
interstitial fluids (citrate and phosphate, for instance) in such a manner that it
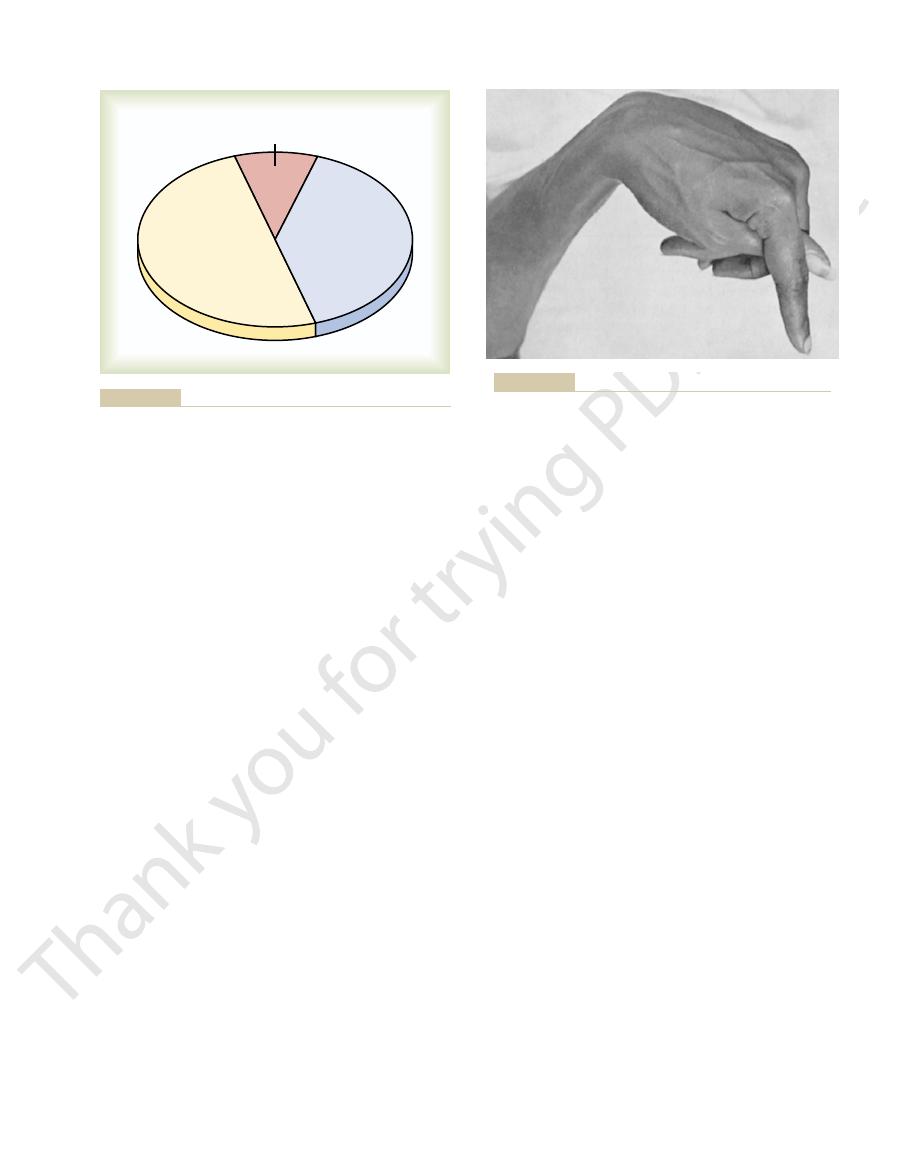
(2) About 9 per cent of the calcium (0.2 mmol/L) is diffusible through the cap-
proteins and in this form is nondiffusible through the capillary membrane.
(1) About 41 per cent (1 mmol/L) of the calcium is combined with the plasma
The calcium in the plasma is present in three forms, as shown in Figure 79–1.
Calcium in the Plasma and Interstitial Fluid
ulated as calcium concentration, phosphate serves several important functions
15 per cent is in the cells, and less than 1 per cent is in the extracellular fluid.
Approximately 85 per cent of the body’s phosphate is stored in bones, 14 to
as large reservoirs, releasing calcium when extracellular fluid concentration
cent is in the cells, and the rest is stored in bones. Therefore, the bones can serve
0.1 per cent of the total body calcium is in the extracellular fluid, about 1 per
gressive depression of the nervous system; conversely, decreases in calcium con-
neurons, are very sensitive to changes in calcium ion concentrations, and
and transmission of nerve impulses, to name just a few. Excitable cells, such as
including contraction of skeletal, cardiac, and smooth muscles; blood clotting;
is essential, because calcium plays a key role in many physiologic processes,
9.4 mg/dl, which is equivalent to 2.4 mmol calcium per liter. This precise control
Extracellular fluid calcium concentration normally is regulated very precisely,
Overview of Calcium and Phosphate
homeostasis are closely associated, they are discussed together in this chapter.
ulated by the hormones just noted. Because phosphate homeostasis and calcium
uptake and release of calcium, each of which is reg-
the intestine, renal excretion of calcium, and bone
calcium ion concentration, for example, is deter-
are all closely intertwined. Extracellular
, and
lism, formation of bone and teeth, and regulation of
The physiology of calcium and phosphate metabo-
and Teeth
Metabolism, Vitamin D, Bone,
Parathyroid Hormone, Calcitonin,
C
H
A
P
T
E
R
7
9
978
Calcium and Phosphate
vitamin D, parathyroid hormone (PTH)
calci-
tonin
mined by the interplay of calcium absorption from
Regulation in the Extracellular Fluid
and Plasma
seldom rising or falling more than a few per cent from the normal value of about
increases in calcium ion concentration above normal (hypercalcemia) cause pro-
centration (hypocalcemia) cause the nervous system to become more excited.
An important feature of extracellular calcium regulation is that only about
decreases and storing excess calcium.
