
Chapter 15
Digestive
system

Ch. 15 – Part 1
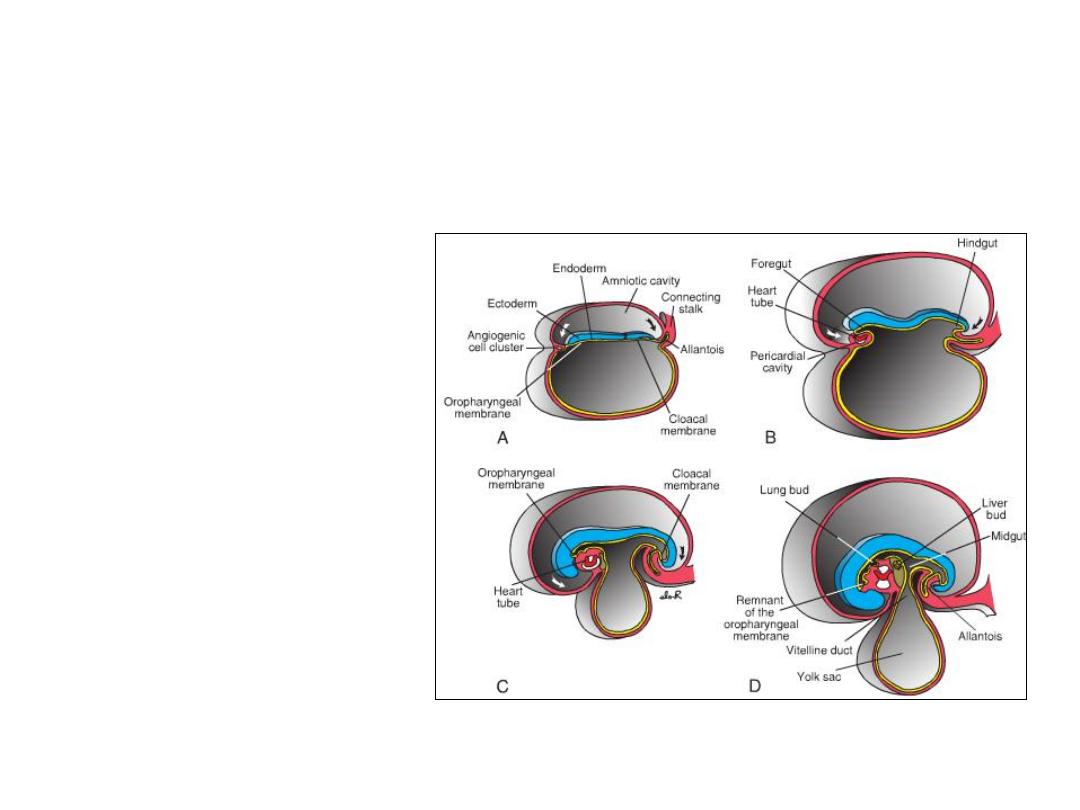
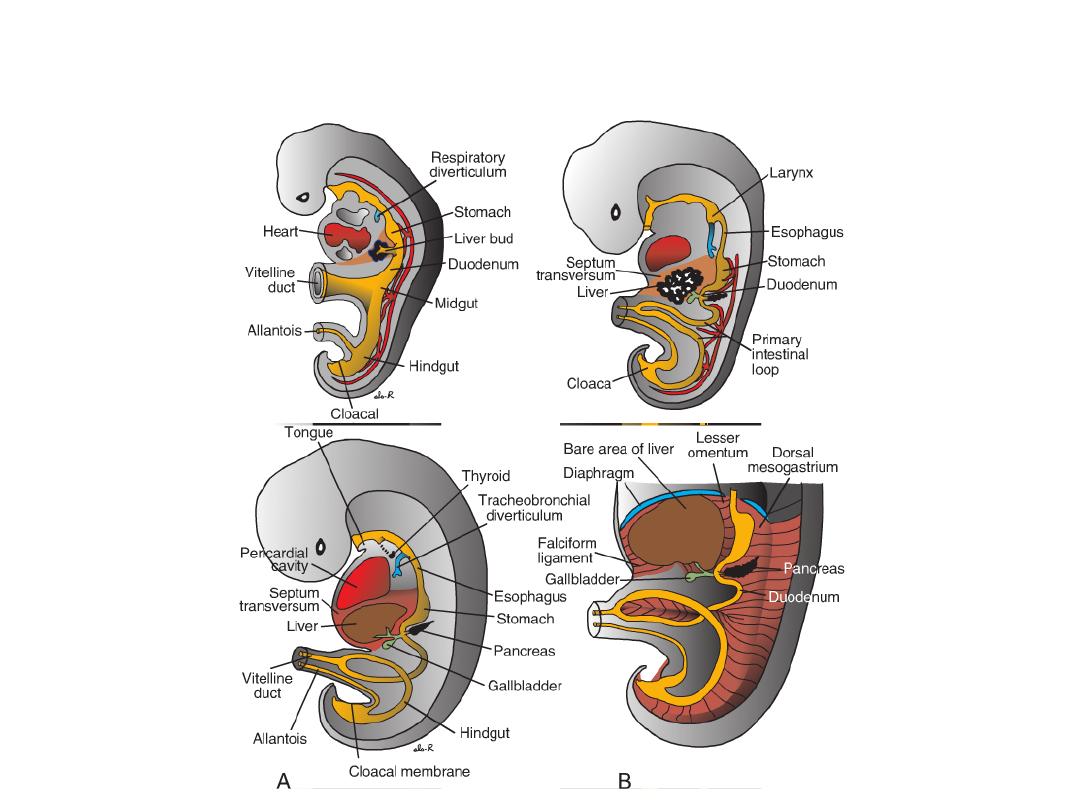
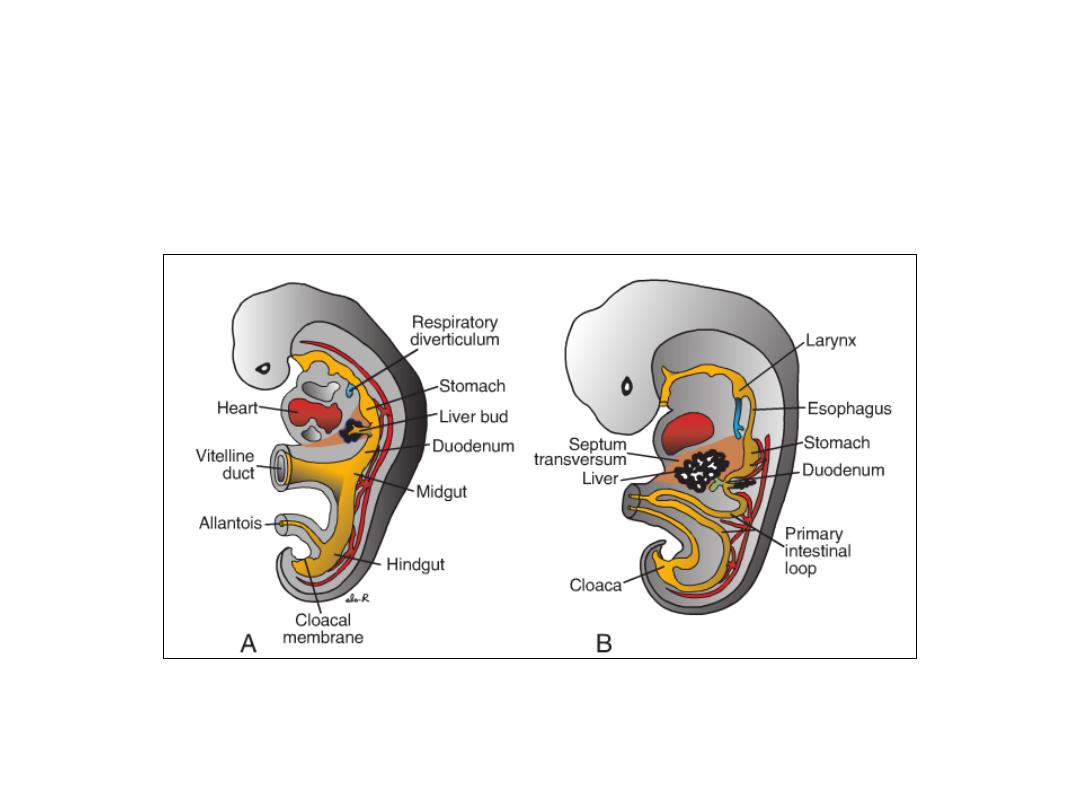
Figure: Sagittal sections
through embryos at
various stages of
development
demonstrating the effect of
cephalocaudal and lateral
folding on the position of
the endoderm-lined cavity.
Note formation of the
foregut, mid-gut, and
hindgut.
A. Presomite embryo.
B. Embryo with seven
somites.
C. Embryo with 14 somites.
D. At the end of the first
month.
• As a result of cephalo-caudal and lateral folding of the embryo, a portion
of the endoderm-lined yolk sac cavity is incorporated into the embryo to
form the primitive gut.
• Two other portions of the endoderm-lined cavity, the yolk sac and the
allantois, remain outside the embryo.
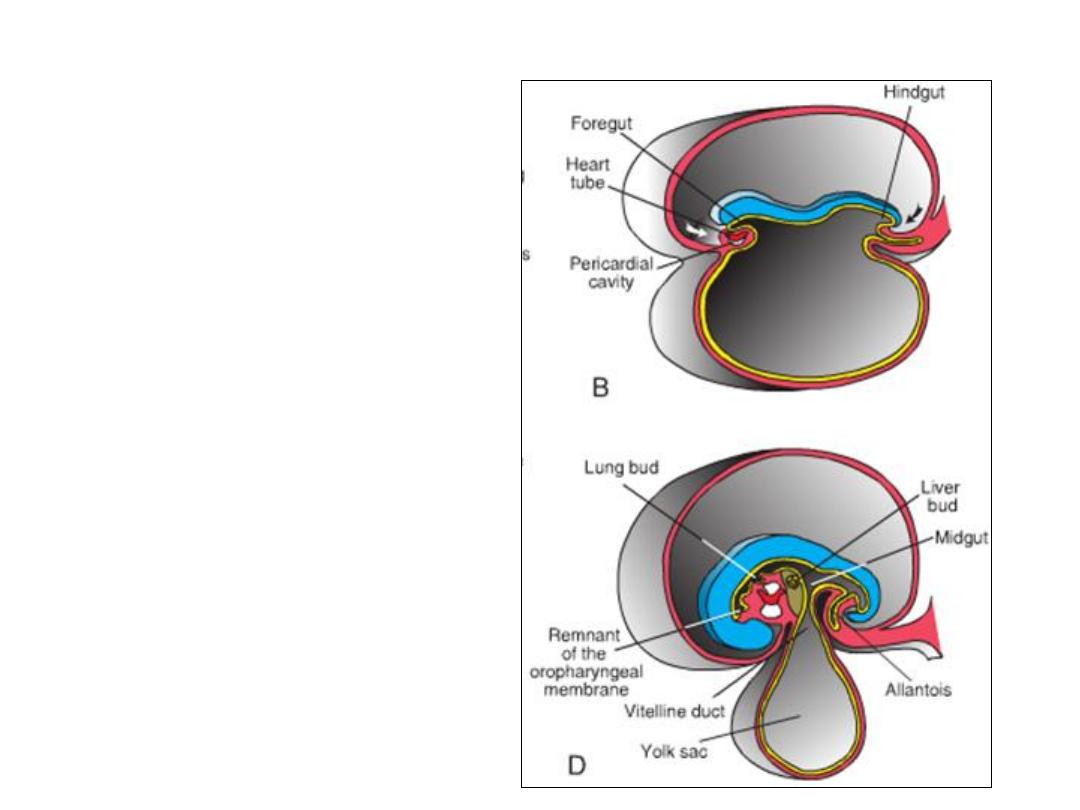
DIVISIONS OF THE GUT TUBE
•In the cephalic and caudal parts
of the embryo, the primitive gut
forms a blind-ending tube, the
foregut and hindgut,
respectively. The middle part,
the midgut, remains temporally
connected to the yolk sac by
means of the vitelline duct, or
yolk stalk.
1- THE FOREGUT:
•The pharyngeal gut, or pharynx,
extends from the oropharyngeal
membrane to the respiratory
diverticulum and is part of the
foregut.
•The remainder of the foregut
lies caudal to the pharyngeal
tube and extends as far caudally
as the liver outgrowth.
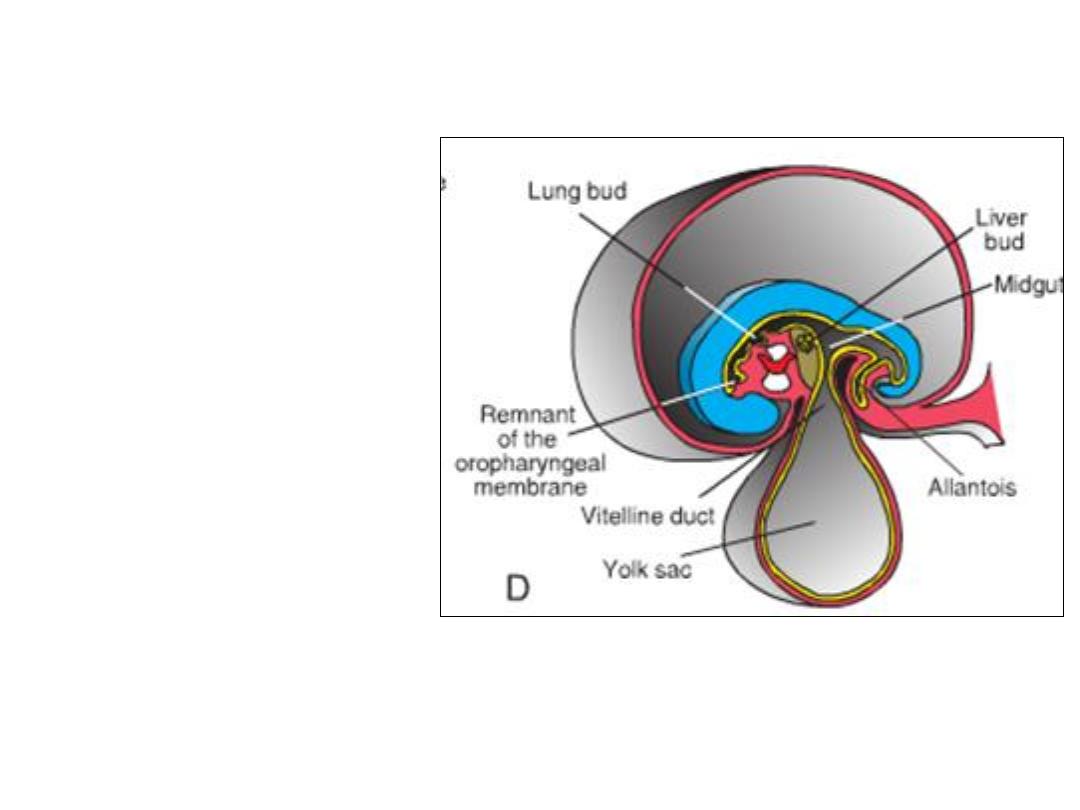
DIVISIONS OF THE GUT TUBE
2- THE MIDGUT begins caudal to
the liver bud and extends to
the junction of the right two
thirds and left third of the
transverse colon in the adult.
3- THE HINDGUT extends from
the left third of the transverse
colon to the cloacal
membrane.

• Endoderm: forms the epithelial lining of the digestive tract
and gives rise to the specific cells (the parenchyma) of glands,
such as hepatocytes and the exocrine and endocrine cells of
the pancreas.
• Visceral mesoderm: forms
– Muscle, connective tissue, and peritoneal components of the wall of
the gut
– stroma (connective tissue) for the glands

MOLECULAR REGULATION OF GUT TUBE DEVELOPMENT
1- Regional specification of the gut tube into different components occurs
during the time that the lateral body folds are bringing the two sides of
the tube together.
2- Specification is initiated by transcription factors expressed in the different
regions of the gut tube:
– SOX2 “specifies” the esophagus and stomach;
– PDX1 ,the duodenum;
– CDXC , the small intestine; and
– CDXA , the large intestine and rectum.
3- This initial patterning is stabilized by Epithelial–mesenchymal interaction
(between the endoderm and visceral mesoderm adjacent to the gut tube)
and is initiated by sonic hedgehog (SHH) expression throughout the gut
tube.
