by
standard karyotyping and needs FISH
• Cardiac defects, DiGeorge syndrome, velocardiofacial,
CATCH*

• 22q11.2 deletion syndrome, also known
as DiGeorge Syndrome, Velocardiofacial
Syndrome, conotruncal anomaly face syndrome,
Congenital Thymic Aplasia, Strong Syndrome,
Thymic hypoplasia, and DiGeorge anomaly. It
also has the mnemonic C-A-T-C-H, for :
• Cardiac Abnormality (especially Fallot's Tetralogy)
Thymic aplasia
Cleft palate
Hypocalcemia

SEX CHROMOSOME DISORDERS
• Problems related to sexual development and
fertility
• Discovered at time of puberty
• Retardation related to the number of X
chromosomes
• If you have at least ONE “Y” chromosome,
you are male

• Sexuality can be defined in many ways, having
at least ONE “Y” chromosome is a good
definition of being male.

HERMAPHRODITES
• GENETIC SEX is determined by the PRESENCE or
ABSENCE of a “Y” chromosome, but there is also,
GONADAL (phenotypic), and DUCTAL sex
• TRUE HERMAPHRODITE: OVARIES AND TESTES, often
on opposite sides
• PSEUDO-HERMAPHRODITE:
– MALE: TESTES with female characteristics (Y-)
– FEMALE: OVARIES with male characteristics (XX)

• “Pseudo”-hermaphrodites are MUCH more
common that TRUE hermaphrodites.

MOLECULAR DX by DNA PROBES
• BIRTH DEFECTS, PRE- or POST- NATAL
• TUMOR CELLS
• CLASSIFICATIONS of TUMORS
• IDENTIFICATION of PATHOGENS
• DONOR COMPATIBILITY
• PATERNITY
• FORENSIC

• My #1 peeve, is people who identify
pathology with forensic pathology. It shows
they have been watching WAY too much TV.

Genetic analysis:
In general, genetic testing can be divided into
prenatal and postnatal analysis.
It may involve
• Karyotype analysis
• FISH,
• Molecular diagnostics (PCR), or
• a combination of these techniques.

Karyotype preparation & analysis:
• Cells (from blood, amniotic fluid, etc) are grown
in vitro (in a cell culture dish) to increase their
number
• Cell division is then arrested in metaphase with
colchicine (prevents mitotic spindle from
forming)
• Cells are centrifuged and lysed to release
chromosomes
• Chromosomes are stained with Geimsa stain,
photographed, and grouped by size and banding
patterns

• Limitations of karyotype analysis:
• * Resolution of this technique is fairly low
• * It is applicable only to cells that are dividing
or can be induced to divide in vitro.

FISH Flourescence insitu hybridization
• FISH utilizes DNA probes that recognize
sequences specific to chromosomal regions.
• The probe binds to its complementary
sequence on the chromosome and thus labels
the specific chromosomal region that can be
visualized under a fluorescent microscope.

Polymerase chain reaction PCR:
• Many genetic diseases are caused by
alterations at the nucleotide level (i.e.,
mutations) that cannot be detected by FISH

PCR, involves amplification of DNA, is used in
molecular diagnosis.
If RNA is used as the substrate, it is first reverse transcribed to
obtain cDNA and then amplified by PCR. This method is often
abbreviated as RT-PCR. One prerequisite for direct detection is
that the sequence of the normal gene must be known.

• Advantages over other techniques:
• * It is remarkably sensitive.
• * The use of polymerase chain reaction (PCR)
allows several million-fold amplification of
DNA or RNA, making it possible to utilize as
few as 1 or 100 cells for analysis. A few drops
of blood or a piece of biopsy tissue can supply
sufficient DNA for PCR amplification.