Although extracellular fluid phosphate concentration is not nearly as well reg-
and is controlled by many of the same factors that regulate calcium.
illary membrane but is combined with anionic substances of the plasma and
seldom evident in patients, such as marked dilatation
ally be reduced beyond the usual lethal levels, very
In laboratory animals, in which calcium can gradu-
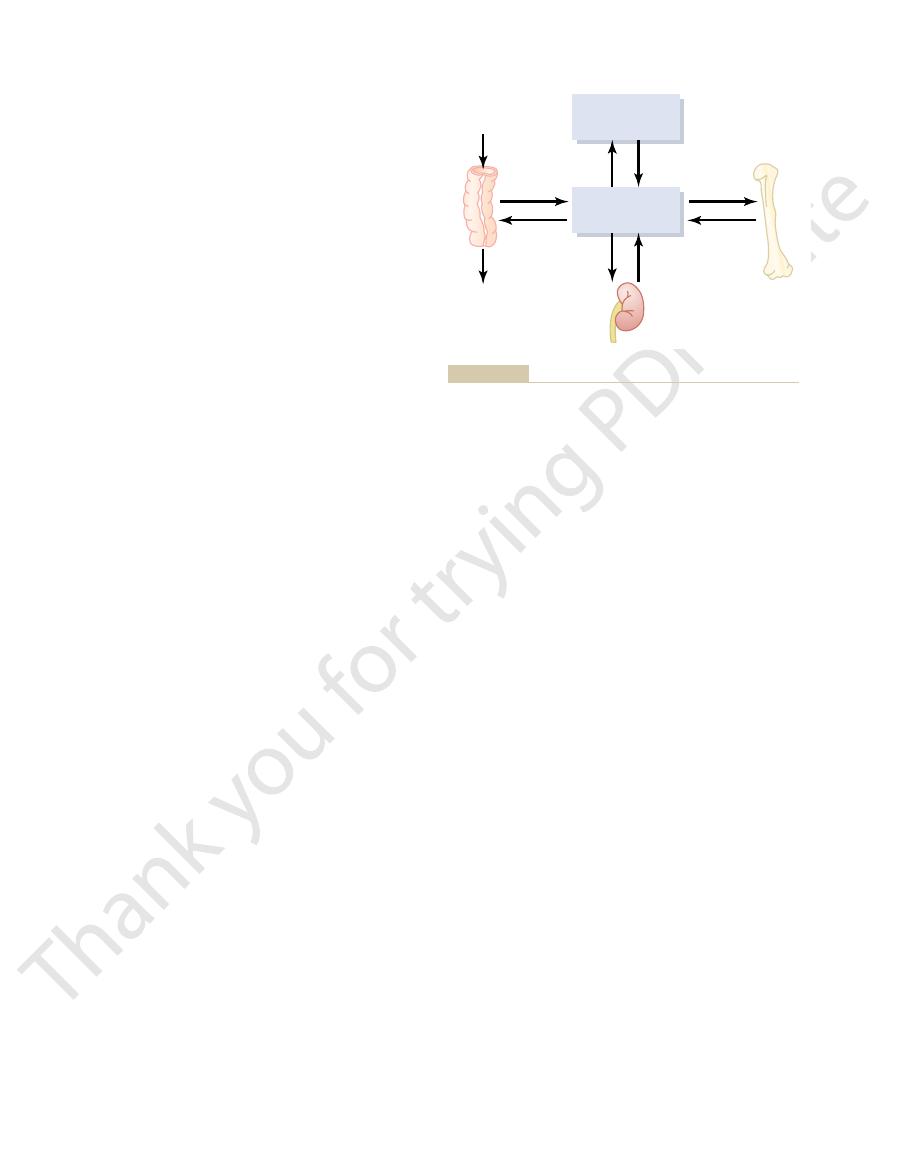
at about 4 mg/dl.
normal calcium concentration, and it is usually lethal
to about 6 mg/dl, which is only 35 per cent below the
tion of calcium falls from its normal level of 9.4 mg/dl
Tetany ordinarily occurs when the blood concentra-
the body. This is called “carpopedal spasm.”
Figure 79–2 shows tetany in the hand, which usually
tetany. It also occasionally causes seizures because of
contraction.
Consequently,
hypocalcemia causes
excitable that they begin to discharge spontaneously,
below normal, the peripheral nerve fibers become so
ions, allowing easy initiation of action potentials. At
progressively more excitable, because this causes
falls below normal, the nervous system becomes
When
Hypocalcemia Causes Nervous System Excitement and Tetany.
bone mineralization, as explained later in the chapter.
immediate physiologic effects. In addition, chronic
body. In contrast, even slight increases or decreases of
Concentrations in the Body Fluids
of Altered Calcium and Phosphate
Non-Bone Physiologic Effects
4 mg/dl in adults and 4 to 5 mg/dl in children.
is about 4 mg/dl, varying between normal limits of 3 to
(100 ml) of blood. The average total quantity of inor-
line. These relations were presented in the discussion
, whereas the oppo-
becomes more acidic, there is a relative increase in
Furthermore, when the pH of the extracellular fluid
quantity of each of these two types of phosphate ions.
of phosphate in the extracellular fluid rises, so does the
is about 0.26 mmol/L. When the total quantity
is about 1.05 mmol/L, and the concentration of
. The concentration of
forms: HPO
Inorganic Phosphate in the
effect of calcium on the heart, the nervous system, and
most functions of calcium in the body, including the
This ionic calcium is the form that is important for
2.4 mEq/L, because it is a divalent ion), a level only
concentration of about 1.2 mmol/L (or
Thus, the plasma and interstitial fluids have a normal
is not ionized. (3) The remaining 50 per cent of the
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
979
calcium in the plasma is both diffusible through the
capillary membrane and ionized.
calcium ion
one half the total plasma calcium concentration.
bone formation.
Extracellular Fluids
Inorganic phosphate in the plasma is mainly in two
4
-
and H
2
PO
4
-
HPO
4
-
H
2
PO
4
-
H
2
PO
4
_
and a decrease in HPO
4
-
site occurs when the extracellular fluid becomes alka-
of acid-base balance in Chapter 30.
Because it is difficult to determine chemically the
exact quantities of HPO
4
-
and H
2
PO
4
-
in the blood,
ordinarily the total quantity of phosphate is expressed
in terms of milligrams of phosphorus per deciliter
ganic phosphorus represented by both phosphate ions
Changing the level of phosphate in the extracellular
fluid from far below normal to two to three times
normal does not cause major immediate effects on the
calcium ion in the extracellular fluid can cause extreme
hypocalcemia or hypophosphatemia greatly decreases
the extracellular fluid concentration of calcium ions
increased neuronal membrane permeability to sodium
plasma calcium ion concentrations about 50 per cent
initiating trains of nerve impulses that pass to the
peripheral skeletal muscles to elicit tetanic muscle
its action of increasing excitability in the brain.
occurs before tetany develops in most other parts of
extreme hypocalcemia can cause other effects that are
Protein-bound calcium
41%
(1.0 mmol/L)
Ionized calcium
50%
(1.2 mmol/L)
Calcium complexed to
anions 9% (0.2 mmol/L)
calcium complexed to anions, and nondiffusible protein-bound
), diffusible but un-ionized
Figure 79–1
Distribution of ionized calcium (Ca
++
calcium in blood plasma.