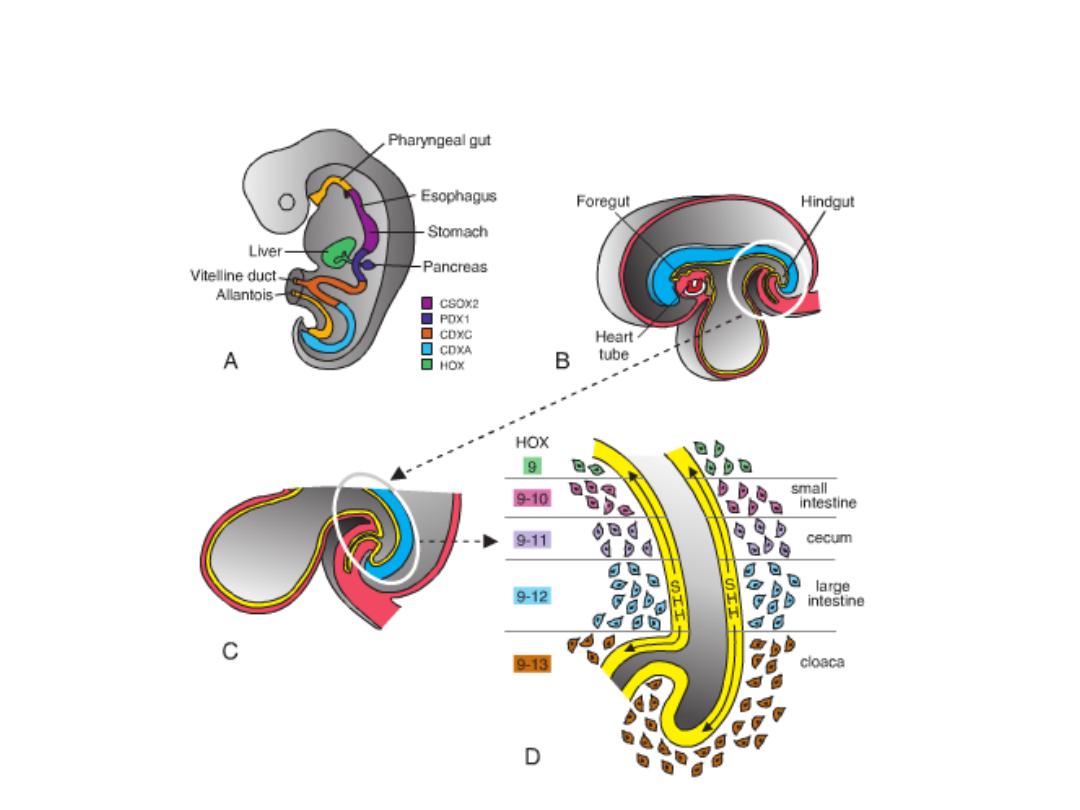
MOLECULAR REGULATION OF GUT TUBE
DEVELOPMENT

MESENTERIES
• Portions of the gut tube and its derivatives are suspended from the dorsal
and ventral body wall by mesenteries.
• Mesenteries: double layers of peritoneum that enclose an organ and
connect it to the body wall.
• Such organs are called intraperitoneal, whereas organs that lie against the
posterior body wall and are covered by peritoneum on their anterior
surface only (e.g., the kidneys) are considered retroperitoneal.
• Peritoneal ligaments are double layers of peritoneum (mesenteries) that
pass from one organ to another or from an organ to the body wall.
• Mesenteries and ligaments provide pathways for vessels, nerves, and
lymphatics to and from abdominal viscera.
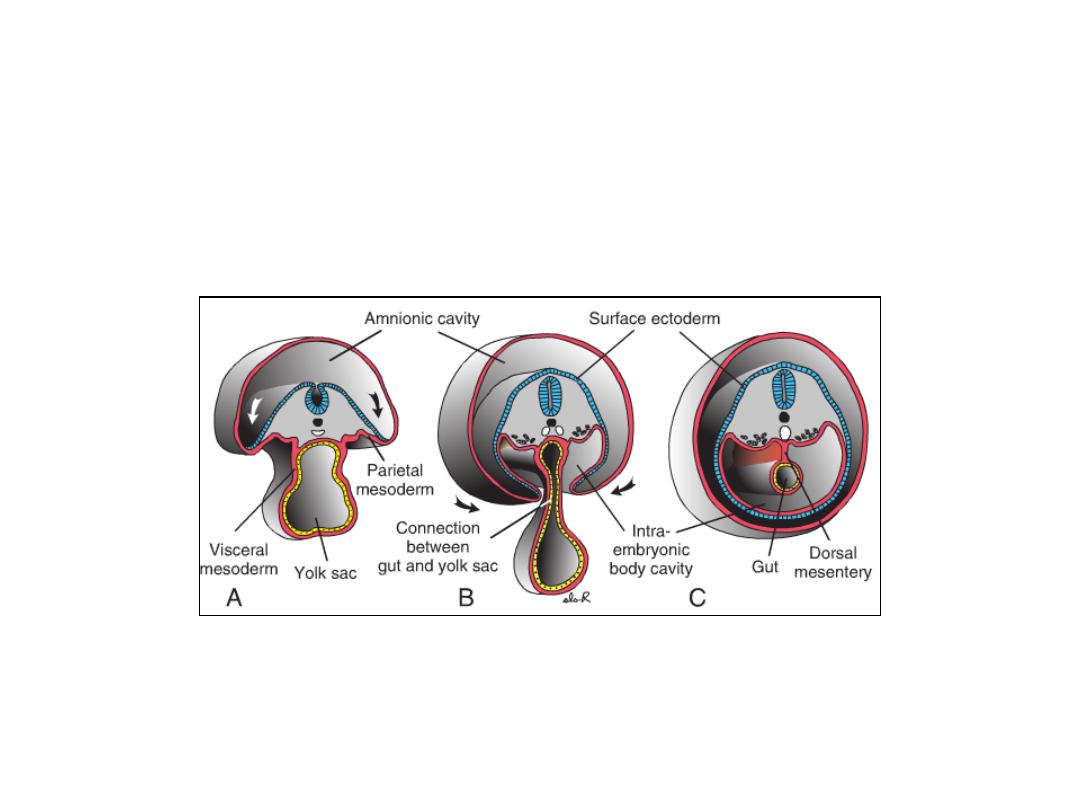
Figure: Transverse sections through embryos at various stages of development.
A. The intra-embryonic cavity, bordered by visceral and somatic layers of lateral plate mesoderm,
is in open communication with the extra embryonic cavity.
B. The intra-embryonic cavity is losing its wide connection with the extra embryonic cavity.
C. At the end of the fourth week, visceral mesoderm layers are fused in the midline and form a
double-layered membrane (dorsal mesentery) between right and left halves of the body cavity.
Ventral mesentery exists only in the region of the septum transversum (not shown).
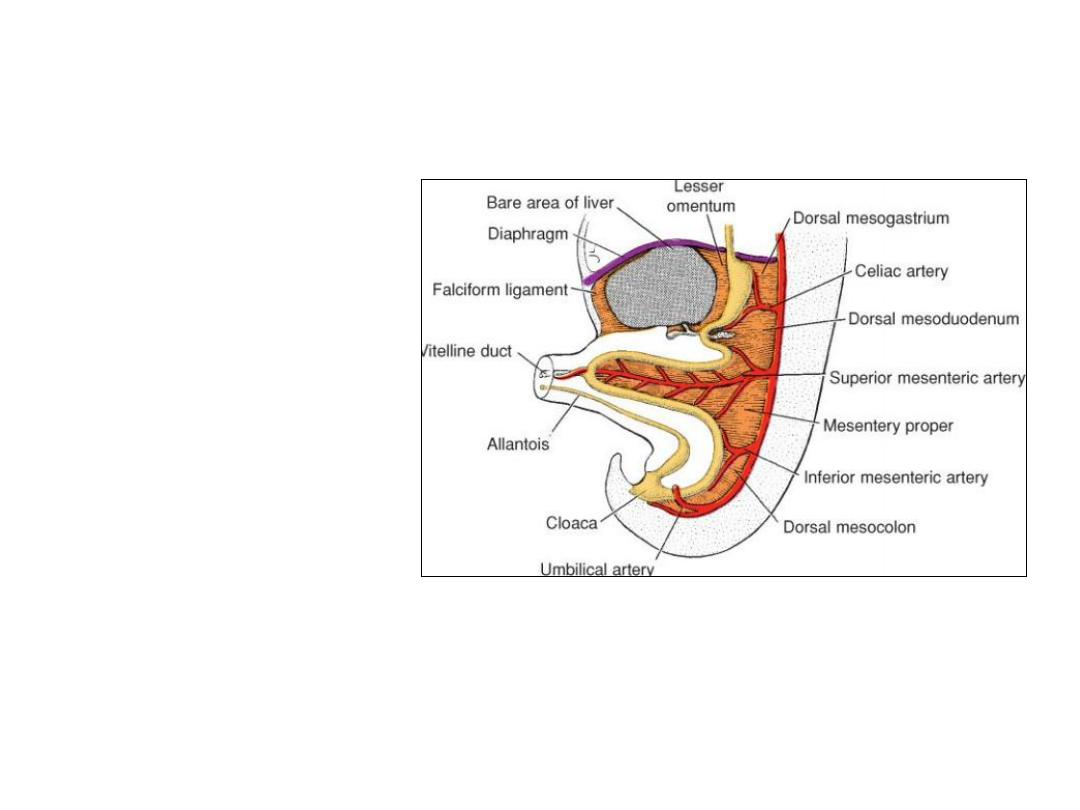
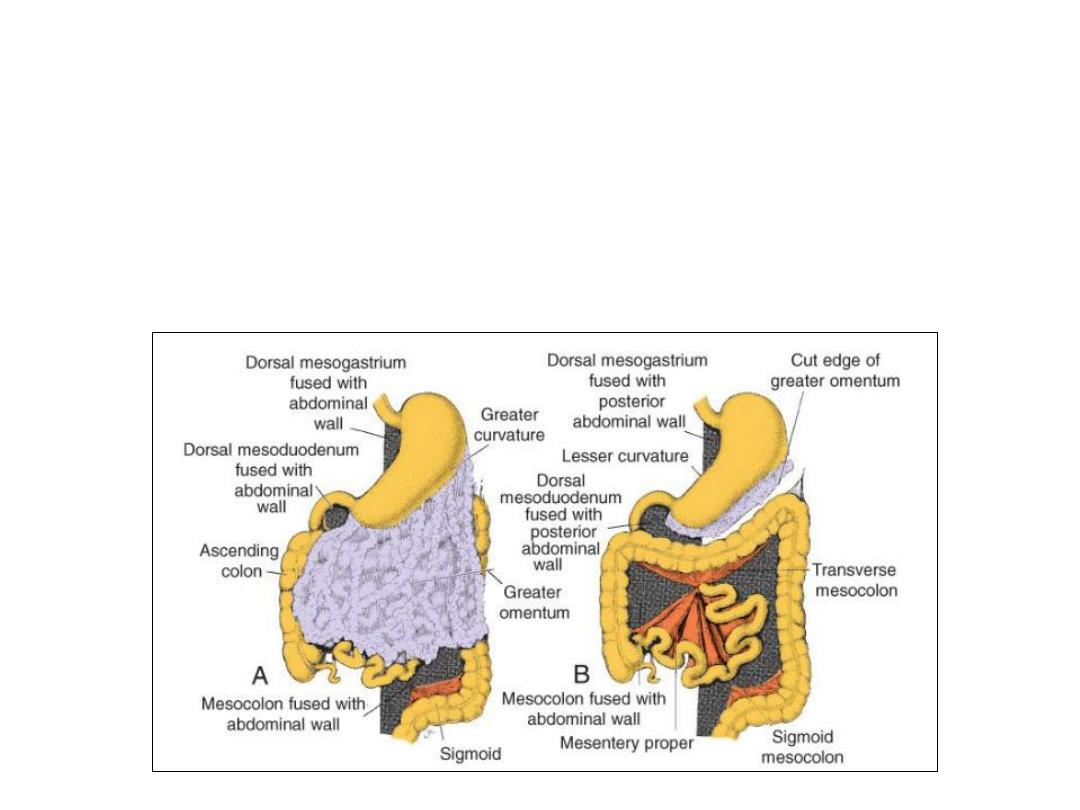
DORSAL MESENTRY
By the fifth week
THE DORSAL MESENTERY:
extends from the lower end of
the esophagus to the cloacal
region of the hindgut.
Dorsal mesogastrium or
greater omentum: In the
region of the stomach.
Dorsal mesoduodenum: In
the region of the duodenum
Mesentery proper: Dorsal
mesentery of the jejunum &
ileum.
Dorsal mesocolon: in the
region of the colon
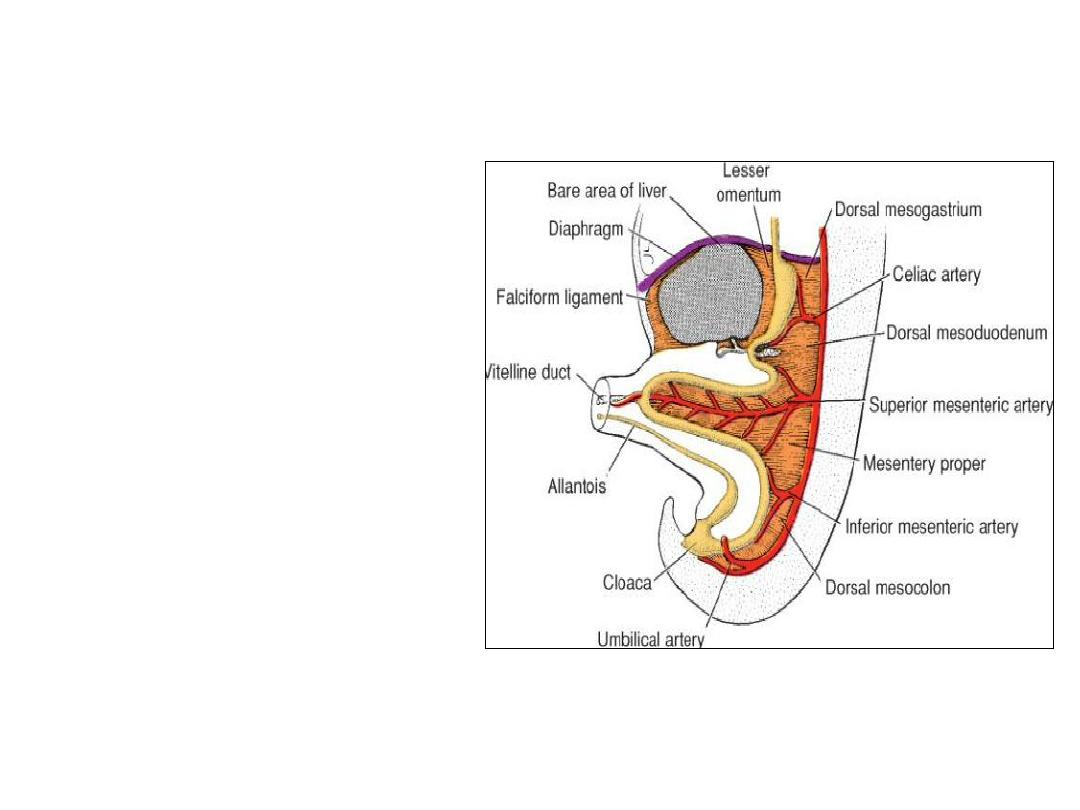
VENTRAL MESENTRY
Exists only in the region of the
terminal part of the esophagus,
the stomach, and the upper part
of the duodenum,
- Derived from the septum
transversum.
Growth of the liver into the
mesenchyme of the septum
transversum divides the ventral
mesentery into
(a) the lesser omentum,
extending from the lower portion
of the esophagus, the stomach,
and the upper portion of the
duodenum to the liver and
(b) the falciform ligament,
extending from the liver to the
ventral body wall.
