
Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Transplantation
transferring an organ, tissue, or cell from one place to
another.
Autotransplants
Transfer of tissue or organs from one part of an individual
to another part of the same individual. They are the most
common type of transplants and include skin grafts, vein
grafts for bypasses, bone and cartilage transplants, and
nerve transplants. Because the donor and the recipient are
the same person and no immunologic disparity exists, no
immunosuppression is required.
Allotransplants
Transfer from one individual to a different individual of the
same species —the most common scenario for most solid
organ transplants performed today. Immunosuppression is
required for allograft recipients to prevent rejection.
Xenotransplants
nvolve transfer across species barriers. Currently,
xenotransplants are largely relegated to the laboratory,
given the complex, potent immunologic barriers to success.
Orthotopic graft:
a graft placed in its normal anatomical site
Heterotopic graft: a graft placed in a site different from
that where the organ is normally located.
Structural grafts biologic e.g. arterial and heart valve grafts
or synthetic, e.g. Dacron vascular prosthesis.
HISTORY
The first human-to-human kidney transplants
by Yu Yu Voronoy in the 1930s (which were unsuccessful
because of failure to address the immunologic barriers).
1954 Joseph Murray performed first human kidney
transplant with long-term success between identical twins
(Boston, MA, USA).

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
1963 Thomas Starzl performed the first human liver
transplant (Denver, CO, USA)
1966 Richard Lillehei and William Kelly performed the first
human whole-organ pancreas transplant (along with a
kidney transplant) (Minneapolis, MN, USA)
1967 Christiaan Barnard performed the first human heart
transplant (Cape Town, South Africa)
1968 Derom performed the first human lung transplant
(Ghent,
Belgium)
1974 Sutherland and Najarin performed the first human
pancreatic islet transplant (Minneapolis, MN, USA)
1981 Reitz and Shumway performed the first successful
human heart–lung transplant (Stanford, CA, USA)
Many new agents have been developed and approved for use
in clinical transplantation; scores of others are currently
being tested in clinical trials. These agents have allowed for
progressively more specific targeting of the immune system
pathways involved in the rejection process. As a result,
rejection rates have substantially declined for all types of
transplants, and graft survival rates have increased.
TRANSPLANT ANTIGENS
The main antigens involved in triggering rejection are coded
for by a group of genes known as the major histocompatibility
complex (MHC). These antigens, and hence genes, define the
"foreign" nature of one individual to another within the
same species. In humans, the MHC complex is known as the
human leukocyte antigen (HLA) system. It comprises a series
of genes located on chromosome 6.
The HLA antigens are grouped into two classes,
which differ in their structure and cellular distribution.
Class I molecules (named HLA-A, -B, and -C) are found on
the membrane of all nucleated cells.
Class II molecules (named HLA-DR, -DP, and -DQ)
generally are expressed by antigen-presenting cells (APCs)
such as B lymphocytes, monocytes, and dendritic cells.

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
ABO blood group antigens
The ABO blood group antigens are expressed not only by
red blood cells but also by most other cell types. For all types
of organ allograft it is vitally important to ensure that
recipients receive a graft that is ABO blood group
compatible otherwise naturally occurring anti-A or anti-B
antibodies will likely cause hyperacute graft rejection.
There is no need to take account of Rhesus antigen
compatibility.
REJECTION
Rejection can be classified into four types, based on timing
and pathogenesis: hyperacute , accelerated acute , acute , and
chronic .
I.
Hyperacute
occurs within minutes after the transplanted organ is
reperfused
is due to the presence of preformed antibodies in the
recipient.
These antibodies may be directed against the donor's HLA
antigens or they may be anti-ABO blood group antibodies.
Either way, they bind to the vascular endothelium in the
graft and activate the complement cascade, leading to
platelet activation and to diffuse intravascular coagulation.
The result is a swollen, darkened graft, which undergoes
ischemic necrosis.
This type of rejection generally is not reversible, so
prevention is key.
Prevention is best done by making sure the graft is ABO-
compatible and by performing a pretransplant crossmatch.
The cross-match is an in vitro test that involves mixing the
donor's cells with the recipient's serum to look for evidence
of donor cell destruction by recipient antibodies. A positive
cross-match indicates the presence of preformed antibodies
in the recipient that are specific to the donor, thus a high
risk of hyperacute rejection if the transplant is performed.
Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
II.
Accelerated Acute
within the first few days posttransplant
involves both cellular and antibody-mediated injury.
It is more common when a recipient has been sensitized by
previous exposure to antigens present in the donor, resulting
in an immunologic memory response.
III. Acute
within days to 6 months posttransplant.
It is predominantly a cellmediated process, with
lymphocytes being the main cells involved.
Biopsy of the affected organ demonstrates a cellular
infiltrate, with membrane damage and apoptosis of graft
cells.
Usually reversible.
IV. Chronic
occurs after 6 months posttransplant.
Now chronic rejection is an increasingly common problem.
Histologically, the process is characterized by atrophy,
fibrosis, and arteriosclerosis.
Clinically, graft function slowly deteriorates over months to
years posttransplant.
A number of risk factors have been identified for chronic
rejection of a kidney transplant:
• previous episodes of acute rejection;
• poor HLA match;
• long cold ischaemia time;
• cytomegalovirus (CMV) infection;
• raised blood lipids;
• inadequate immunosuppression (including poor
compliance).
Graft-versus-host disease