Figure 79–2
Hypocalcemic tetany in the hand, called carpopedal spasm.
greatly increase phosphate excretion by the kidneys,
However, as discussed later in the chapter, PTH can
the kidneys.
proportional to the additional increase. Thus, the
centration, the rate of phosphate loss is directly
phate is lost in the urine. But above this critical con-
the critical value of about 1 mmol/L, all the phosphate
as explained in Chapter 29. That is,
flow mechanism,
over-
excretion, is PTH.
nephron, and therefore controlling the rate of calcium
calcium excretion markedly. We shall see later in the
urine. Conversely, even a minute increase in blood
tion is great, so that almost no calcium is lost in the
When calcium concentration is low, this reabsorp-
selective, depending on the calcium ion concentration
ducts, reabsorption of the remaining 10 per cent is very
Then in the late distal tubules and early collecting
tubules, loops of Henle, and early distal tubules.
in the urine. Approximately 90 per cent of the calcium
the filtered calcium, and about 100 mg/day is excreted
Normally, the renal tubules reabsorb 99 per cent of
glomeruli into the renal tubules.
not filtered by the glomerular capillaries. The rest is
excreted in the urine. About 41 per cent of the plasma
10 per cent (100 mg/day) of the ingested calcium is
urine.
calcium, almost all the dietary phosphate is absorbed
easily. Except for the portion of phosphate that is
is excreted in the feces (Figure 79–3).
90 per cent (900 mg/day) of the daily intake of calcium
testinal juices and sloughed mucosal cells. Thus, about
excreted in the feces. An additional 250 mg/day of
absorbed; the calcium remaining in the intestine is
cent (350 mg/day) of the ingested calcium is usually
calcium absorption by the intestines, and about 35 per
However, as discussed later,
calcium ions are poorly absorbed from the intestines.
in 1 liter of milk. Normally, divalent cations such as
each for calcium and phosphorus, about the amounts
The usual rates of intake are about 1000 mg/day
Absorption and Excretion of Calcium
parathyroid poisoning.
tals are likely to precipitate throughout the body; this
about 17 mg/dl in the blood, calcium phosphate crys-
above 15 mg/dl. When the level of calcium rises above
blood level of calcium rises above about 12 mg/dl, and
These depressive effects begin to appear when the
lack of appetite and constipation, probably because of
sluggish. Also, increased calcium ion concentration
above normal, the nervous system becomes depressed
When the level of calcium in the body fluids rises
Hypercalcemia Depresses Nervous System and Muscle Activity.
addition to nerve cells), and impaired blood clotting.
of the heart, changes in cellular enzyme activities,
980
Unit XIV
Endocrinology and Reproduction
increased membrane permeability in some cells (in
and reflex activities of the central nervous system are
decreases the QT interval of the heart and causes
depressed contractility of the muscle walls of the gas-
trointestinal tract.
they can become marked as the calcium level rises
condition is discussed later in connection with
and Phosphate
Intestinal Absorption and Fecal Excretion of Calcium and Phos-
phate.
vitamin D promotes
calcium enters the intestines via secreted gastroin-
Intestinal absorption of phosphate occurs very
excreted in the feces in combination with nonabsorbed
into the blood from the gut and later excreted in the
Renal Excretion of Calcium and Phosphate.
Approximately
calcium is bound to plasma proteins and is therefore
combined with anions such as phosphate (9 per cent)
or ionized (50 per cent) and is filtered through the
in the glomerular filtrate is reabsorbed in the proximal
in the blood.
calcium ion concentration above normal increases
chapter that the most important factor controlling this
reabsorption of calcium in the distal portions of the
Renal phosphate excretion is controlled by an
when phosphate concentration in the plasma is below
in the glomerular filtrate is reabsorbed and no phos-
kidneys regulate the phosphate concentration in the
extracellular fluid by altering the rate of phosphate
excretion in accordance with the plasma phosphate
concentration and the rate of phosphate filtration by
thereby playing an important role in the control of
plasma phosphate concentration as well as calcium
concentration.
Bone and Its Relation
to Extracellular Calcium
and Phosphate
Bone is composed of a tough organic matrix that is
greatly strengthened by deposits of calcium salts.