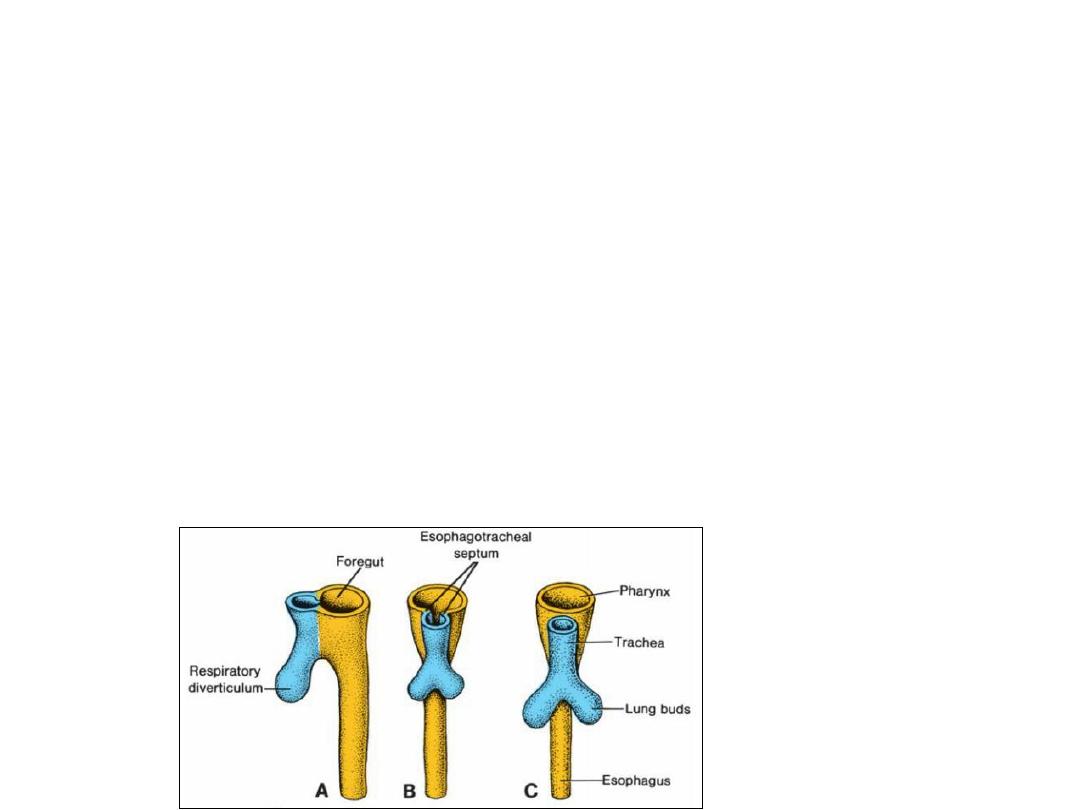
FOREGUT
•Esophagus
•When the embryo is approximately 4 weeks old, the respiratory diverticulum (lung bud)
appears at the ventral wall of the foregut at the border with the pharyngeal gut.
•The tracheo-esophageal septum gradually partitions this diverticulum from the dorsal part
of the foregut.
• In this manner, the foregut divides into a ventral portion, the respiratory primordium, and
a dorsal portion, the esophagus.
•At first, the esophagus is short, but with descent of the heart and lungs, it lengthens
rapidly.
•The muscular coat, which is formed by surrounding splanchnic mesenchyme, is striated in
its upper two thirds and innervated by the vagus; the muscle coat is smooth in the lower
third and is innervated by the splanchnic plexus.
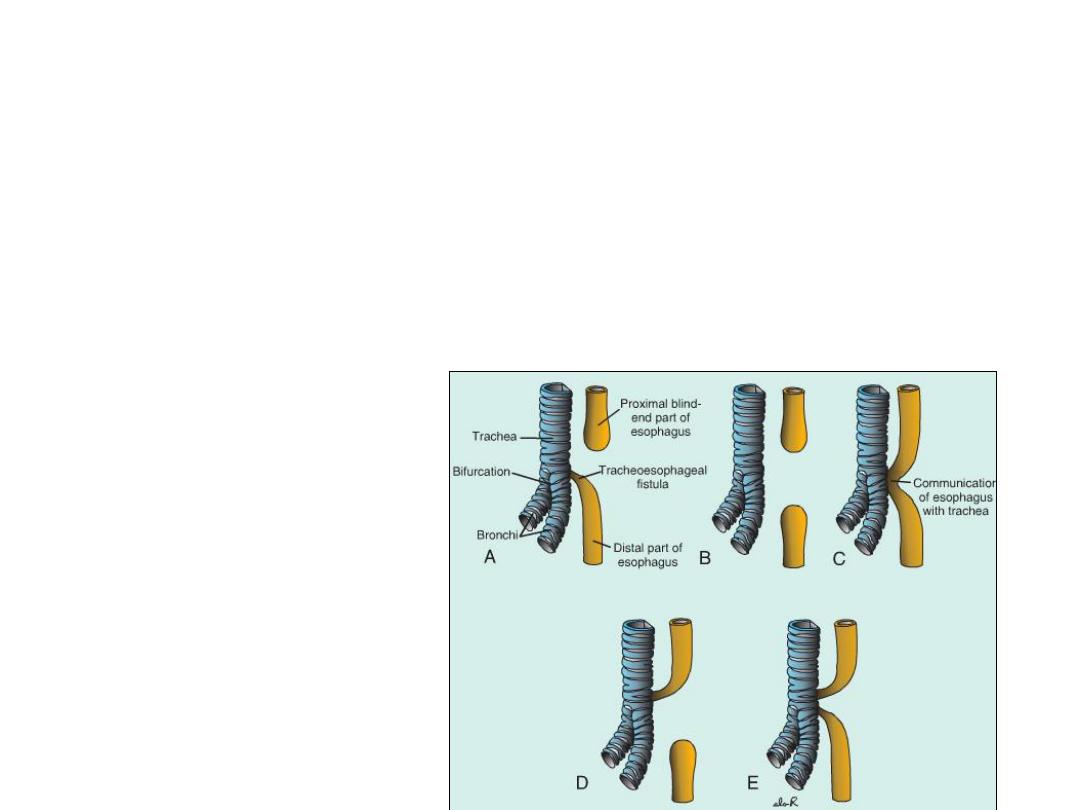
Figure: Variations of
esophageal atresia
and/or tracheo-
esophageal fistula in
order of their frequency
of appearance: A,90%;
B,4%; C,4%; D,1%; and
E,1%.
Clinical Correlates
Esophageal Abnormalities
Esophageal atresia and/or tracheoesophageal fistula results either from
spontaneous posterior deviation of the tracheoesophageal septum or from some
mechanical factor pushing the dorsal wall of the foregut anteriorly.
In its most common form, the proximal part of the esophagus ends as a blind
sac, and the distal part is connected to the trachea by a narrow canal just above
the bifurcation.
Atresia of the esophagus prevents normal passage of amniotic fluid into the
intestinal tract, resulting in accumulation of excess fluid in the amniotic sac
(polyhydramnios) .

• The lumen of the esophagus may narrow, producing esophageal stenosis,
usually in the lower third. Stenosis may be caused by incomplete
recanalization, vascular abnormalities, or accidents that compromise
blood flow.
• Occasionally, the esophagus fails to lengthen sufficiently, and the stomach
is pulled up into the esophageal hiatus through the diaphragm. The result
is a congenital hiatal hernia.
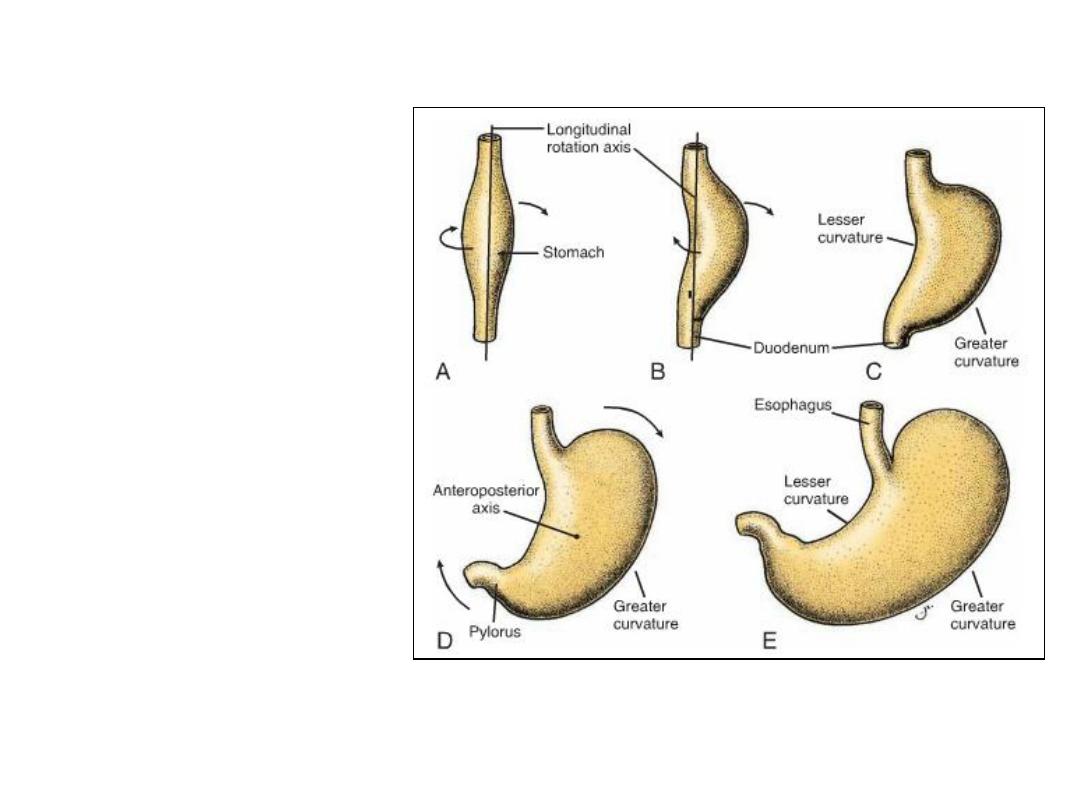
Stomach rotation
•The stomach appears as a fusiform
dilation of the foregut in the
fourth week of development.
•The stomach rotates 90°clockwise
around its longitudinal axis,
causing its left side to face
anteriorly and its right side to face
posteriorly.
•Hence, the left vagus nerve,
initially innervating the left side of
the stomach, now innervates the
anterior wall; similarly, the right
nerve innervates the posterior
wall.
•During this rotation, the original
posterior wall of the stomach
grows faster than the anterior
portion, forming the greater and
lesser curvatures.
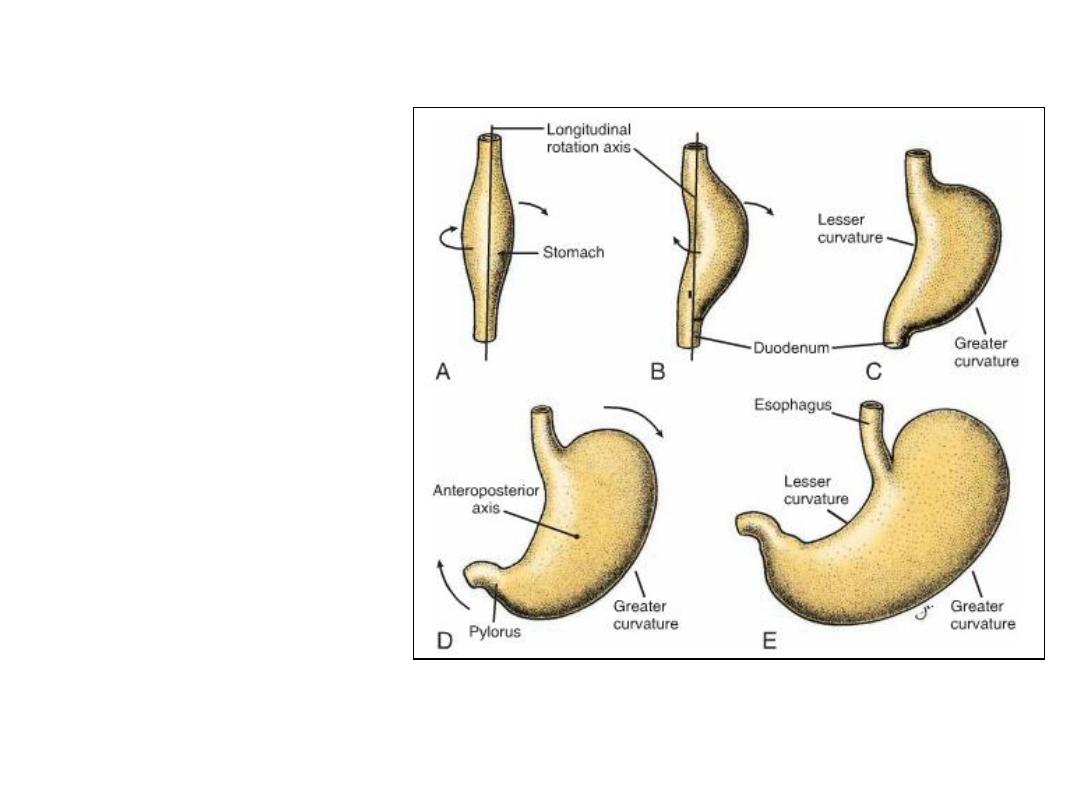
Stomach rotation
•The cephalic and caudal
ends of the stomach
originally lie in the midline,
but during further growth,
the stomach rotates
around an anteroposterior
axis, such that the caudal
or pyloric part moves to
the right and upward, and
the cephalic or cardiac
portion moves to the left
and slightly downward.
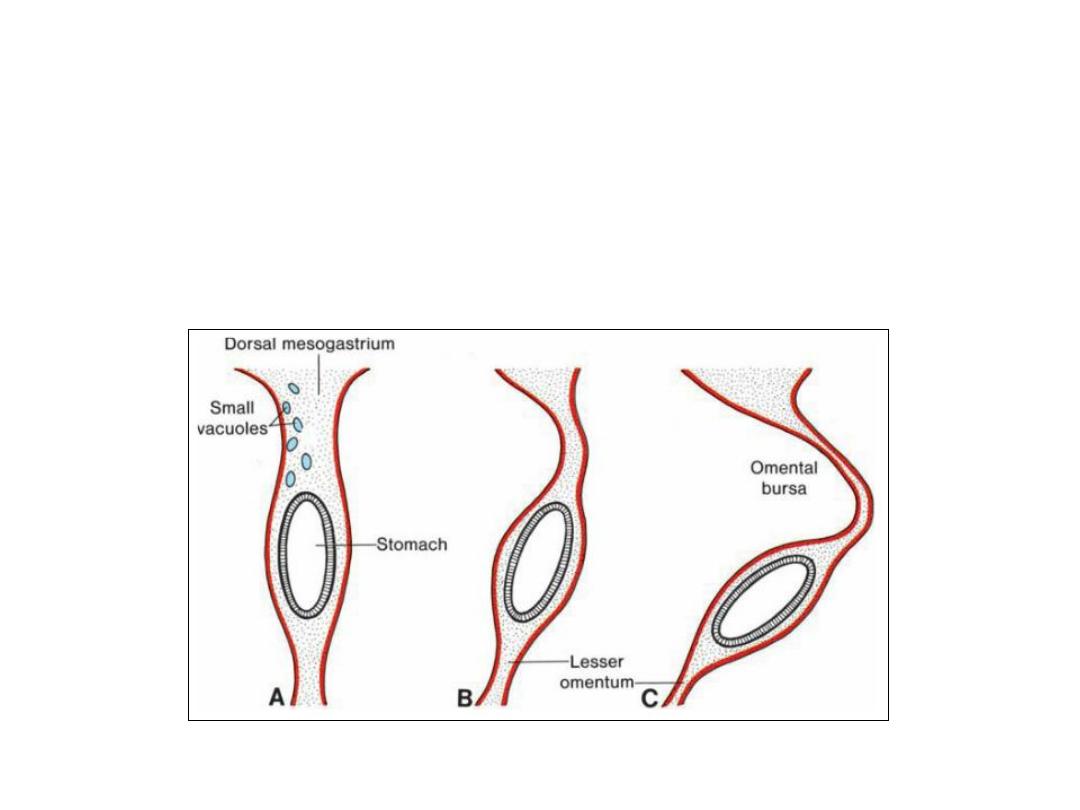
Formation of omental bursa (lesser sac)
• Rotation about the longitudinal axis pulls the dorsal mesogastrium to the
left, creating a space behind the stomach called the omental bursa (lesser
peritoneal sac).
• This rotation also pulls the ventral mesogastrium to the right.