Methods of prenatal screening
• Amniocentesis
• Chorionic Villus biopsy
• Cord blood
• Ultrasound Sonography
• Maternal Blood Test
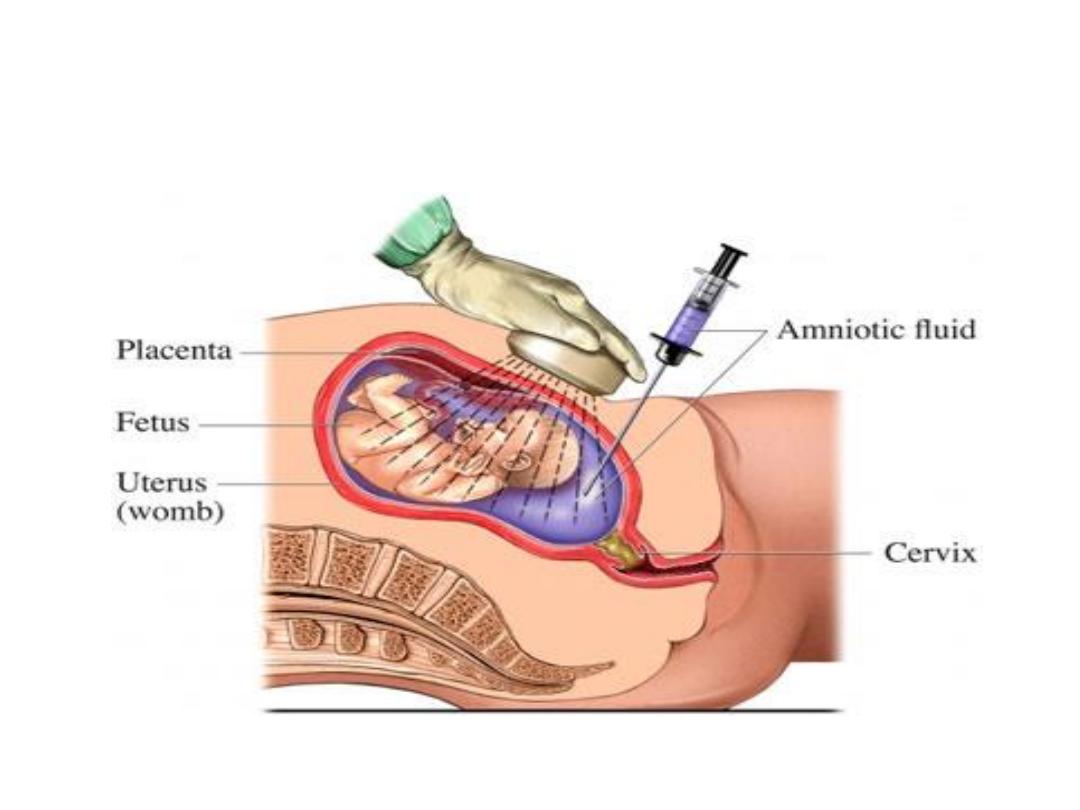
Amniocentesis

Indications for genetic analysis
Prenatal genetic analysis:
• It can be performed on cells obtained by
amniocentesis, on chorionic villus biopsy, or
umbilical cord blood.

• A mother of advanced
age
(>34 years),
because of greater risk of trisomies
• A parent with a previous child with a
chromosomal abnormality
• A parent who is a
carrier
of an X-linked genetic
disorder (to determine fetal sex).

Postnatal genetic testing
• Postnatal genetic analysis is usually performed
on peripheral blood lymphocytes .
• Multiple congenital anomalies in family.
• Unexplained mental retardation.
• Suspected sex chromosome abnormality
• Infertility
• Multiple spontaneous abortions.

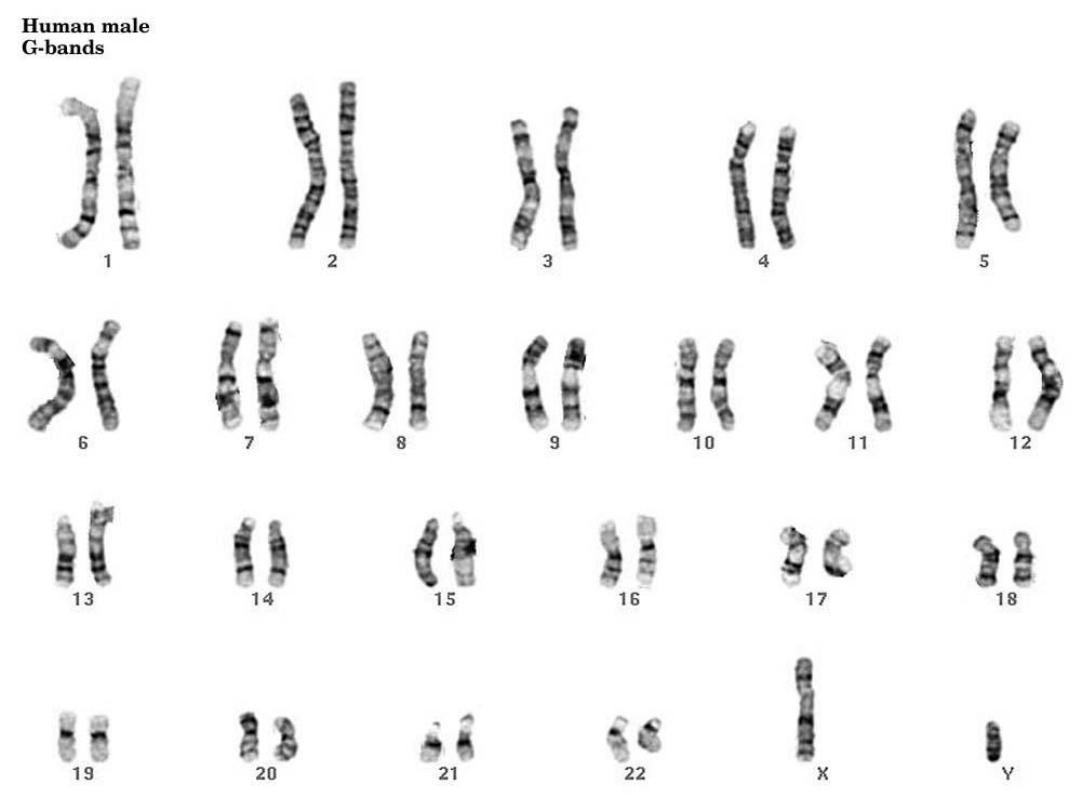
KARYOTYPING
• Defined as the study of CHROMOSOMES
• 46 = (22x2) + X + Y
• Conventional notation is “46,XY” or
“46,XX”
• Giemsa-banding, 500 bands per haploid
recognizable
• Short (“p”-etit) arm = p,
• long arm = q