Cells
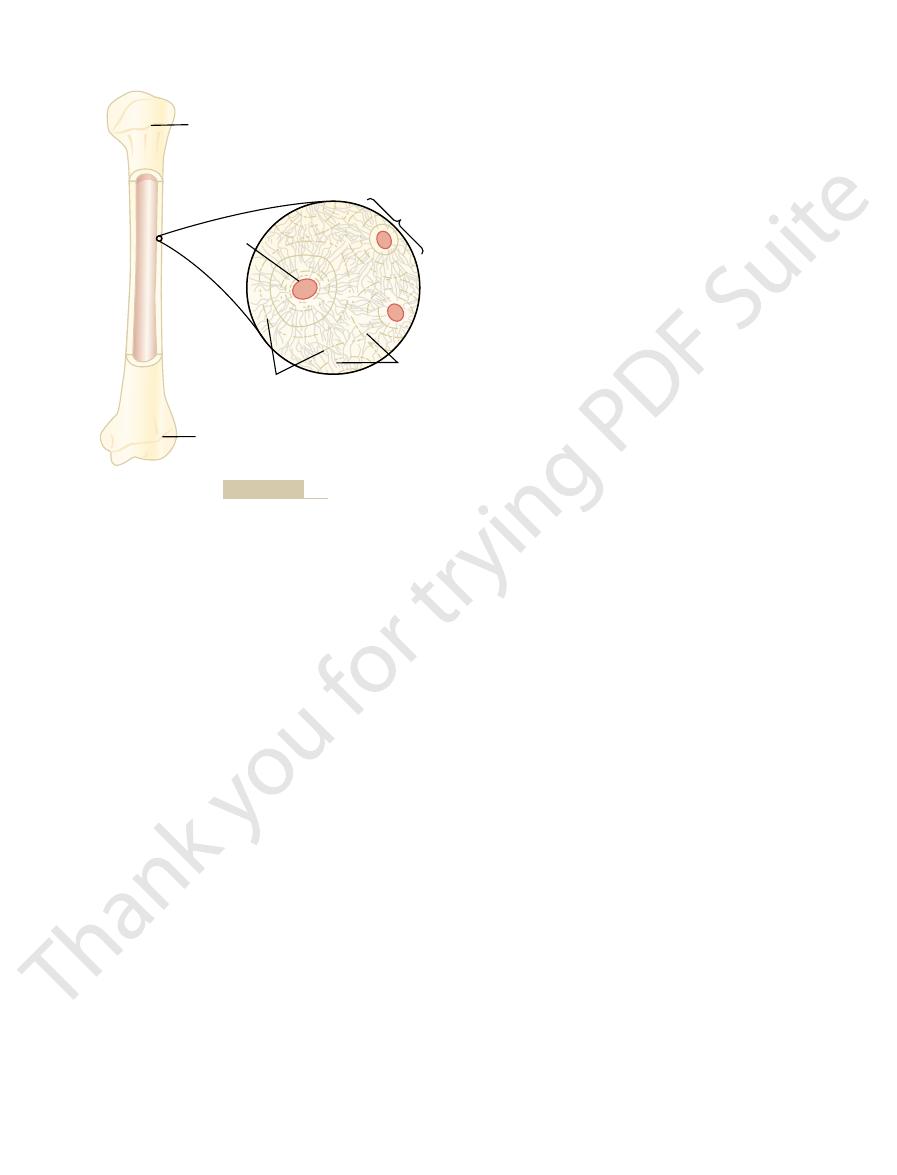
(13,000 mg)
Bone
(1,000,000 mg)
Deposition
(500 mg/day)
Absorption
(500 mg/day)
Reabsorption
(9880 mg/day)
Filtration
(9980 mg/day)
Absorption
(350 mg/day)
Calcium
intake
(350 mg/day)
Feces
(900 mg/day)
Urine
(100 mg/day)
Kidneys
Secretion
(250 mg/day)
Extracellular
fluid
(1300 mg)
amounts by reducing tubular reabsorption of calcium.
feces, although the kidneys have the capacity to excrete large
that most of the ingested calcium is normally eliminated in the
ments in a person ingesting 1000 mg of calcium per day. Note
Overview of calcium exchange between different tissue compart-
Figure 79–3

Once the pyrophosphate has been neutralized, the
causing precipitation of calcium salts. The osteoblasts
theory holds that at the time of formation, the colla-
deposited in osteoid is not fully understood. One
The mechanism that causes calcium salts to be
the amorphous form. This is important because
months. A few per cent may remain permanently in
and reprecipitation, these salts are converted into
of substitution and addition of atoms, or reabsorption
O, and others. Then by a process
O, Ca
(noncrystalline), a mixture of salts such as CaHPO
The initial calcium salts to be deposited are not
hydroxyapatite
vals along each collagen fiber, forming minute nidi
collagen fibers. The precipitates first appear at inter-
Within a few days after the osteoid is formed,
and become quiescent. At this stage they are called
readily precipitate in it. As the osteoid is formed, some
, a cartilage-like
. The collagen
The initial stage in bone
ration of the ions.
. Therefore,
pyrophosphate
tation; one such inhibitor is
However, inhibitors are present in almost all tissues of
required to cause precipitation of hydroxyapatite.
The
in Bone—Equilibrium with the
of Calcium and Phosphate
Precipitation and Absorption
have great compressional strength. These combined
have great tensile strength, whereas the calcium salts
The collagen fibers of bone, like those of tendons,
overlap one another, also causing hydroxyapatite crys-
addition, the segments of adjacent collagen fibers
which is essential in providing strength to the bone. In
tals and collagen fibers from slipping out of place,
vents “shear” in the bone; that is, it prevents the crys-
the fiber, bound tightly to it.This intimate bonding pre-
Tensile and Compressional Strength of Bone.
tually develops in most cases.
deposited, an osteogenic sarcoma (bone cancer) even-
of the bone tissues, and if a sufficient amount is
. Deposition of radioactive sub-
the major radioactive products released by explosion of
, and
many ions normally foreign to bone, such as
into distinct crystals of their own. This ability of many
by them. Therefore, they are believed to be conjugated
are also present among the bone salts, although x-ray
, and
tional conditions, the Ca/P ratio on a weight basis
like a long, flat plate. The relative ratio of calcium to
angstroms thick, and 100 angstroms wide—is shaped
Each crystal—about 400 angstroms long, 10 to 30
, is the
hydroxyapatite
crystalline salt, known as
. The formula for the major
The crystalline salts deposited in the
the deposition of calcium salts.
these is not known, although they do help to control
. The precise function of each of
chondroitin sulfate
, especially
The ground substance is composed of extracellular
. The collagen fibers extend primarily along the
, and the remainder is a
The organic matrix of bone is 90
matrix in relation to salts.
per cent matrix and 70 per cent salts.
Average
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
981
compact bone contains by weight about 30
Newly formed
bone may have a considerably higher percentage of
Organic Matrix of Bone.
to 95 per cent collagen fibers
homogeneous gelatinous medium called ground sub-
stance
lines of tensional force and give bone its powerful
tensile strength.
fluid plus proteoglycans
and hyaluronic acid
Bone Salts.
organic matrix of bone are composed principally of
calcium and phosphate
following:
Ca
10
(PO
4
)
6
(OH)
2
phosphorus can vary markedly under different nutri-
varying between 1.3 and 2.0.
Magnesium, sodium, potassium
carbonate ions
diffraction studies fail to show definite crystals formed
to the hydroxyapatite crystals rather than organized
types of ions to conjugate to bone crystals extends to
strontium,
uranium, plutonium, the other transuranic elements,
lead, gold, other heavy metals
at least 9 of 14 of
the hydrogen bomb
stances in the bone can cause prolonged irradiation
Each collagen
fiber of compact bone is composed of repeating peri-
odic segments every 640 angstroms along its length;
hydroxyapatite crystals lie adjacent to each segment of
tals to be overlapped like bricks keyed to one another
in a brick wall.
properties plus the degree of bondage between the
collagen fibers and the crystals provide a bony struc-
ture that has both extreme tensile strength and
extreme compressional strength.