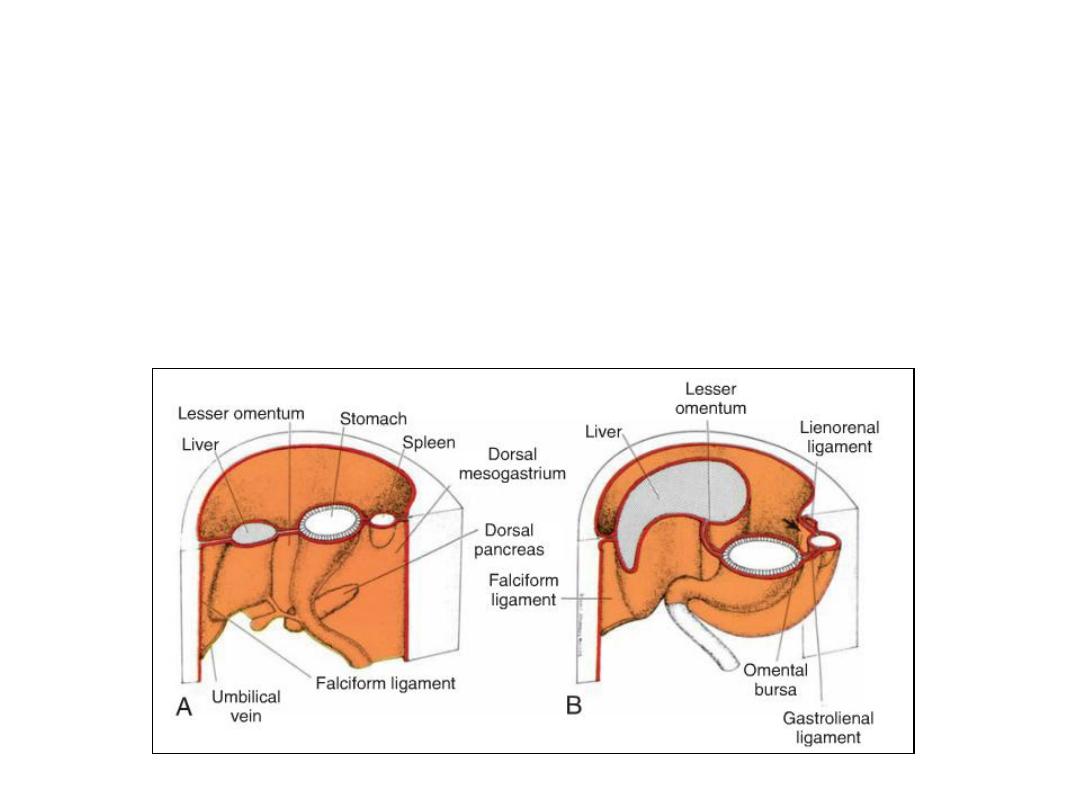
Spleen development
• The spleen primordium appears as a mesodermal proliferation between
the two leaves of the dorsal mesogastrium.
• With continued rotation of the stomach, the spleen, which remains
intraperitoneal, is then connected to the body wall in the region of the
left kidney by the lienorenal ligament and to the stomach by the
gastrolienal ligament.
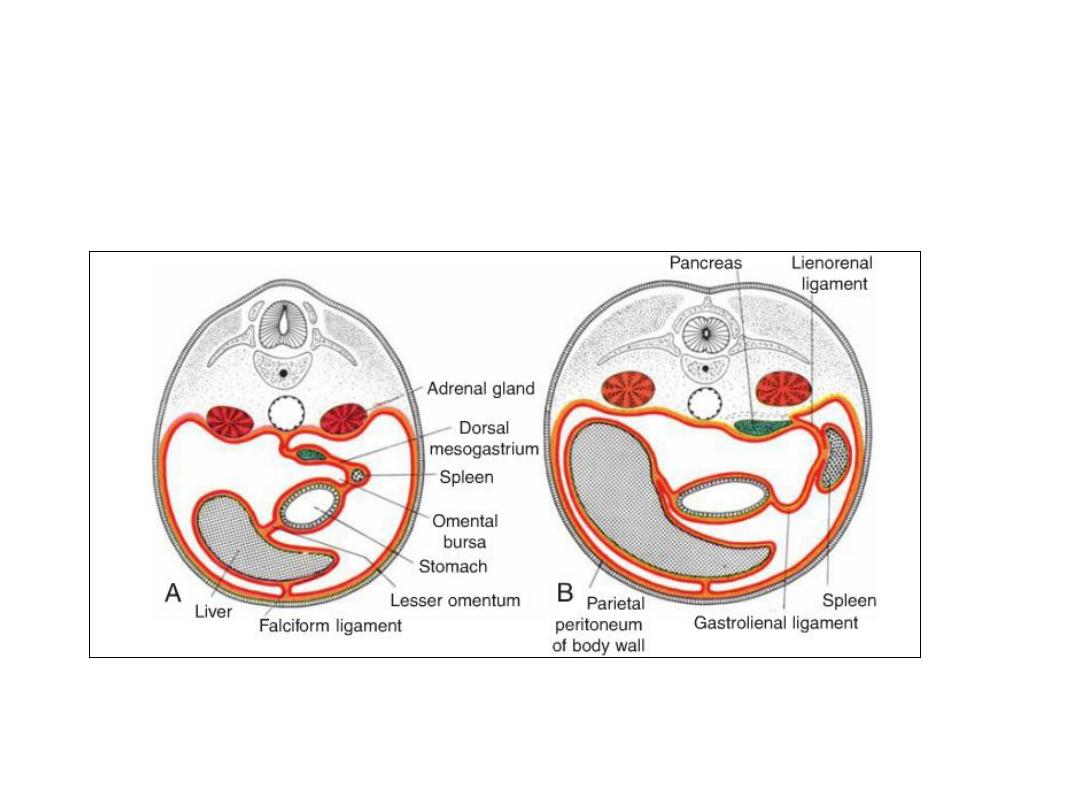
Organs, such as the pancreas, that are originally covered by
peritoneum, but later fuse with the posterior body wall to become
retroperitoneal, are said to be secondarily retroperitoneal.
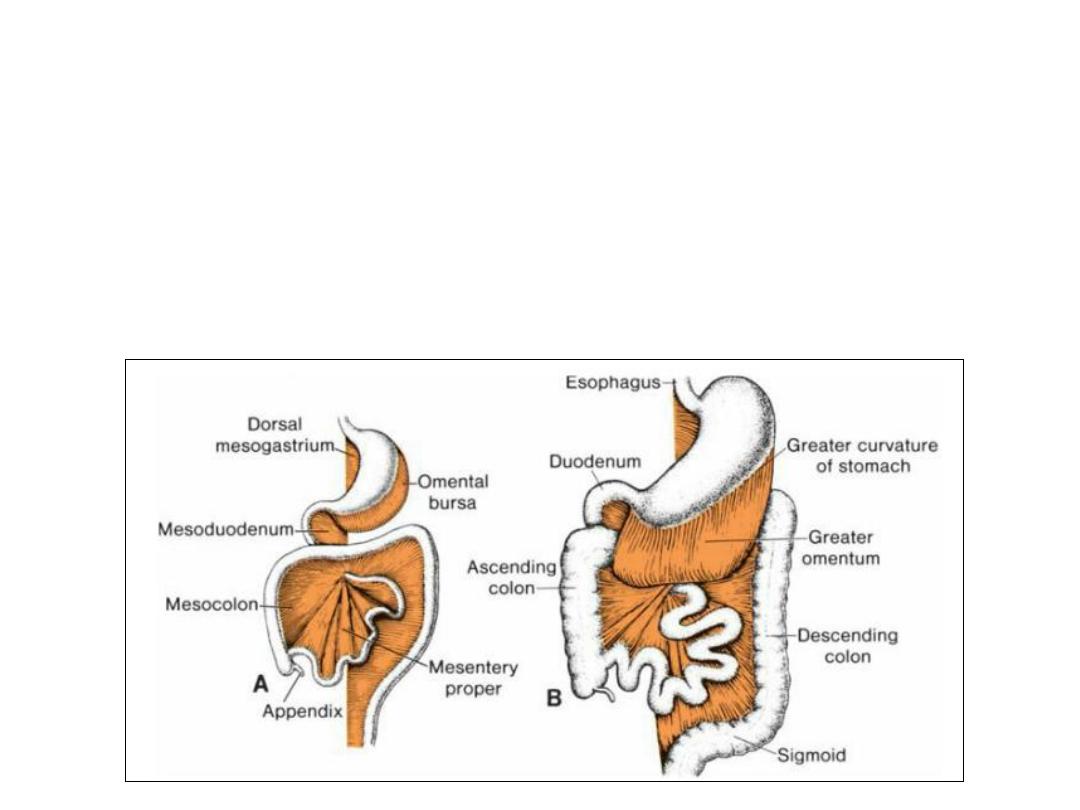
Formation of greater omentum
• As a result of rotation of the stomach about its anteroposterior axis, the
dorsal mesogastrium bulges down. It continues to grow down and forms a
double-layered sac extending over the transverse colon and small
intestinal loops like an apron. This double-leafed apron is the greater
omentum; later, its layers fuse to form a single sheet hanging from the
greater curvature of the stomach.
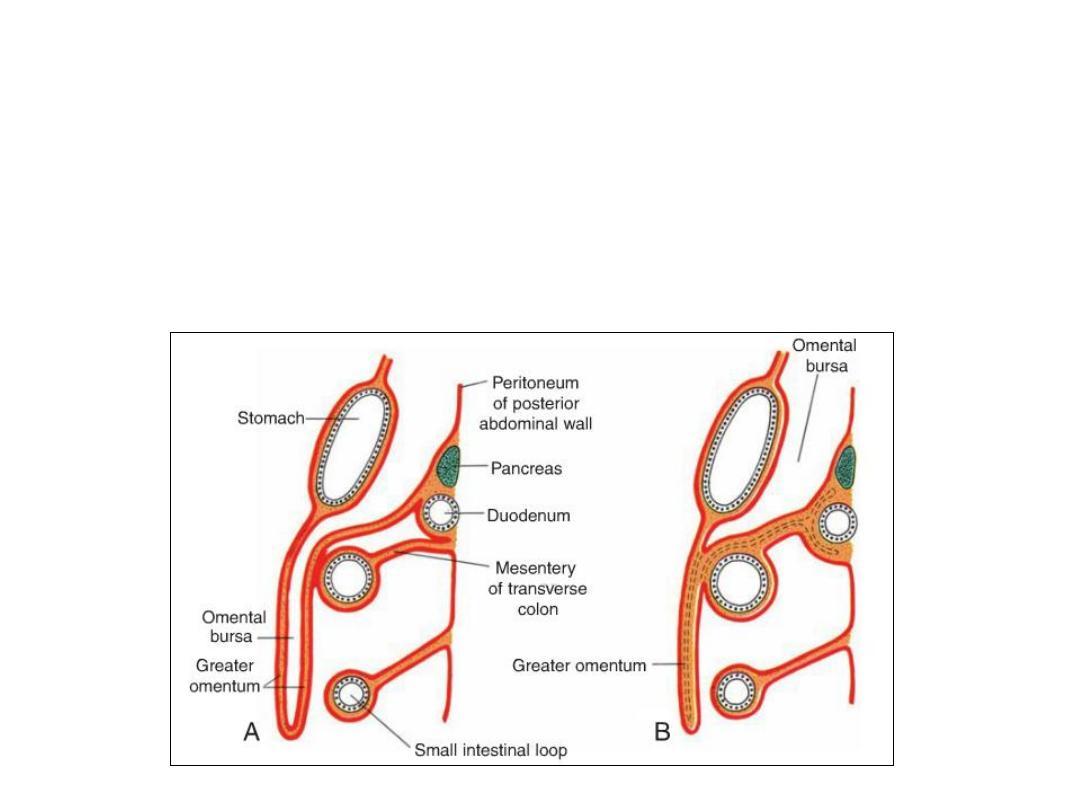
Formation of greater omentum
• The posterior layer of the greateromentum also fuses with the mesentery
of the transverse colon.
The lesser omentum and falciform ligament form from the ventral mesogastrium,
which itself is derived from mesoderm of the septum transversum.
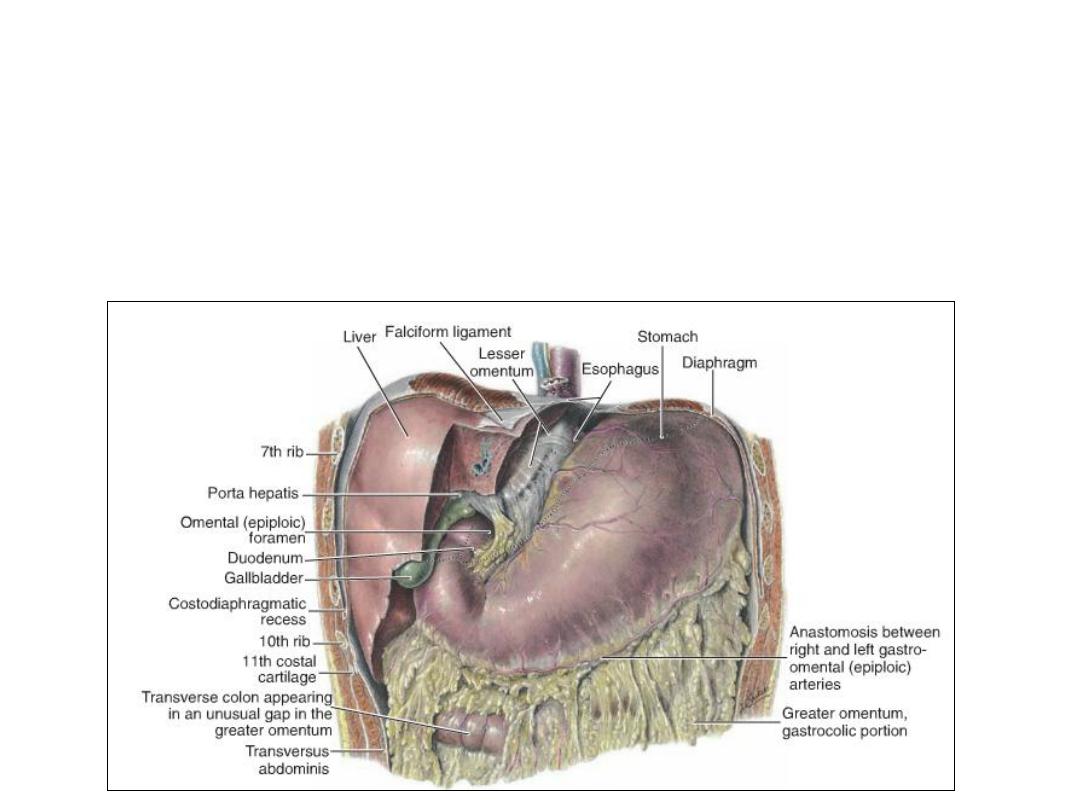
• The free margin of the falciform ligament contains the umbilical vein, which
is obliterated after birth to form the round ligament of the liver (ligamentum
teres hepatis) .
• The free margin of the lsser omentum connecting the duodenum and liver
(hepatoduodenal ligament) contains the bile duct, portal vein, and hepatic
artery (portaltriad) This free margin also forms the roof of the epiploic
foramen of Winslow, which is the opening connecting the omental bursa
(lesser sac) with the rest of the peritoneal cavity (greater sac).

Clinical Correlates
• Stomach Abnormalities
• Pyloric stenosis: occurs when the circular and, to a lesser degree, the
longitudinal musculature of the stomach in the region of the pylorus
hypertrophies. One of the most common abnor-malities of the stomach in
infants, pyloric steno-sis is believed to develop during fetal life. There is an
extreme narrowing of the pyloric lumen, and the passage of food is
obstructed, resulting in severe vomiting. In a few cases, the pylorus is
atretic.