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
The donor liver and small bowel both contain large numbers
of immunocompetent lymphocytes and these may
react against HLA antigens expressed by recipient tissues
leading to graft-versus-host disease (GVHD).
When GVHD develops it frequently involves the skin,
causing a characteristic rash on the palms and soles. It may
also involve the liver (after small bowel transplantation) and
the gastrointestinal tract (after liver transplantation).
GVHD is a serious and sometimes fatal complication.
IMMUNOSUPPRESSION
The success of modern transplantation is in large part due to
the successful development of effective immunosuppressive
agents. Without these agents, only transplants between
genetically identical individuals would be possible.
CORTICOSTEROIDS
inhibit the production of T-cell lymphokines, which are
needed to amplify macrophage and lymphocyte responses.
Steroids in high doses are the first-line choice of many
clinicians for the initial treatment of acute cellular rejection.
Steroids also are an integral part of most maintenance
immunosuppressive regimens.(e.g., prednisone at 30 mg/d in
adults), tapering to the maintenance dose of 5 to 15 mg/d
over 3 to 6 months.
Common side effects include
mild cushingoid facies and habitus, acne, increased appetite,
mood changes, hypertension, proximal muscle weakness,
Osteoporosis, glucose intolerance, and impaired wound
healing. Less common are posterior subcapsular cataracts,
glaucoma, and aseptic necrosis of the femoral heads.
These serious side effects have fueled the current interest in
withdrawing patients from steroids within a few months
posttransplant, or avoiding steroids altogether.
AZATHIOPRINE (Imuran)
Antimetabolite, converted in the liver to its active metabolite
6-mercaptopurine. Interferes with DNA and RNA synthesis

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Used in maintenance protocols
1–3 mg/kg per day for maintenance
Side effects: bone marrow suppression leukopenia,
thrombocytopenia, and anemia. Liver dysfunction, GI
disturbances (nausea and vomiting), pancreatitis, and
alopecia.
With the introduction of newer agents such as
mycophenolate mofetil (MMF), the use of AZA has
decreased significantly, and may be discontinued
altogether in the near future.
CYCLOSPORINE (Sandimmune)
inhibits the activity of calcineurin. Doing so impairs
expression of several critical T-cell activation genes, the
most important being for IL-2(Interleukin-2).
As a result, T-cell activation is suppressed.
The metabolism of cyclosporine is via the cytochrome P-450
system, therefore several drug interactions are possible.
Adverse effects of cyclosporine can be classified as renal or
nonrenal.
Nephrotoxicity is the most important and troubling adverse
effect of cyclosporine.
nonrenal side effects most commonly hirsutism and gingival
hyperplasia.
Several neurologic complications, including headaches,
tremor, and seizures, also have been reported.
Other nonrenal side effects include hyperlipidemia,
hepatotoxicity, and hyperuricemia.
Oral dose is 8–10 mg/kg per day (given in two divided doses)
TACROLIMUS (Prograf)
Tacrolimus (FK506) is a metabolite of the soil fungus
Streptomyces tsukubaensis.
Tacrolimus, like cyclosporine inhibits calcineurin and IL-2
synthesis.