Extracellular Fluids
Hydroxyapatite Does Not Precipitate in Extracellular Fluid
Despite Supersaturation of Calcium and Phosphate Ions.
concentrations of calcium and phosphate ions in extra-
cellular fluid are considerably greater than those
the body as well as in plasma to prevent such precipi-
hydroxyapatite crystals fail to precipitate in normal
tissues except in bone despite the state of supersatu-
Mechanism of Bone Calcification.
production is the secretion of collagen molecules
(called collagen monomers) and ground substance
(mainly proteoglycans) by osteoblasts
monomers polymerize rapidly to form collagen fibers;
the resultant tissue becomes osteoid
material differing from cartilage in that calcium salts
of the osteoblasts become entrapped in the osteoid
osteocytes.
calcium salts begin to precipitate on the surfaces of the
that rapidly multiply and grow over a period of days
and weeks into the finished product,
crystals.
hydroxyapatite crystals but amorphous compounds
4
·
2H
2
3
(PO
4
)
2
· 3H
2
the hydroxyapatite crystals over a period of weeks or
these amorphous salts can be absorbed rapidly when
there is need for extra calcium in the extracellular
fluid.
gen fibers are specially constituted in advance for
supposedly also secrete a substance into the osteoid to
neutralize an inhibitor (believed to be pyrophosphate)
that normally prevents hydroxyapatite crystallization.

Figure 79–5.
, as shown in
remains of the original cavity. Each new area of bone
, is all that
these vessels run, called the
vessels supplying the area. The canal through which
cavity until the tunnel is filled. Deposition of new bone
Bone deposition then continues for several months, the
osteoblasts instead; then new bone begins to develop.
and is several millimeters long. At the end of this time,
eats away at the bone for about 3 weeks, creating a
once a mass of osteoclasts begins to develop, it usually
clasts usually exist in small but concentrated masses, and
that the total mass of bone remains constant. Osteo-
deposition and absorption are equal to each other, so
Normally, except in growing bones, the rates of bone
particles of bone matrix and crystals, eventually also dis-
osteoclastic cells also imbibe by phagocytosis minute
bone, and the acids cause solution of the bone salts. The
The enzymes digest or dissolve the organic matrix of the
released from the mitochondria and secretory vesicles.
and (2) several acids, including citric acid and lactic acid,
enzymes, released from the lysosomes of the osteoclasts,
The villi secrete two types of substances: (1) proteolytic
forming a so-called ruffled border adjacent to the bone.
clasts send out villus-like projections toward the bone,
absorption is believed to be the following: The osteo-
adjacent to the osteoclasts. The mechanism of this
Histologically, bone absorption occurs immediately
osteoclasts.
of the bone surfaces of an adult. Later in the chapter we
monocyte-like cells formed in the bone marrow. The
(as many as 50 nuclei), derivatives of monocytes or
clasts, which are large phagocytic, multinucleated cells
formed constantly.
time in an adult), so that at least some new bone is being
the bones and in the bone cavities. A small amount of
79–4). Osteoblasts are found on the outer surfaces of
are active (Figure
, and it is continually
Deposition and Absorption of
The importance of exchangeable calcium is that it
other amorphous calcium salts.
calcium. This calcium is deposited in the bones in a
exchangeable calcium is in the bone. It normally
and the gastrointestinal tract. However, most of the
the calcium found in all tissue cells, especially in highly
with the calcium ions in the extracellular fluids.
exchangeable
minutes to about 1 hour. These effects result in great
removed from the circulating body fluids, the calcium
normal. Likewise, if large quantities of calcium ions are
or more, the calcium ion concentration returns to
to high levels. However, within 30 minutes to 1 hour
If soluble calcium salts are injected intravenously, the
Calcium Exchange Between Bone and
tissues, thereby allowing precipitation.
in these instances, the inhibitor factors that normally
degenerating tissues or in old blood clots. Presumably,
like tubes. Likewise, calcium salts frequently deposit in
abnormal conditions, they do precipitate. For instance,
never precipitate in normal tissues besides bone, under
Abnormal Conditions.
Precipitation of Calcium in Nonosseous Tissues Under
982
Unit XIV
Endocrinology and Reproduction
natural affinity of the collagen fibers for calcium salts
causes the precipitation.
Although calcium salts almost
they precipitate in arterial walls in the condition called
arteriosclerosis and cause the arteries to become bone-
prevent deposition of calcium salts disappear from the
Extracellular Fluid
calcium ion concentration may increase immediately
ion concentration again returns to normal within 30
part from the fact that the bone contains a type of
calcium that is always in equilibrium
A small portion of this exchangeable calcium is also
permeable types of cells such as those of the liver
amounts to about 0.4 to 1 per cent of the total bone
form of readily mobilizable salt such as CaHPO
4
and
provides a rapid buffering mechanism to keep the
calcium ion concentration in the extracellular fluids
from rising to excessive levels or falling to very low
levels under transient conditions of excess or
decreased availability of calcium.
Bone—Remodeling of Bone
Deposition of Bone by the Osteoblasts.
Bone is continually
being deposited by osteoblasts
being absorbed where osteoclasts
osteoblastic activity occurs continually in all living
bones (on about 4 per cent of all surfaces at any given
Absorption of Bone—Function of the Osteoclasts.
Bone is also
being continually absorbed in the presence of osteo-
osteoclasts are normally active on less than 1 per cent
see that PTH controls the bone absorptive activity of
soluting these and releasing the products into the blood.
Bone Deposition and Absorption Are Normally in Equilibrium.
tunnel that ranges in diameter from 0.2 to 1 millimeter
the osteoclasts disappear and the tunnel is invaded by
new bone being laid down in successive layers of con-
centric circles (lamellae) on the inner surfaces of the
ceases when the bone begins to encroach on the blood
haversian canal
deposited in this way is called an osteon
Osteoblasts
Osteoclasts
Vein
Bone
Fibrous periosteum
Figure 79–4
Osteoblastic and osteoclastic activity in the same bone.
can increase many times,
plasma, an effect that is shown in Figure 79–7. Note
First, the feedback mechanism precisely regulates
reasons.
inhibitory effect on the conversion reactions. This
this occurs in the liver. The process is a limited one,
The first step in the activation of cholecal-
the Liver.
tical to the cholecalciferol formed in the skin, except
sun prevents vitamin D deficiency. The additional
the sun. Consequently, appropriate exposure to the
stance normally in the skin, by ultraviolet rays from
, a sub-
7-dehydrocholesterol
cholecalciferol
functions. Vitamin D
D family, and they all perform more or less the same
stance from vitamin D. Let us discuss these steps.