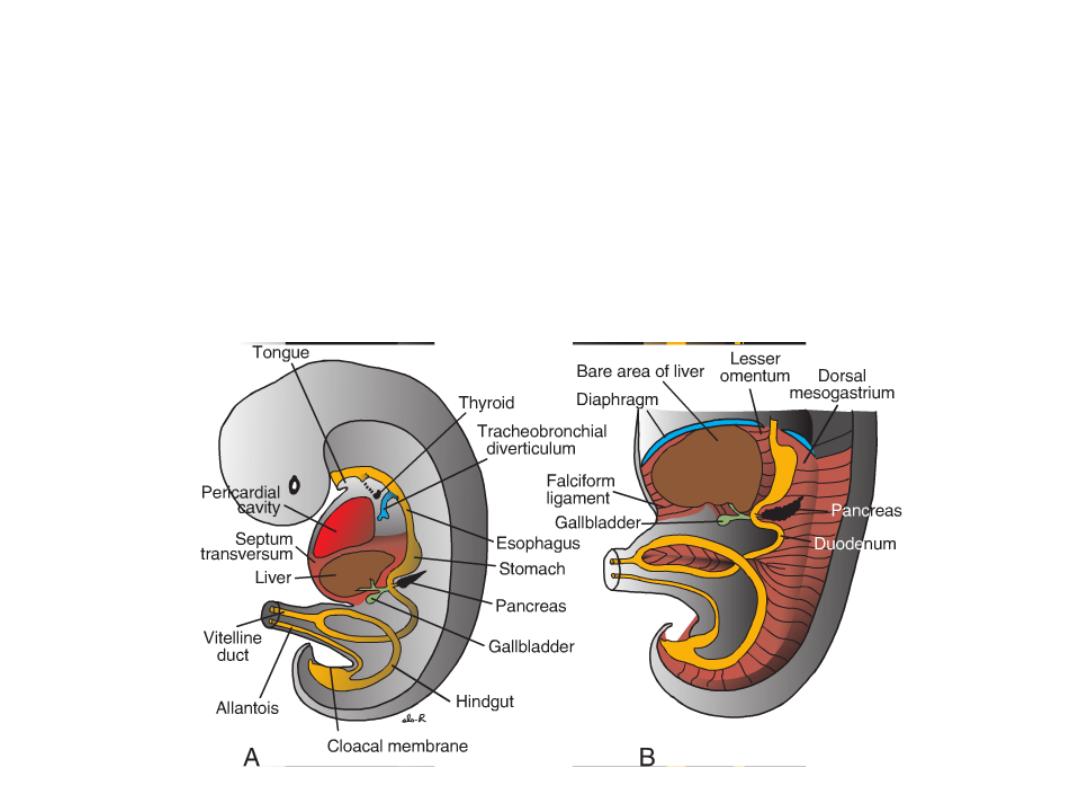
Duodenum
The terminal part of the foregut and the cephalic part of the midgut
form the duodenum. The junction of the two parts is directly distal to
the origin of the liver bud.
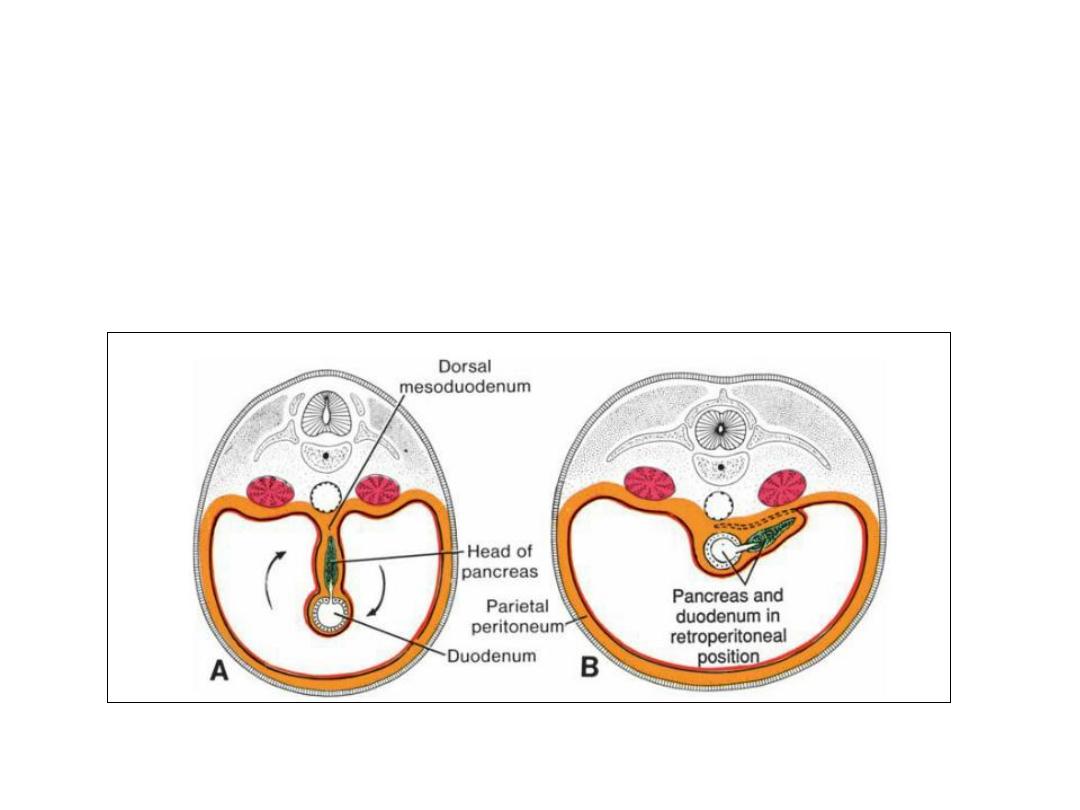
Duodenum
As the stomach rotates, the duodenum takes on the form of a C-
shaped loop and rotates to the right. This rotation, together with rapid
growth of the head of the pancreas, swings the duodenum from its
initial midline position to the right side of the abdominal cavity .
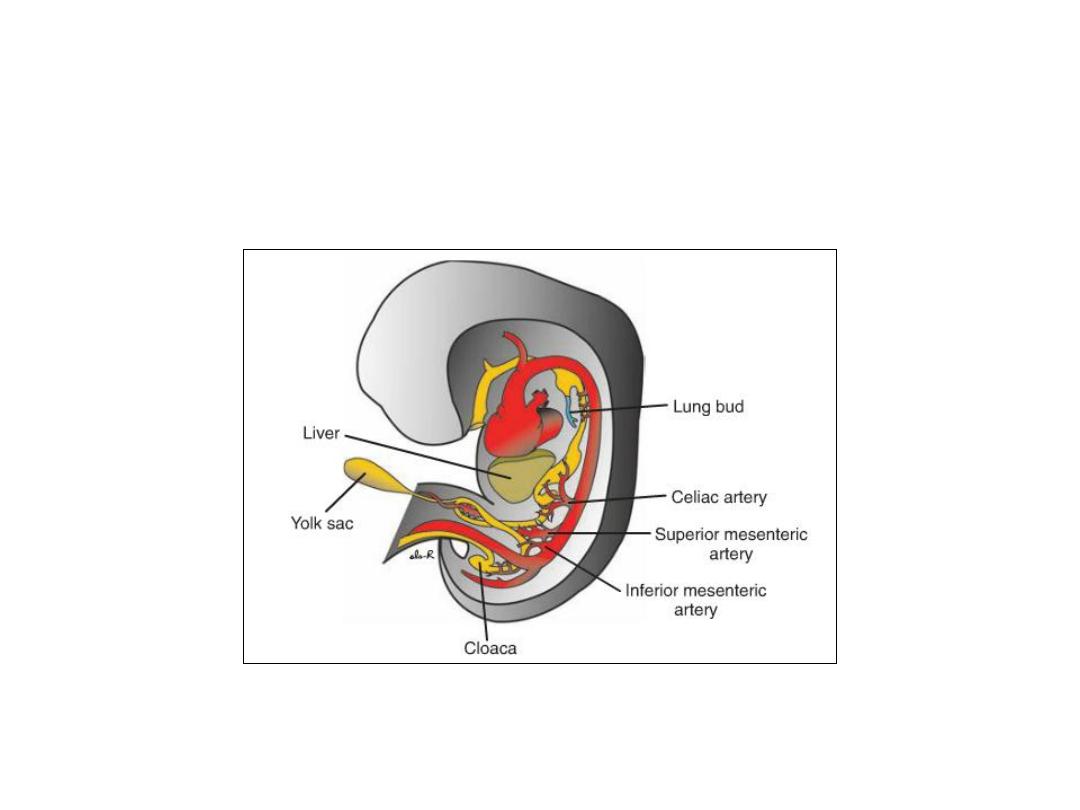
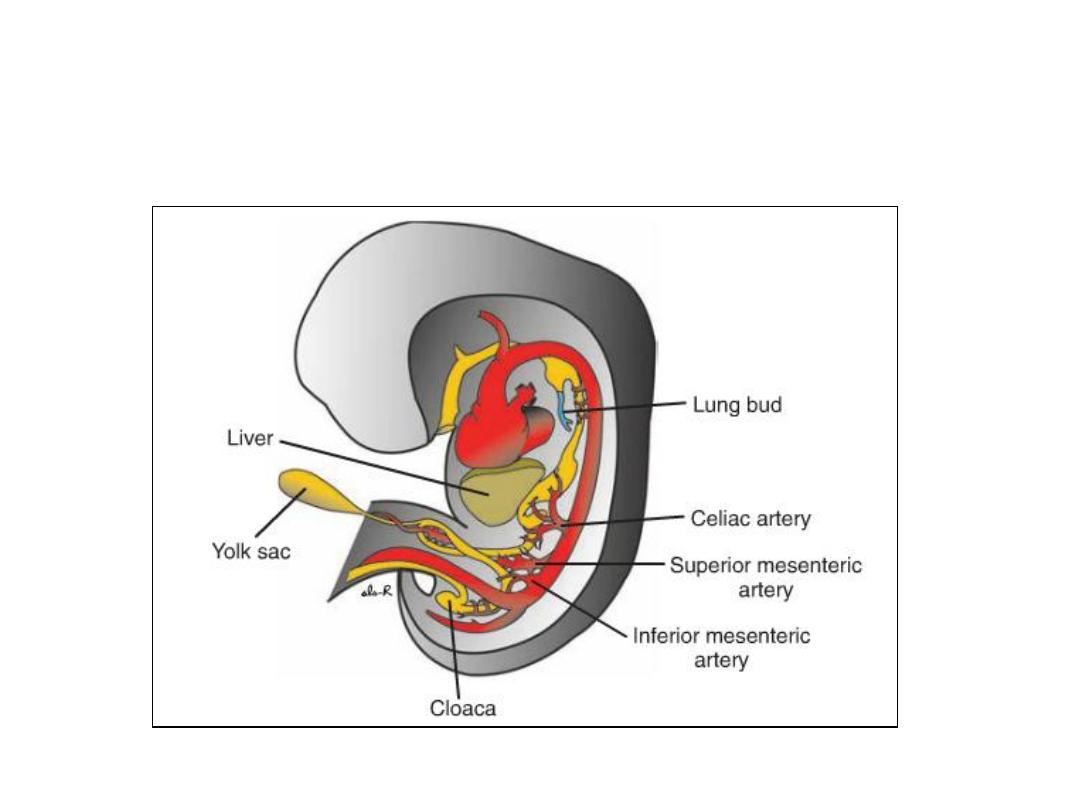
Duodenum
• Since the foregut is supplied by the celiac artery and the midgut is
supplied by the superior mesenteric artery, the duodenum is supplied by
branches of both arteries.
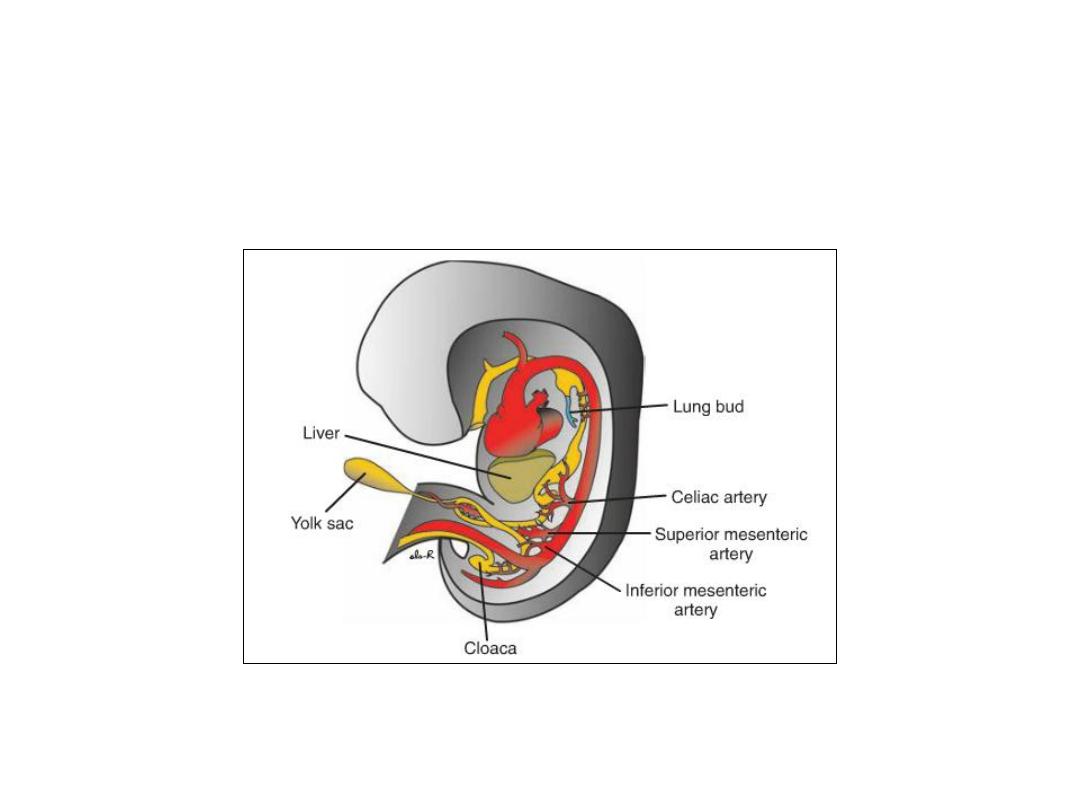
Figure: Embryo during the sixth week of development, showing blood
supply to the segments of the gut. The superior mesenteric artery supplies
the midgut. The celiac and inferior mesenteric arteries supply the foregut
and hindgut, respectively.
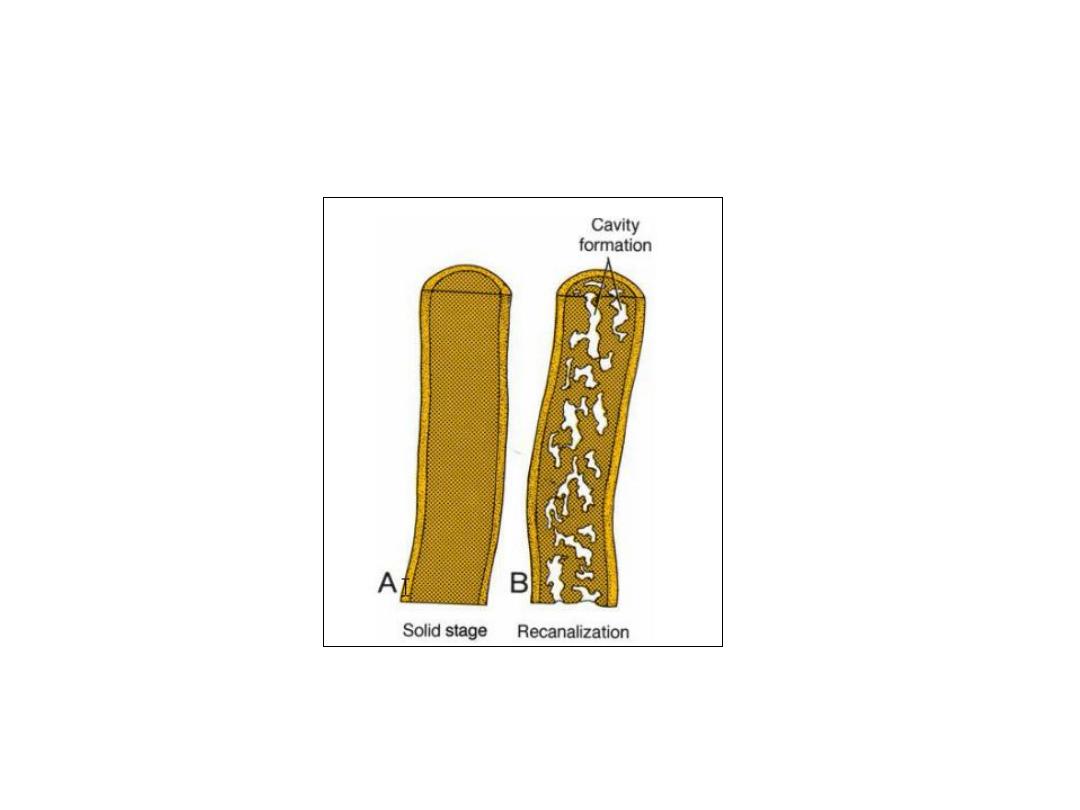
During the second month, the lumen of the duodenum is obliterated
by proliferation of cells in its walls. However, the lumen is recanalized
shortly thereafter.