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Tacrolimus is 100 times more potent than cyclosporine on a
molar basis.
Side effects: Nephrotoxicity, Hypertension
hypercholesterolemia, and hypomagnesemia.
IV 0.05–0.1 mg/kg per day.
SIROLIMUS (Rapamune)
A macrolide antibiotic derived from a soil actinomycete
(previously known as rapamycin ) is structurally similar to
tacrolimus and binds to the same immunophilin (FKBP).
mTOR inhibitors.
Unlike tacrolimus, it does not affect calcineurin activity.
The net result is to prevent progression from the G1 to the S
phase of the cell cycle, halting cell division.
Side effects: Thrombocytopenia, elevation of the serum
triglyceride and cholesterol levels.
May allow early withdrawal of steroids.
2–4 mg/d.
MYCOPHENOLATE MOFETIL (CellCept)
is isolated from the mold Penicillium glaucum .
Antimetabolite inhibits inosine monophosphate
dehydrogenase, which is a crucial, rate-limiting enzyme in
de novo synthesis of purines.
adverse events with MMF are similar to those seen with
AZA. Notable exceptions are GI side effects (diarrhea,
gastritis, and vomiting), which are more common with
MMF. Clinically, significant leucopenia also is more
common, affecting about one third of recipients.
1 g bid PO (may need 1.5 g in black recipients).
POLYCLONAL ANTIBODIES
Polyclonal antibodies are produced by immunizing animals
such as horses or rabbits with human lymphoid tissue,
allowing for an immune response, removing the resultant
immune sera, and purifying the sera in an effort to remove
unwanted antibodies.