. Figure 79–6 shows the suc-
25-dihydroxycholecalciferol
Instead, vitamin D must first be converted through a
the active substance that actually causes these effects.
tion, as discussed later. However, vitamin D itself is not
absorption from the intestinal tract; it also has impor-
Vitamin D has a potent effect to increase calcium
Vitamin D
break and often shortens convalescence.
bones, which accelerates osteoblastic activity at the
This causes stress on the opposed ends of the broken
the patient can continue to use the bone immediately.
stress to accelerate the rate of fracture healing. This is
callus.
bone. This is called a
salts, develops between the two broken ends of the
matrix, followed shortly by the deposition of calcium
“bone membrane.” Therefore, within a short time, a
stem cells in the surface tissue lining bone, called the
, which are bone
intraosseous osteoblasts involved in the break. Also,
Fracture of a bone
remodeling of bone at younger ages.
straight, especially in children because of the rapid
tion on the outer side, the bone can become almost
not compressed. After many years of increased deposi-
increased deposition of bone, and increased absorption
leg breaks in its center and then heals at an angle, the
certain circumstances. For instance, if a long bone of the
tion and calcification of bone.
bone remains thick and normally calcified. Therefore,
decalcified within a few weeks, whereas the opposite
continues to walk on the opposite leg, the bone of the
nonathletes. Also, if a person has one leg in a cast but
that the bone must carry. For instance, the bones of
deposition and absorption are slow.
son with the bones of the elderly, in whom the rates of
absorption are rapid, show little brittleness in compari-
bones of children, in whom the rates of deposition and
normal toughness of bone is maintained. Indeed, the
the old organic matrix degenerates. In this manner, the
tively brittle and weak, new organic matrix is needed as
stress patterns. Third, because old bone becomes rela-
loads. Second, even the shape of the bone can be
Consequently, bones thicken when subjected to heavy
its strength in proportion to the degree of bone stress.
cally important functions. First, bone ordinarily adjusts
The continual deposi-
Value of Continual Bone Remodeling
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
983
tion and absorption of bone have several physiologi-
rearranged for proper support of mechanical forces by
deposition and absorption of bone in accordance with
Control of the Rate of Bone Deposition by Bone “Stress.”
Bone
is deposited in proportion to the compressional load
athletes become considerably heavier than those of
leg in the cast becomes thin and as much as 30 per cent
continual physical stress stimulates osteoblastic deposi-
Bone stress also determines the shape of bones under
compression stress on the inside of the angle causes
occurs on the outer side of the angle where the bone is
tion on the inner side of the angulated bone and absorp-
Repair of a Fracture Activates Osteoblasts.
in some way maximally activates all the periosteal and
immense numbers of new osteoblasts are formed almost
immediately from osteoprogenitor cells
large bulge of osteoblastic tissue and new organic bone
Many bone surgeons use the phenomenon of bone
done by use of special mechanical fixation apparatuses
for holding the ends of the broken bone together so that
tant effects on both bone deposition and bone absorp-
succession of reactions in the liver and the kidneys to
the final active product, 1,
,
also called 1,25(OH)
2
D
3
cession of steps that lead to the formation of this sub-
Cholecalciferol (Vitamin D
3
) Is Formed in the Skin.
Several
compounds derived from sterols belong to the vitamin
3
(also called
) is
the most important of these and is formed in the skin
as a result of irradiation of
vitamin D compounds that we ingest in food are iden-
for the substitution of one or more atoms that do not
affect their function.
Cholecalciferol Is Converted to 25-Hydroxycholecalciferol in
ciferol is to convert it to 25-hydroxycholecalciferol;
because the 25-hydroxycholecalciferol has a feedback
feedback effect is extremely important for two
the concentration of 25-hydroxycholecalciferol in the
that the intake of vitamin D
3
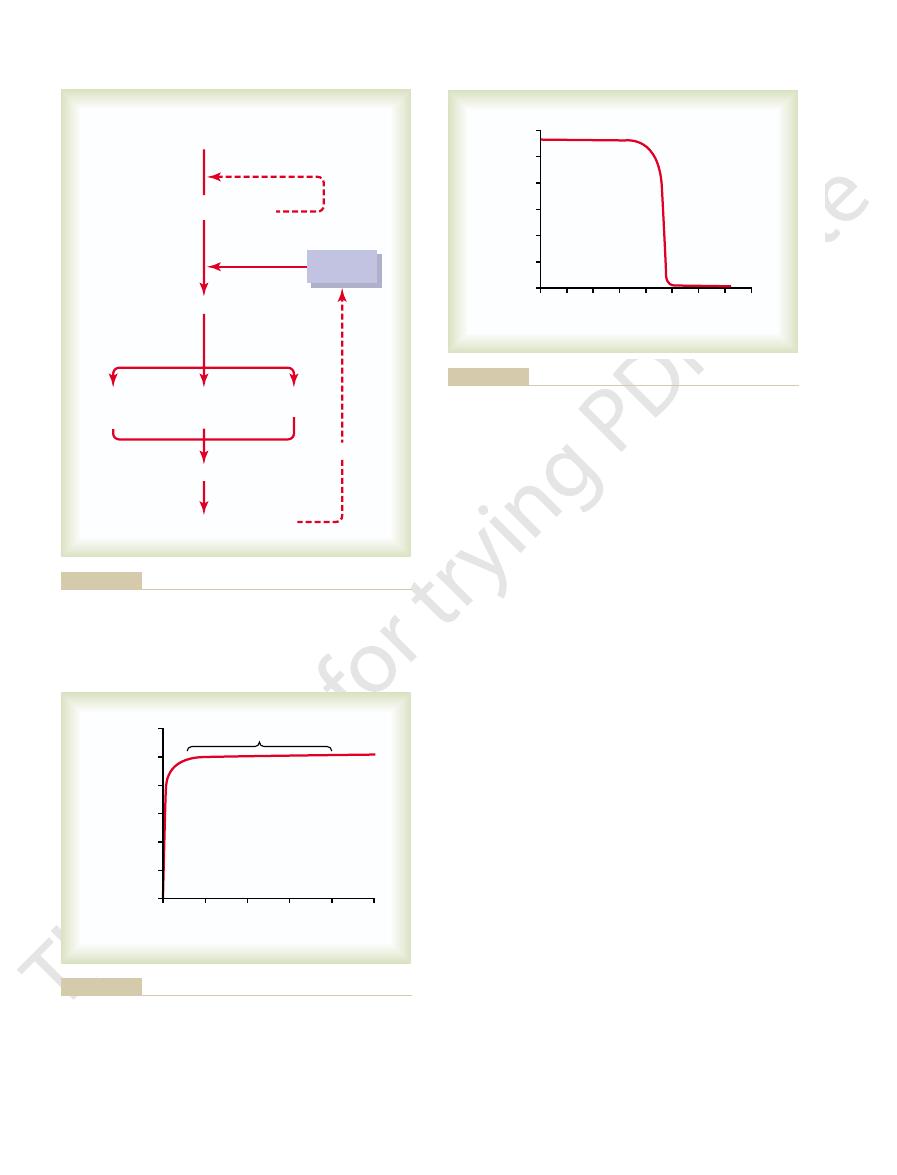
Epiphyseal line
Osteon
Canaliculi
Lacunae
Haversian
canal
Magnified
section
Epiphyseal line
Structure of bone.