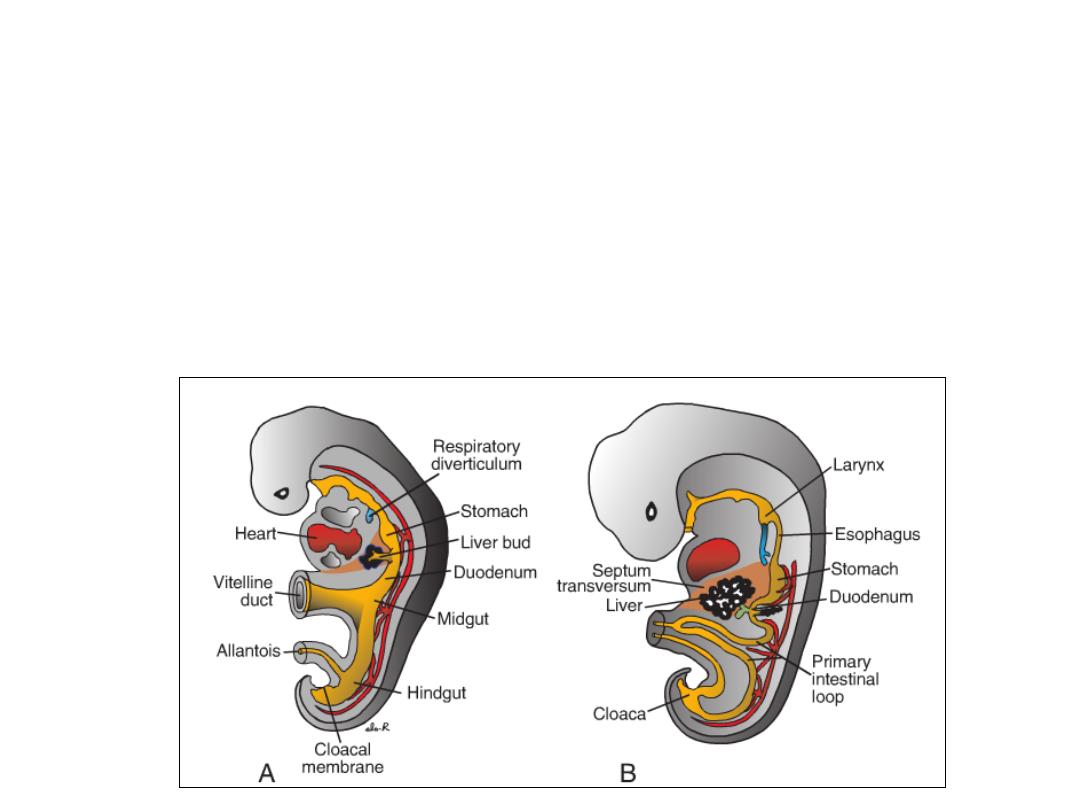
Liver & gall bladder
•
The liver primordium appears in the middle of the third week as an outgrowth of the
endodermal epithelium at the distal end of the foregut.
•
This outgrowth, the hepatic diverticulum, or liver bud, consists of rapidly proliferating cells
that penetrate the septum transversum, that is, the mesodermal plate between the
pericardial cavity and the stalk of the yolk sac.
•
While hepatic cells continue to penetrate the septum, the connection between the hepatic
diverticulum and the foregut (duodenum) narrows, forming the bile duct.
•
A small ventral outgrowth is formed by the bile duct, and this outgrowth gives rise to the gall
bladder and the cystic duct.
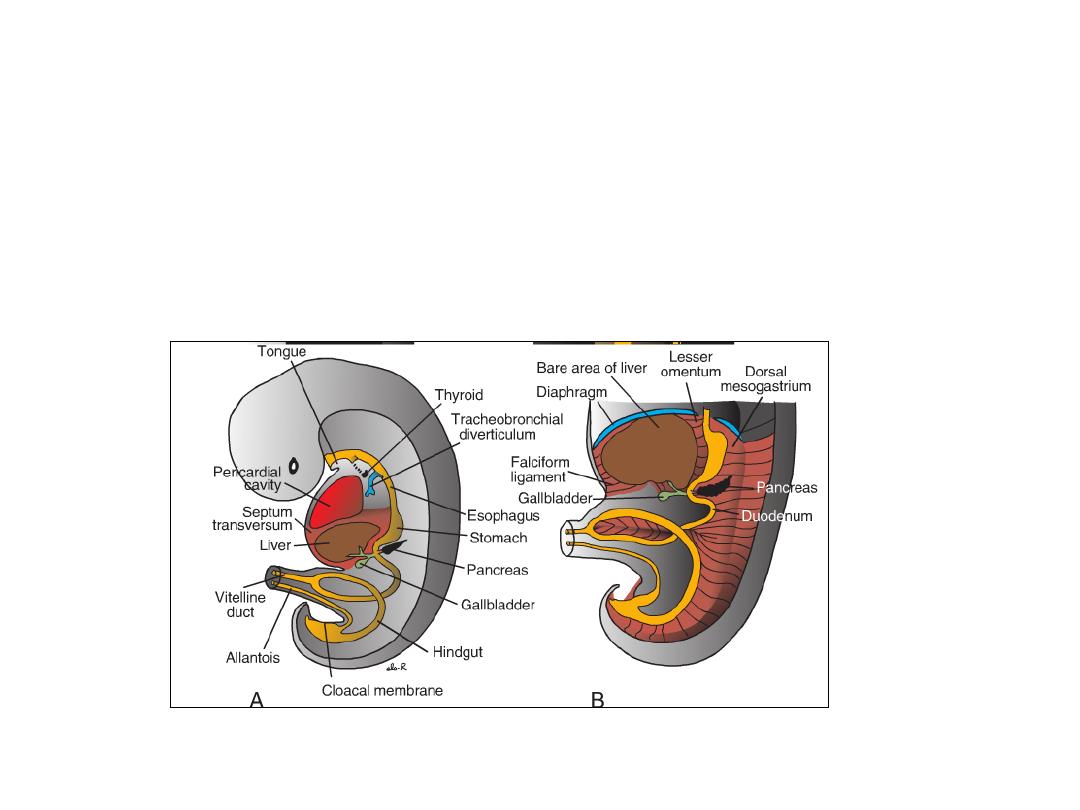
• During further development, epithelial liver cords intermingle with the
vitelline and umbilical veins, which form hepatic sinusoids.
• Liver cords differentiate into the parenchyma (liver cells) and form the
lining of the biliary ducts (endoderm).
• Hematopoietic cells, Kupffer cells, and connective tissue cells are derived
from mesoderm of the septum transversum.

• In this region, the liver remains in contact with the rest of the original
septum transversum. This portion of the septum, which consists of
densely packed mesoderm, will form the central tendon of the
diaphragm.
• The surface of the liver that is in contact with the future diaphragm is
never covered by peritoneum; it is the bare area of the liver.
• Hematopoietic function: Large nests of proliferating cells, which produce
red and white blood cells, lie between hepatic cells and walls of the
vessels. This activity gradually subsides during the last 2 months of
intrauterine life, and only small hematopoietic islands remain at birth.
• Another important function of the liver begins at approximately the 12th
week, when bile is formed by hepatic cells.
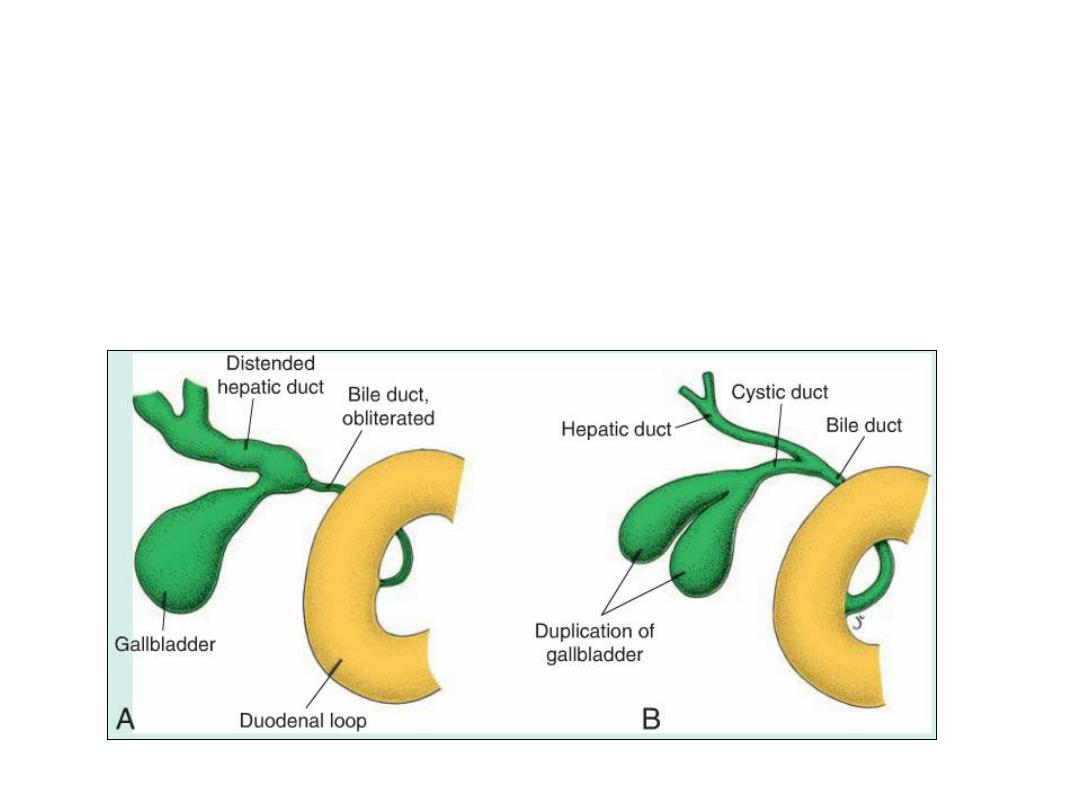
Clinical correlates
• Accessory hepatic ducts
• Duplication of the gallbladder
• Extrahepatic biliary atresia,
• Intrahepatic biliary duct atresia and hypoplasia.
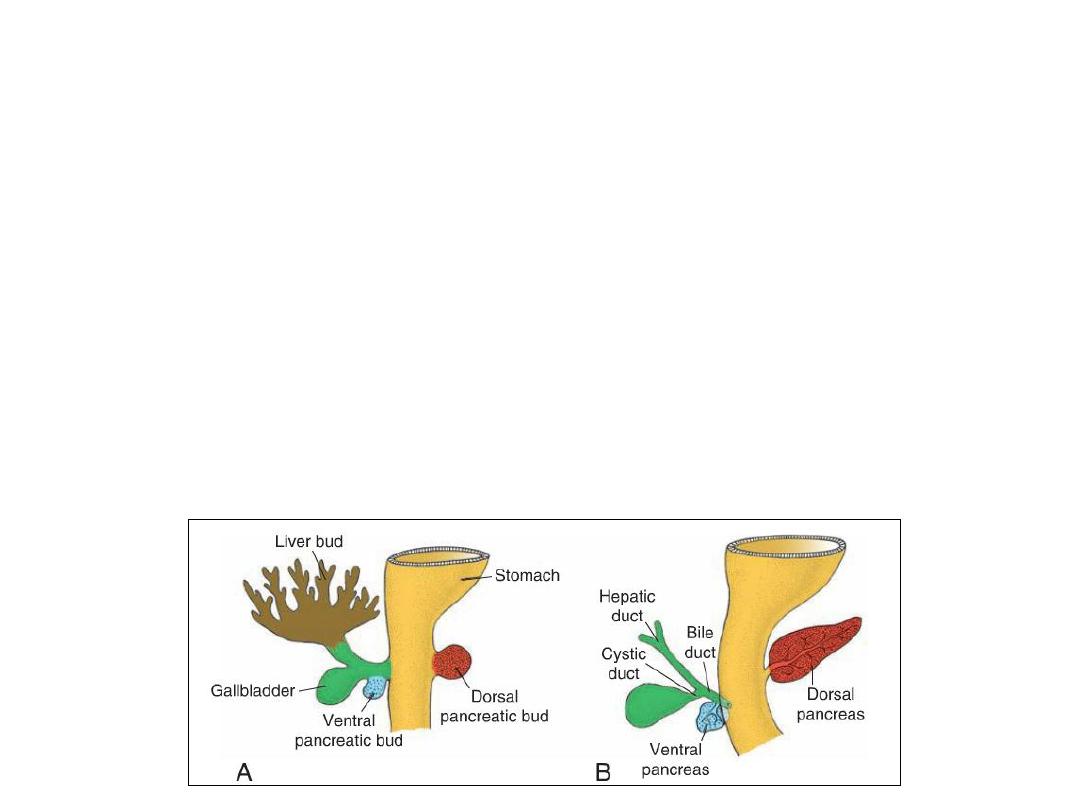
PANCREAS
•
The pancreas is formed by two buds, dorsal and ventral, originating from the
endodermal lining of the duodenum.
•
Whereas the dorsal pancreatic bud is in the dorsal mesentery, the ventral
pancreatic bud is close to the bile duct.
•
When the duodenum rotates to the right and becomes C-shaped, the ventral
pancreatic bud moves dorsally in a manner similar to the shifting of the entrance
of the bile duct.
•
Finally, the ventral bud comes to lie immediately below and behind the dorsal bud.
•
Later, the parenchyma and the duct systems of the dorsal and ventral pancreatic
buds fuse.
•
The ventral bud forms the uncinate process and inferior part of the head of the
pancreas. The remaining part of the gland is derived from the dorsal bud.
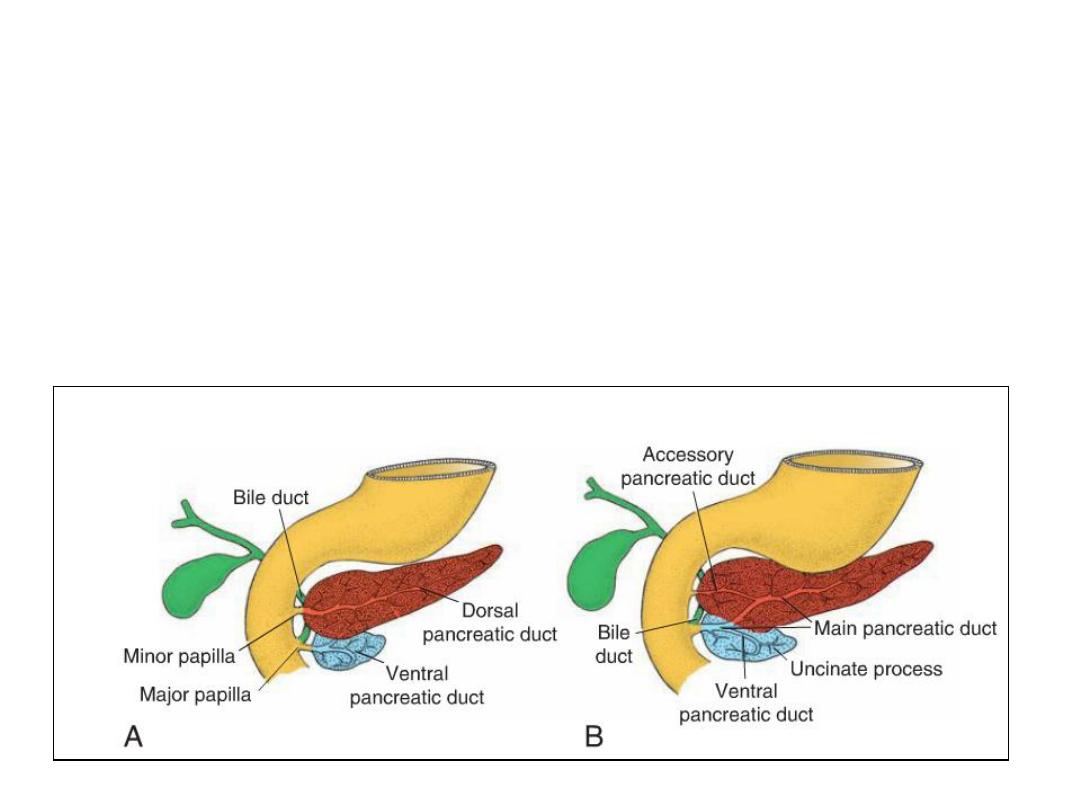
• The main pancreatic duct (of Wirsung) is formed by the distal part of the
dorsal pancreatic duct and the entire ventral pancreatic duct.