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Polyclonal antibodies have been successfully used as
induction agents to prevent rejection and to treat acute
rejection episodes.
MONOCLONAL ANTIBODIES
Monoclonal antibodies directed against the IL-2 receptor on
T lymphocytes (CD25) are commonly given at the time of
transplantation.
Side effects of non-specific immunosuppression
Infection
■ Transplant recipients are at high risk of opportunistic
infection, especially by viruses
■ Viral infection may result from reactivation of latent virus
or from primary infection
■ CMV is a major problem
■ Bacterial and fungal infections are also common
■ Risk of infection is highest during first 6 months after
transplantation
■ Chemoprophylaxis is important in high-risk patients
■ Pre-transplant vaccination against community-acquired
infection should be considered
Malignancy
■ Recipients are at risk of post-transplant
lymphoproliferative disorder
■ There is a high risk of squamous cancer of the skin and
recipients should be reviewed regularly
ORGAN PROCUREMENT AND
PRESERVATION
Most of the organs used for transplantation are obtained
from either:
1. Live related donors.
2. Live unrelated donors.
3. brainstem-dead, heart-beating deceased donors (brain
death).
Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
4. Non-heart beating deceased donors(cardiac death).
When testing for brain death, hypothermia, medication side
effects, drug overdose, and intoxication must be excluded.
Clinical testing for brainstem death
1.Absence of cranial nerve reflexes
■ Pupillary reflex
■ Corneal reflex
■ Pharyngeal (gag) and tracheal (cough) reflex
■ Oculovestibular (caloric) reflex
2.Absence of motor response
The absence of a motor response to painful stimuli
3.Absence of spontaneous respiration
Apnoea test: After pre-ventilation with 100% oxygen for at
least 5 min, the patient is disconnected from the ventilator
for 10 min to confirm absence of respiratory effort, during
which time the arterial Pco2 level should be > 60 mmHg to
ensure adequate respiratory stimulation.
Confirmatory tests must verify the absence of intracranial
blood flow on brain flow studies or the
presence of an isoelectric electroencephalogram reading.
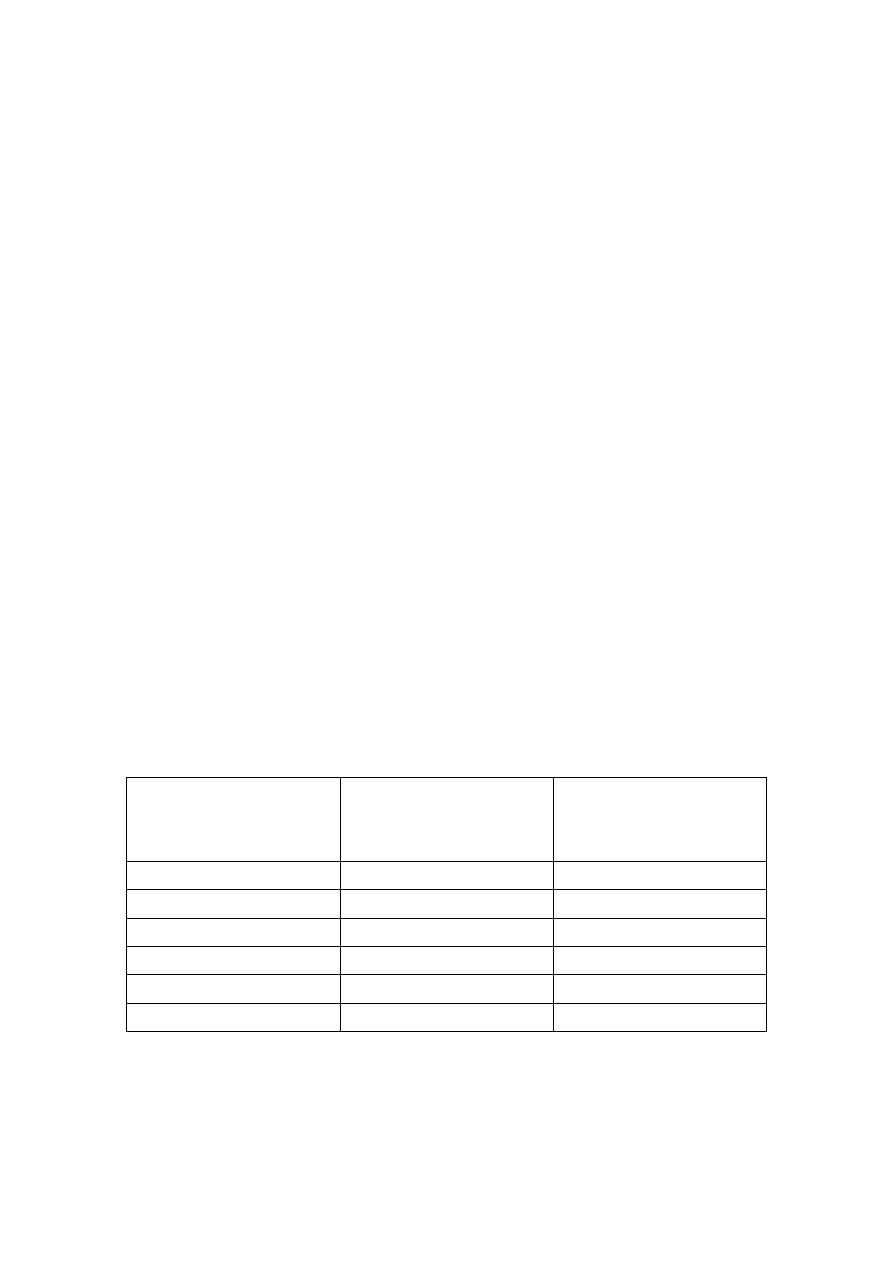
Maximum and optimal cold storage times
Organ
Optimal storage
time (hours)
Safe maximum
storage time
(hours)
Kidney
< 24
48
Liver
< 12
24
Pancreas
< 10
24
Small intestine
< 4
8
Heart
< 3
6
Lung
< 3
8
KIDNEY TRANSPLANTATION
A kidney transplant now represents the treatment of choice
for patients with end-stage renal disease (ESRD).
Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Compared with dialysis, it is associated with better patient
survival and superior quality of life, and is more cost
effective.
Untreated malignancy and active infection are absolute
contraindications to a transplant, because of the requisite
lifelong immunosuppression.
Preoperative Evaluation
The preoperative evaluation can be divided into four parts:
medical, surgical, immunologic, and psychosocial.
The surgical evaluation should identify vascular or urologic
abnormalities that may contraindicate or complicate a
transplant.
The immunologic evaluation involves determining blood
type, tissue type (HLA-A, -B, or -DR antigens), and presence
of any cytotoxic antibodies against HLA antigens (because of
prior transplants, blood transfusions, or pregnancies).
The psychosocial evaluation is necessary to ensure that
transplant candidates understand the nature of the
transplant procedure and its attendant risk. They must be
capable of rigorously adhering to the medical regimen
posttransplant.
Tests Done Before Transplantation
1. Tissue typing.
2. ABO blood group compatibility.
3. Complete blood picture.
4. Renal function tests.
5. To exclude diabetes, hypertension, HIV, Hepatitis B
and C infections.
6. Bilateral renal angiography or MRA to study vascular
pattern.
Complications
1.
HEMORRHAGE
usually it occurs from unligated vessels in the graft hilum or
from the retroperitoneum of the recipient.