Figure 79–5
1,25-dihydroxycholecalciferol. Second, and even more
First, the calcium ion itself has a slight effect in pre-
calcium in the plasma. There are two reasons for this.
Figure 79–8 demonstrates that
ing the functional effects of vitamin D in the body.
Therefore, PTH exerts a potent influence in determin-
erol requires PTH. In the absence of PTH, almost
Note also in Figure 79–6 that the conversion of 25-
effectiveness.
absence of the kidneys, vitamin D loses almost all its
than 1/1000 of the vitamin D effect. Therefore, in the
ous products in the scheme of Figure 79–6 have less
the most active form of vitamin D, because the previ-
. This latter substance is by far
dihydroxycholecalciferol
Figure 79–6
many months.
the vitamin D form, it can be stored in the liver for
it persists in the body for only a few weeks, whereas in
stored in the liver for future use. Once it is converted,
Second, this controlled conversion of vitamin D
is altered over a wide range.
erol remains nearly normal. This high degree of feed-
984
Unit XIV
Endocrinology and Reproduction
and yet the concentration of 25-hydroxycholecalcif-
back control prevents excessive action of vitamin D
when intake of vitamin D
3
3
to
25-hydroxycholecalciferol conserves the vitamin D
Formation of 1,25-Dihydroxycholecalciferol in the Kidneys
and Its Control by Parathyroid Hormone.
also shows the conversion in the proximal tubules
of the kidneys of 25-hydroxycholecalciferol to 1,25-
hydroxycholecalciferol to 1,25-dihydroxycholecalcif-
none of the 1,25-dihydroxycholecalciferol is formed.
Calcium Ion Concentration Controls the Formation of 1,25-
Dihydroxycholecalciferol.
the plasma concentration of 1,25-dihydroxycholecal-
ciferol is inversely affected by the concentration of
venting the conversion of 25-hydroxycholecalciferol to
ATPase
1,25-Dihydroxycholecalciferol
Intestinal absorption of calcium
Plasma calcium ion concentration
25-Hydroxycholecalciferol
Cholecalciferol (vitamin D
3
)
Calcium-
stimulated
Alkaline
phosphatase
Inhibition
Inhibition
Intestinal
epithelium
Kidney
Liver
Skin
Activation
Calcium-
binding
protein
Parathyroid
hormone
and the role of vitamin D in controlling the plasma calcium
to form 1,25-dihydroxycholecalciferol
Figure 79–6
Activation of vitamin D
3
concentration.
0
0.5
1.0
1.5
2.0
0
1.2
1.0
0.8
0.6
0.4
0.2
2.5
Intake of vitamin D
3
(times normal)
Normal range
Plasma 25-
hydroxycholecalciferol
(times normal)
of activated vitamin D that is formed.
changes in vitamin D intake have little effect on the final quantity
of 25-hydroxycholecalciferol. This figure shows that tremendous
Effect of increasing vitamin D
Figure 79–7
3
intake on the plasma concentration
0
2
4
6
8
10
12
14
X
0
1
2
3
4
5
6
16
Plasma calcium (mg/100 mL)
Normal
Plasma 1,25-
hydroxycholecalciferol
(times normal)
greatly increased absorption of calcium from the intestine.
increased formation of activated vitamin D, which in turn leads to
slight decrease in calcium concentration below normal causes
tion of 1,25-dihydroxycholecalciferol. This figure shows that a
Effect of plasma calcium concentration on the plasma concentra-
Figure 79–8

transient hypoparathyroidism. But even a small quan-
causes no major physiologic abnormalities. However,
erally recognized, total or subtotal thyroidectomy fre-
reason, before the importance of these glands was gen-
just another lobule of the thyroid gland. For this
fat. The parathyroid glands are difficult to locate
limeters long, 3 millimeters wide, and 2 millimeters
of the thyroid. Each parathyroid gland is about 6 mil-
there are four parathyroid glands in humans; they are
resultant tetany.
, often with
the extracellular fluid; conversely, hypofunction of the
salts from the bones, with resultant
lar fluid and bone of these ions. Excess activity of the
renal excretion, and exchange between the extracellu-
Parathyroid Hormone
osteocytic cell membranes.
through cell membranes—but in this instance, perhaps
again, the mechanism of the effect is unknown, but it
increase, it enhances the mineralization of bone. Here
intestines. However, even in the absence of such
. One of the ways in which it does this is to
Vitamin D in smaller quantities promotes bone cal-
transport through cellular membranes.
is not known, but it is believed to result from the effect
prevented. The mechanism of this action of vitamin D
D, the effect of PTH in causing bone absorption (dis-
. In the absence of vitamin
extreme quantities of vitamin D
both bone absorption and bone deposition. The
Vitamin D plays important roles in
Hormone Activity.
substances.
stances in the urine. However, this is a weak effect
absorption by the epithelial cells of the renal tubules,
Vitamin D also increases calcium and phosphate
Vitamin D Decreases Renal Calcium and Phosphate Excretion.
phosphate.
hormone’s action on calcium absorption, the cal-
enhanced by vitamin D. It is believed that this results
Although phosphate is usually absorbed easily, phos-
details of all these effects are unclear.
line phosphatase in the epithelial cells. The precise
the formation of (1) a calcium-stimulated ATPase in
from the body, thus causing a prolonged effect on
quantity of this calcium-binding protein. Furthermore,
membrane of the cell by facilitated diffusion. The rate
these cells to transport calcium into the cell cytoplasm,
cells. This protein functions in the brush border of
increasing, over a period of about 2 days, formation of
absorption of calcium. It does this principally by
tions as a type of “hormone” to promote intestinal
to feedback regulation of these substances.