• The proximal part of the dorsal pancreatic duct either is obliterated or
persists as a small channel, the accessory pancreatic duct (of Santorini).
• The main pancreatic duct, together with the bile duct, enters the
duodenum at the site of the major papilla; the entrance of the accessory
duct (when present) is at the site of the minor papilla.
• In about 10% of cases, the duct system fails to fuse, and the original
double system persists.

Endocrine Pancreas
• In the third month of fetal life, pancreatic islets (of Langerhans ) develop
from the parenchymatous pancreatic tissue and scatter throughout the
pancreas.
• Insulin secretion begins at approximately the fifth month. Glucagon and
somatostatin-secreting cells also develop from parenchymal cells.
• Visceral mesoderm surrounding the pancreatic buds forms the pancreatic
connective tissue.
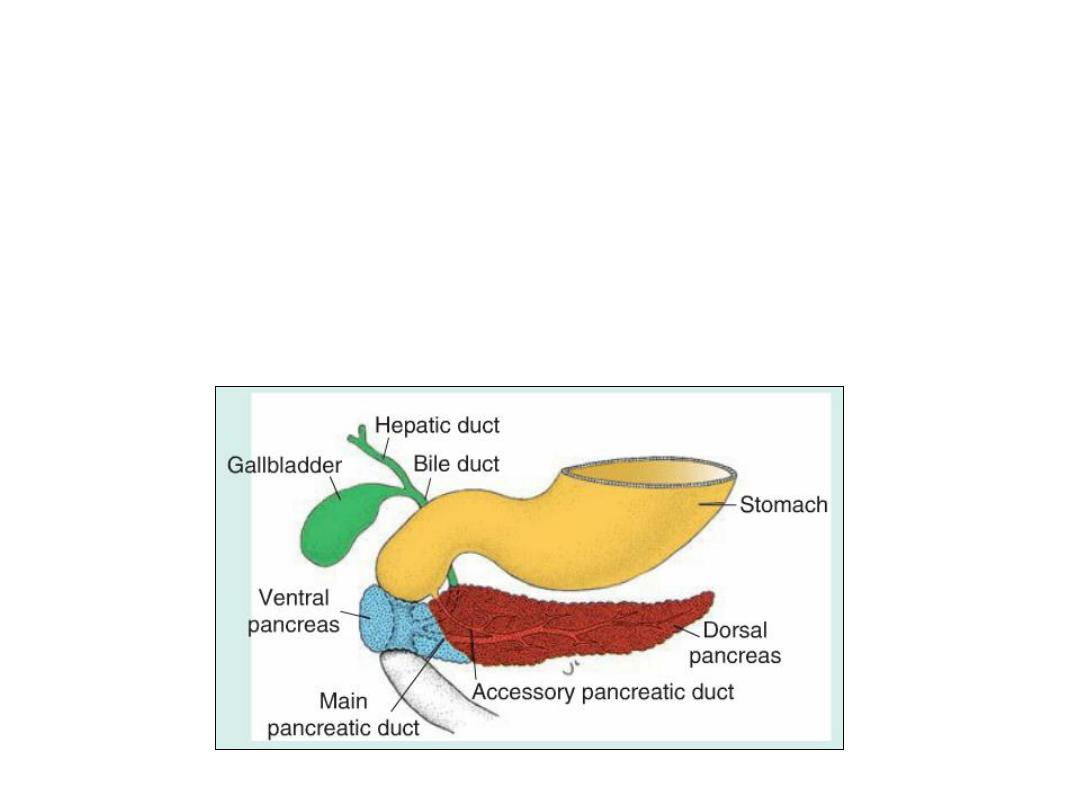
Clinical Correlates
• Annular pancreas
• Accessory pancreatic tissue

Ch. 15 – Part 2
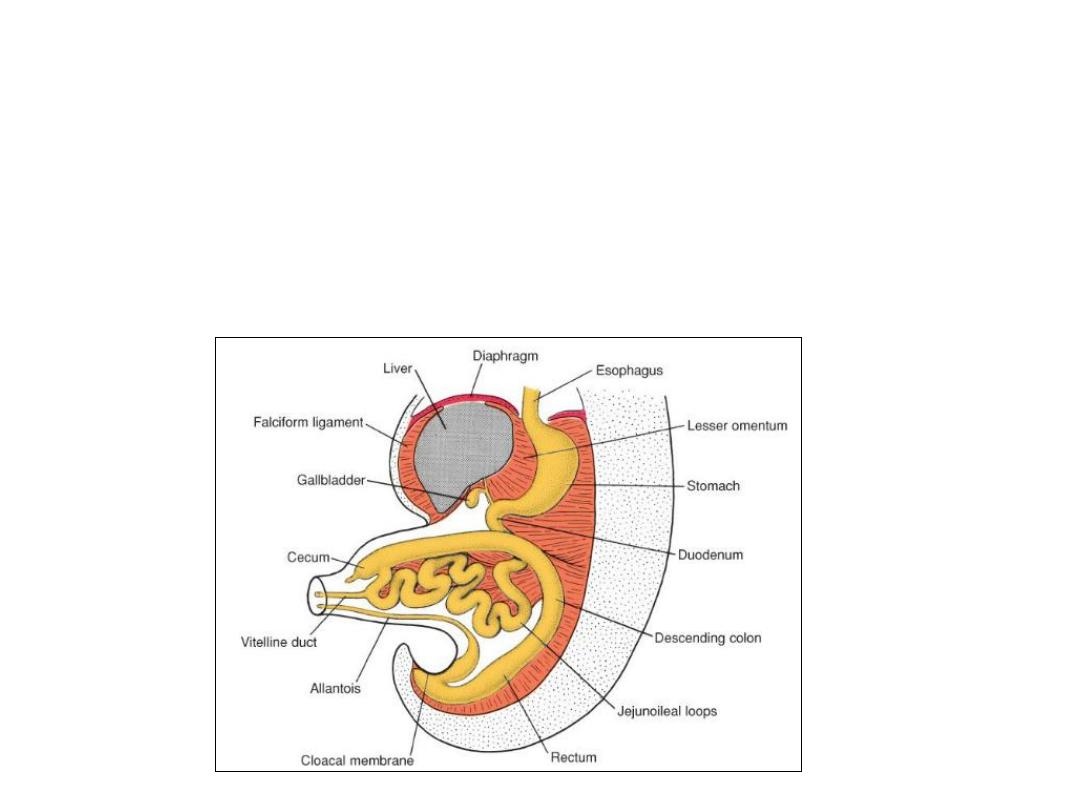
MIDGUT
• In the 5-week embryo, the midgut is suspended from the dorsal
abdominal wall by a short mesentery and communicates with the yolk sac
by way of the vitelline duct or yolk stalk .
• In the adult, the midgut begins immediately distal to the entrance of the
bile duct into the duodenum and terminates at the junction of the
proximal two thirds of the transverse colon with the distal third.
• Over its entire length, the midgut is supplied by the superior mesenteric
artery.
Physiological Herniation
• Development of the primary intestinal loop is characterized by rapid
elongation.
• As a result of the rapid growth and expansion of the liver, the abdominal
cavity temporarily becomes too small to contain all the intestinal loops,
and they enter the extraembryonic cavity in the umbilical cord during the
sixth week of development.
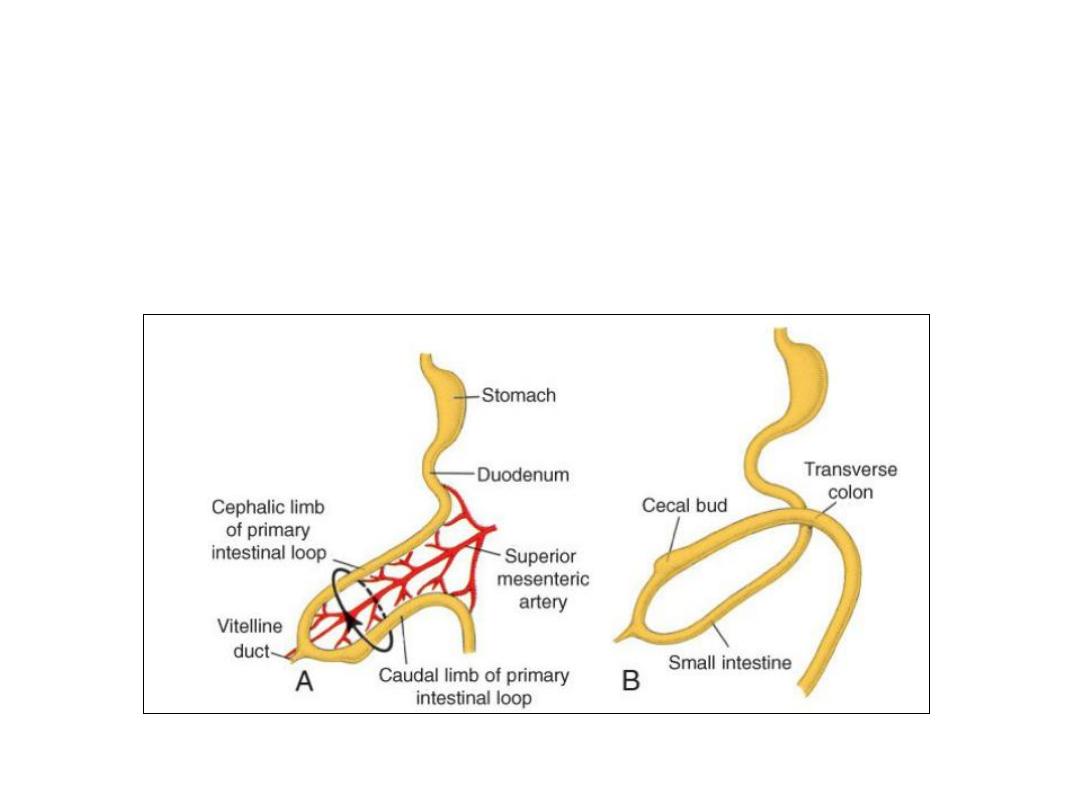
Rotation of the Midgut
• Coincident with growth in length, the primary intestinal loop rotates
around an axis formed by the superior mesenteric artery.
• This rotation is counter clockwise, and it amounts to approximately
270°when it is complete.
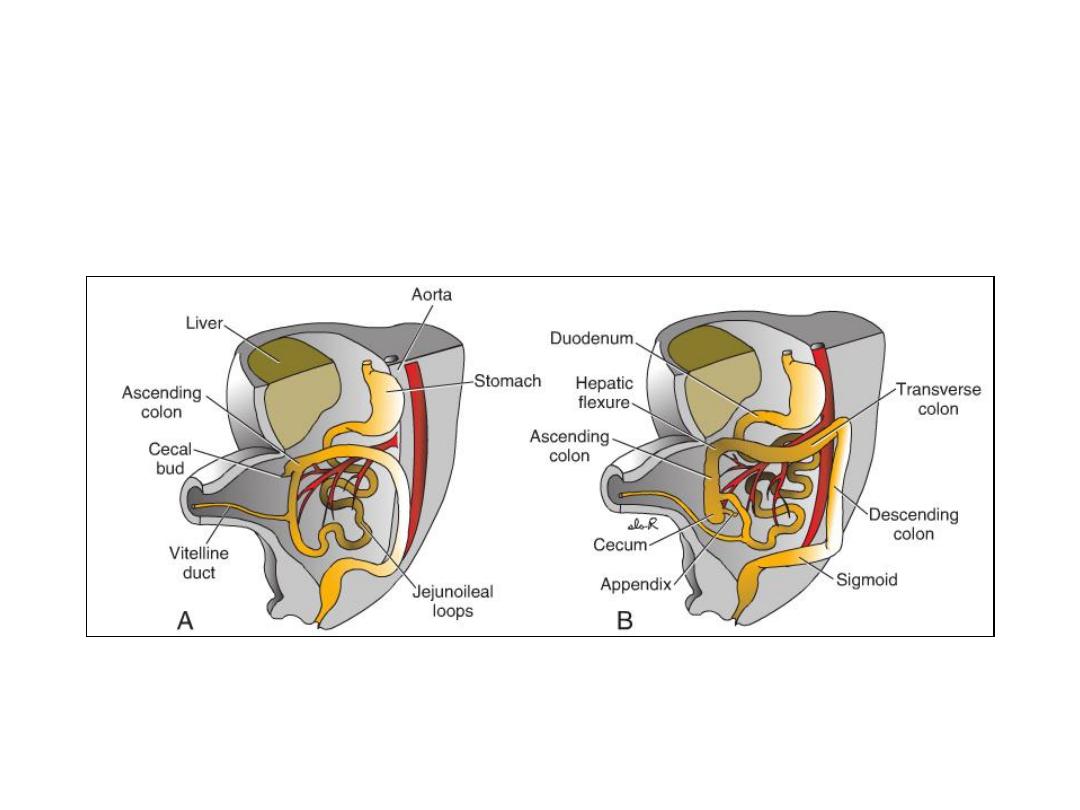
Retraction of Herniated Loops
• During the 10th week, herniated intestinal loops begin to return to the
abdominal cavity.
Mesenteries of the Intestinal Loops
• With retraction of the intestinal loop, the dorsal mesentery twists
around the origin of the superior mesenteric artery.
• After retraction, the ascending and descending colons are permanently
anchored to the posterior abdominal wall in a retroperitoneal position.
• The appendix, lower end of cecum, transverse colon and sigmoid colon
remain intraperitoneal.

Abnormalities of the Mesenteries
• Mobile cecum: Persistence of a portion of the mesocolon (in the
ascending colon).
• Volvulus: In the most extreme form, the mesentery of the ascending colon
failsto fuse with the posterior body wall, and allows abnormal movements
of the gut.
• Retrocolic hernia: entrapment of portions of the small intestine behind
the mesocolon.
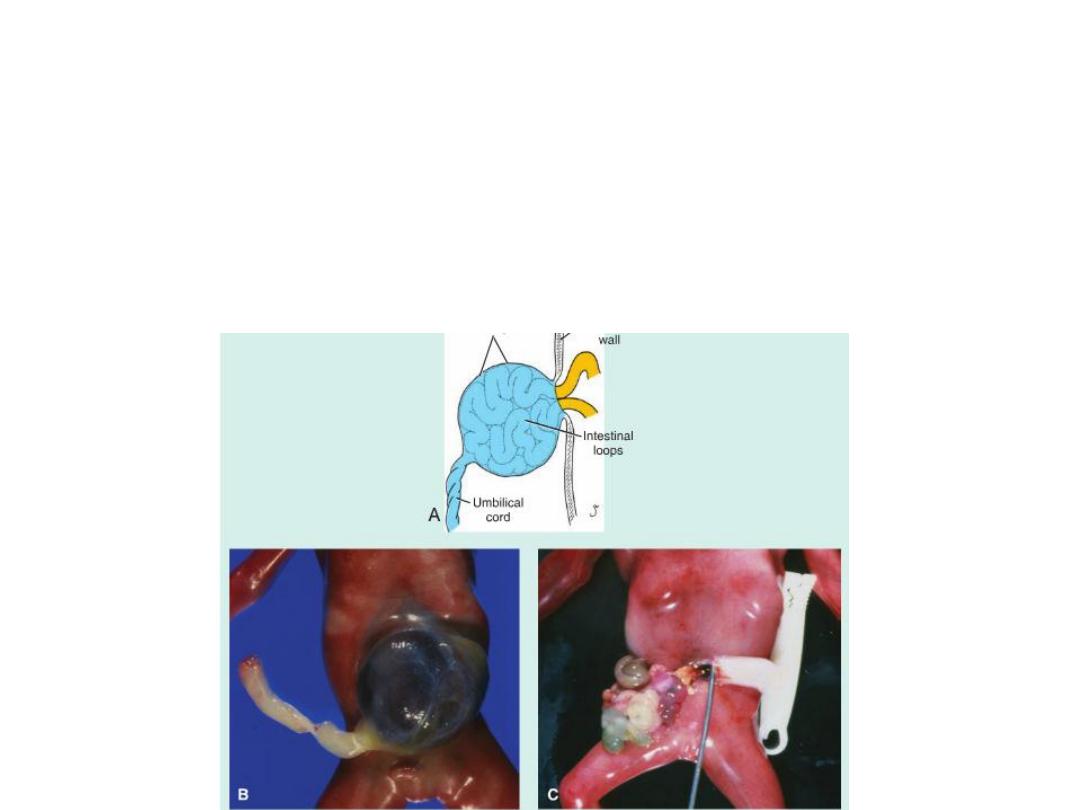
Body Wall Defects: Omphalocele, Gastroschisis.