• The Giemsa stain, named after Gustav Giemsa,
is a VERY common stain in pathology, often
used to identify organisms in cells such as
malaria and helicobacter, and MANY other
things such as parts of cells and connective
tissue. It is a VERY simple stain to do.
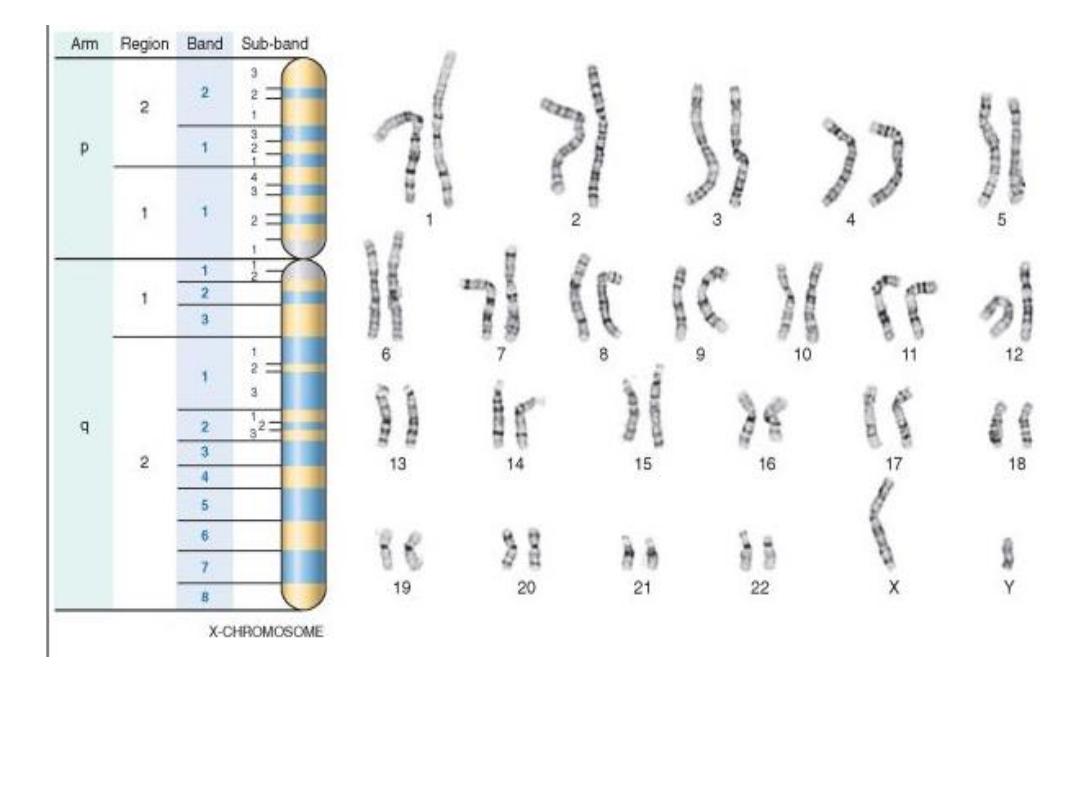
G-banded
karyotype
. Shown is the banding pattern with nomenclature of
arms,
regions, bands, and sub-bands.