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
2.
VASCULAR COMPLICATIONS
Vascular complications can involve
- donor vessels (renal artery thrombosis or stenosis,
renal vein thrombosis),
- recipient vessels [iliac artery thrombosis,
pseudoaneurysms, and deep venous thrombosis
(DVT)], or both.
3.
UROLOGIC COMPLICATIONS
leakage or obstruction, generally occur in 2 to 10% of
kidney recipients.
4.
LYMPHOCELE
The reported incidence of lymphoceles (fluid collections of
lymph that generally result from cut lymphatic vessels in the
recipient) is 0.6 to 18%..
Symptoms are generally related to the mass effect and
compression of nearby structures (e.g., ureter, iliac vein,
allograft renal artery).
Ultrasound is used to confirm a fluid collection.
The standard surgical treatment is creation of a peritoneal
window to allow for drainage of the lymphatic fluid into the
peritoneal cavity, where it can be absorbed. Either a
laparoscopic or an open approach may be used. Another
option is percutaneous insertion of a drainage catheter, with
or without sclerotherapy; however, it is associated with some
risk of recurrence or infection.
5.
OTHER COMPLICATIONS
A wide variety of medical complications can be seen after a
kidney transplant.
a. Infections are probably the most common,
b. complications affecting the cardiac, GI, and neurologic
systems also have been well described posttransplant.
Most centers now report patient survival rates exceeding
95% during the first posttransplant year for all kidney
Recipients and 5 year survival about 80 %.
PANCREAS TRANSPLANTATION

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
A successful pancreas transplant can establish
normoglycemia and insulin independence in diabetic
recipients
drainage procedure must be performed to handle the
pancreatic exocrine secretions. Options include
anastomosing the donor duodenum to the recipient bladder
or to the small bowel.
Complications
THROMBOSIS
HEMORRHAGE
PANCREATITIS
INFECTIONS
UROLOGIC COMPLICATIONS
Bladder drainage of the exocrine pancreas may result in the
following complications:
• bladder/duodenal anastomotic leaks;
• cystitis (because of the effect of pancreatic enzymes);
• urethritis/urethral stricture;
• reflux pancreatitis;
• urinary tract infection;
• haematuria;
• metabolic acidosis (caused by loss of bicarbonate in the
urine).
ISLET CELL TRANSPLANTATION
The pancreas consists of two separate functional systems
(endocrine and exocrine), but it is only the endocrine
component that is of use in the transplant process.
Therefore, the concept of transplanting simply the cells
responsible for the production of insulin is very logical and
attractive.
The purified islets can then be injected into the recipient,
most commonly into the portal vein. The islet cells then
engraft in the hepatic parenchyma and secrete insulin
Potential complications associated with the injection include
portal hypertension, hepatic abscesses, and bacteremia.

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
LIVER TRANSPLANTATION
A liver transplant is indicated for liver failure, whether
acute or chronic. Liver failure
The indications for liver transplantation fall into four
groups:
• cirrhosis;
• acute fulminant liver failure;
• metabolic liver disease;
• primary hepatic malignancy.
Types:
1. whole liver transplant.
2. Partial liver transplant (left lobe or right lobe).
INTESTINAL TRANSPLANTATION
However, intestinal transplants remain the least frequently
performed of all transplants, with the highest rejection rates
and the lowest graft survival rates. About 200 cm of the
distal small bowel is used.
Indications for transplant include the following:
• intestinal atresia;
• necrotising enterocolitis;
• volvulus;
• disorders of motility;
• mesenteric infarction;
• Crohn’s disease;
• trauma;
• desmoid tumours.
HEART AND LUNG TRANSPLANTATION
Heart transplantation is a well-established therapy for end-
stage heart failure.
POSTTRANSPLANT
LYMPHOPROLIFERATIVE DISORDER

Iraqia University College of Medicine Surgery
3
rd
stage Wed. 13
th
– Apr – 2016 Dr. Firas Fadhil
Lymphomas constitute the largest group of noncutaneous
neoplasms in transplant recipients.The vast majority
(>95%) of these lymphomas consist of a spectrum of B-cell
proliferation disorders associated with EBV,
known collectively as posttransplant lymphoproliferative
disorder (PTLD).
Xenotransplantation
(i.e., transplants of organs between different species)
Problems facing xenotransplant
1.
Formidable immunological barrier.
2.
High risk of infection transmitted.