A.Omphalocele showing failure of the intestinal loops to return to the
body cavity after physiologi-calherniation. The herniated loops are
covered by amnion. B.Omphalocele in a newborn.
C.Newborn with gastroschi-sis. Loops of bowel extend through a
closure defect in the ventral body wall and are not covered by
amnion.
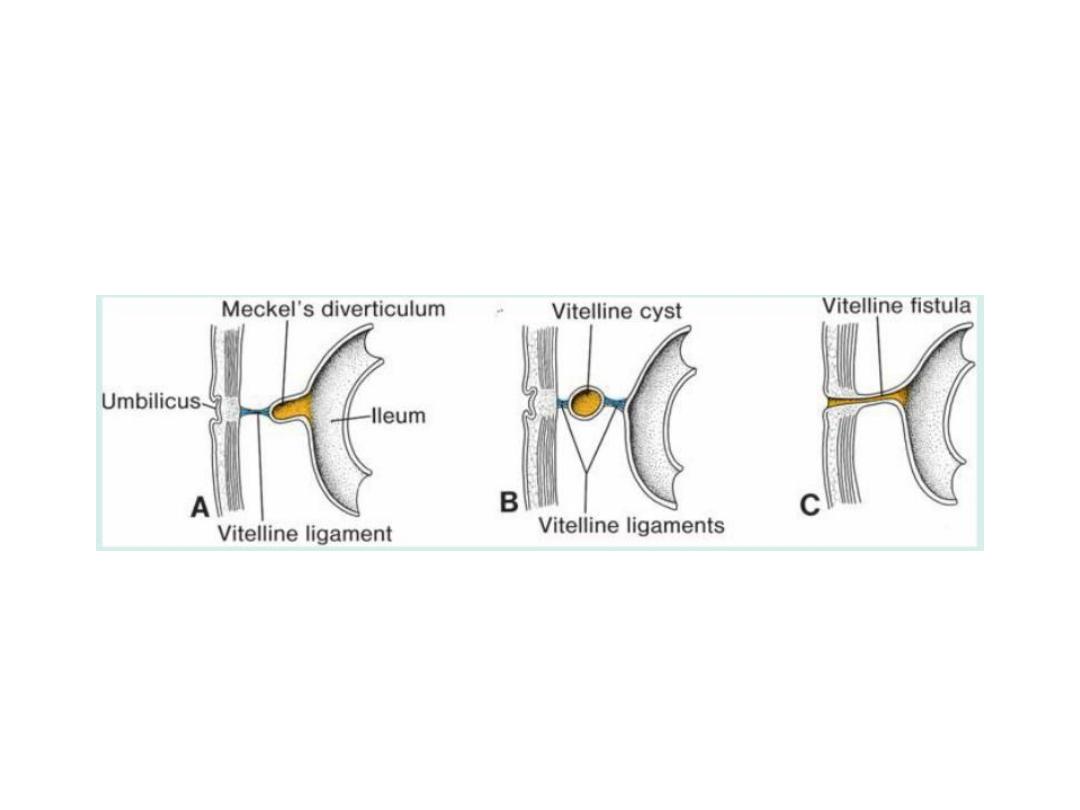
Vitelline Duct Abnormalities
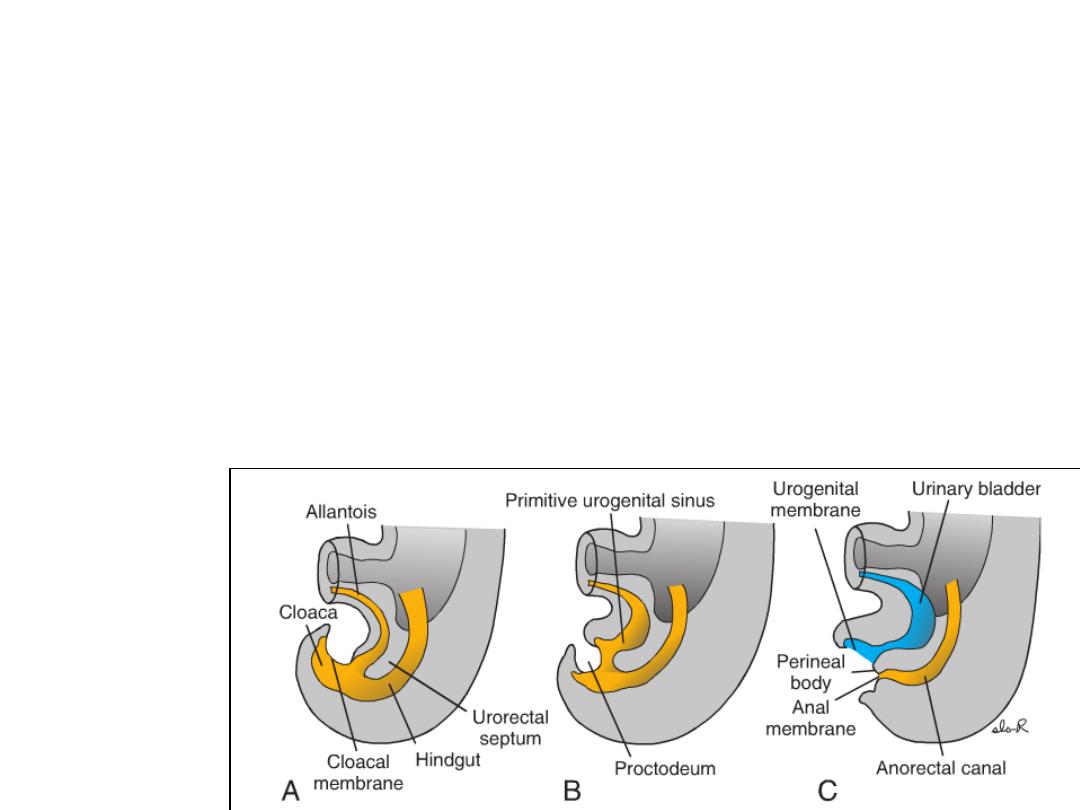
HINDGUT
• The hindgut gives rise to the distal third of the transverse colon, the
descending colon, the sigmoid, the rectum, and the upper part of the anal
canal.
• The terminal portion of the hindgut enters into the posterior region of the
cloaca, the primitive anorectal canal;
• The allantois enters into the anterior portion, the primitive urogenital sinus
• The cloaca itself is an endoderm-lined cavity covered at its ventral
boundary by surface ectoderm. This boundary between the endoderm
and the ectoderm forms the cloacal membrane.
• A layer of mesoderm, the urorectal septum, separates the region
between the allantois and hindgut.
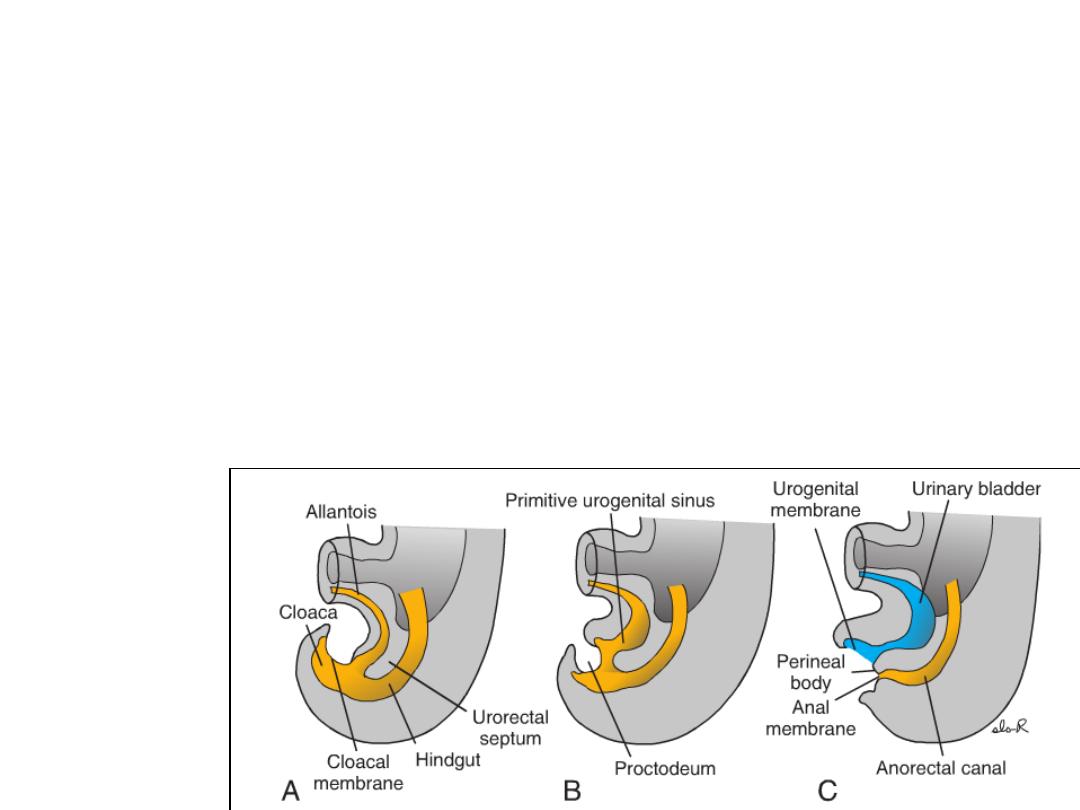
• As the embryo grows and caudal folding continues, the tip of the urorectal
septum comes to lie close to the cloacal membrane.
• At the end of the seventh week, the cloacal membrane ruptures, creating the
anal opening for the hindgut and a ventral opening for the urogenital sinus.
• Between the two, the tip of the urorectal septum forms the perineal body.
• The upper part (two-thirds) of the anal canal is derived from endoderm of the
hindgut; the lower part (one-third) is derived from ectoderm around the
Proctodeum.
• Degeneration of the cloacal membrane (now called the Anal membrane )
establishes continuity between the upper and lower parts of the anal canal.
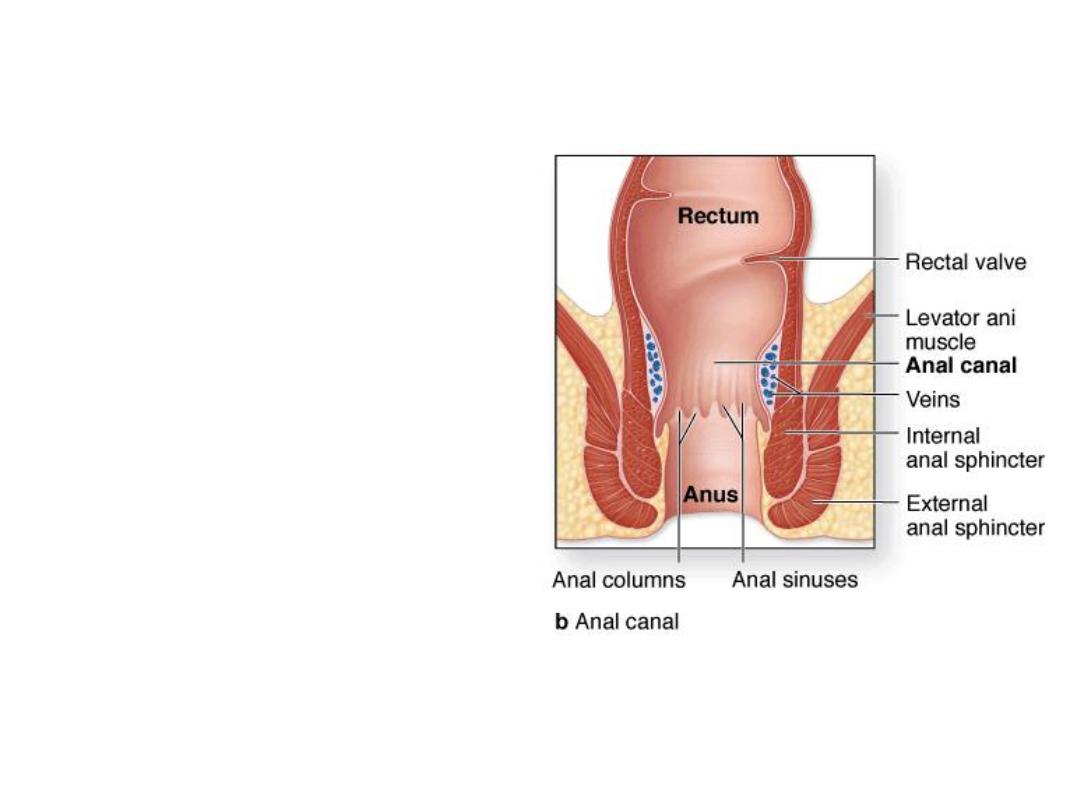
Since the caudal part of the anal canal
originates from ectoderm, it is supplied by
the inferior rectal arteries, branches of
the internal pudendal arteries.
However, the cranial part of the anal
canal originates from endoderm and is
therefore supplied by the superior rectal
artery, a continuation of the inferior
mesenteric artery, the artery of the
hindgut.
The junction between the endodermal and
ectodermal regions of the anal canal is
delineated by the pectinate line, just
below the anal columns.
At this line, the epithelium changes from
columnar to stratified squamous
epithelium.
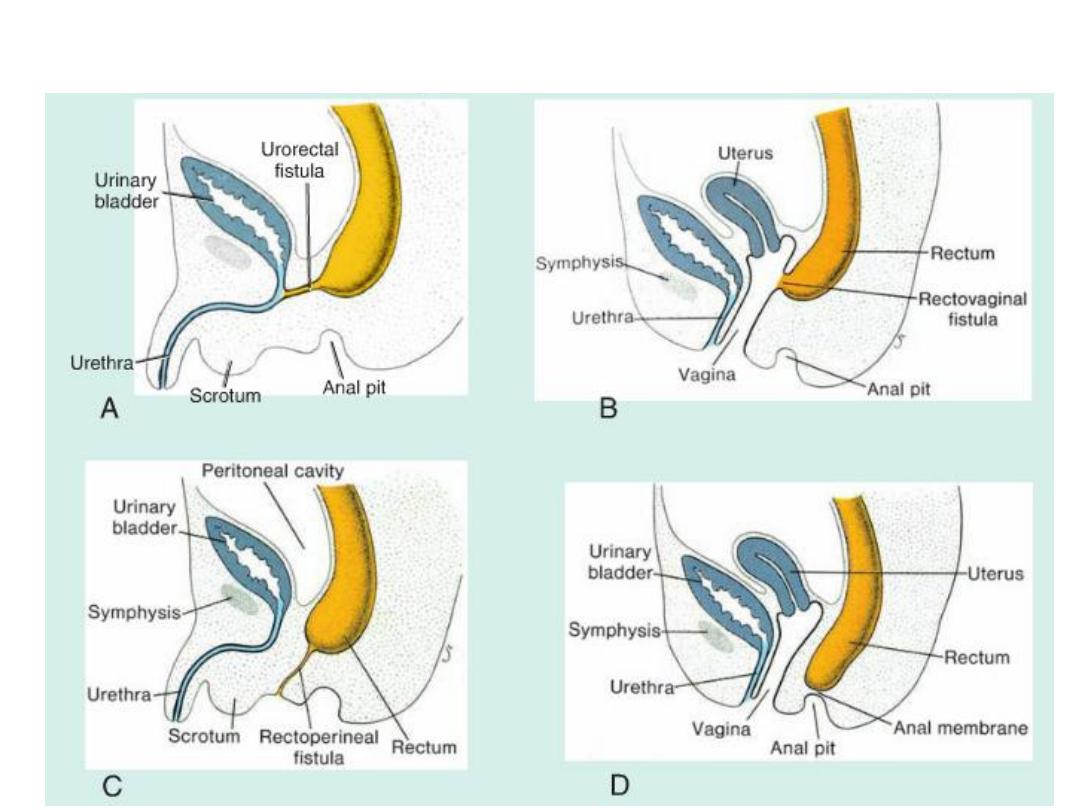
Hindgut Abnormalities

Congenital megacolon
• Is due to an absence of parasympathetic ganglia in the bowel wall
(aganglionic megacolon or hirschsprung disease ).
• These ganglia are derived from neural crest cells that migrate from the
neural folds to the wall of the bowel.
• Mutations in the RET gene, a tyrosine kinase receptor involved in crest
cell migration, can result in congenital megacolon.