ciferol, has several effects on the intestines, kidneys,
The active form of vitamin D, 1,25-dihydroxycholecal-
Actions of Vitamin D
bones, and the renal tubules, thus causing the calcium
the absorption of calcium from the intestines, the
erol is greatly depressed. Lack of this in turn decreases
too high, the formation of 1,25-dihydroxycholecalcif-
When the plasma calcium concentration is already
when PTH is suppressed, the 25-hydroxycholecalcif-
erol in the kidneys. At higher calcium concentrations,
below this level, PTH promotes the conversion of 25-
10 mg/100 ml. Therefore, at calcium concentrations
important, as we shall see later in the chapter, the rate
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
985
of secretion of PTH is greatly suppressed when the
plasma calcium ion concentration rises above 9 to
hydroxycholecalciferol to 1,25-dihydroxycholecalcif-
erol is converted to a different compound—24,25-
dihydroxycholecalciferol—that has almost no vitamin
D effect.
ion concentration to fall back toward its normal level.
and bones that increase absorption of calcium and
phosphate into the extracellular fluid and contribute
“Hormonal” Effect of Vitamin D to Promote Intestinal Calcium
Absorption.
1,25-Dihydroxycholecalciferol itself func-
a calcium-binding protein in the intestinal epithelial
and the calcium then moves through the basolateral
of calcium absorption is directly proportional to the
this protein remains in the cells for several weeks after
the 1,25-dihydroxycholecalciferol has been removed
calcium absorption.
Other effects of 1,25-dihydroxycholecalciferol that
might play a role in promoting calcium absorption are
the brush border of the epithelial cells and (2) an alka-
Vitamin D Promotes Phosphate Absorption by the Intestines.
phate flux through the gastrointestinal epithelium is
from a direct effect of 1,25-dihydroxycholecalciferol,
but it is possible that it results secondarily from this
cium in turn acting as a transport mediator for the
thereby tending to decrease excretion of these sub-
and probably not of major importance in regulating
the extracellular fluid concentration of these
Effect of Vitamin D on Bone and Its Relation to Parathyroid
administration of
causes absorption of bone
cussed in the next section) is greatly reduced or even
of 1,25-dihydroxycholecalciferol to increase calcium
cification
increase calcium and phosphate absorption from the
probably also results from the ability of 1,25-dihy-
droxycholecalciferol to cause transport of calcium ions
in the opposite direction through the osteoblastic or
Parathyroid hormone provides a powerful mechanism
for controlling extracellular calcium and phosphate
concentrations by regulating intestinal reabsorption,
parathyroid gland causes rapid absorption of calcium
hypercalcemia in
parathyroid glands causes hypocalcemia
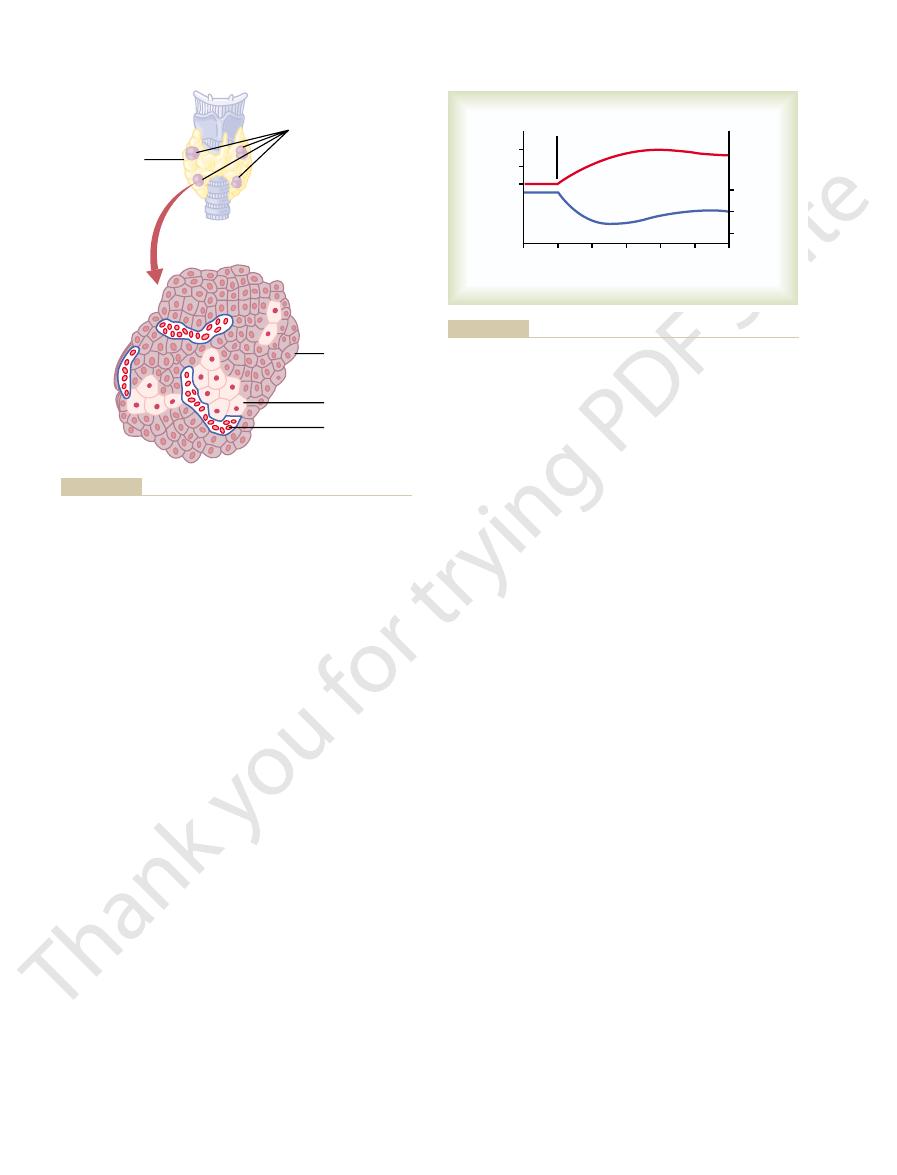
Physiologic Anatomy of the Parathyroid Glands.
Normally
located immediately behind the thyroid gland—one
behind each of the upper and each of the lower poles
thick and has a macroscopic appearance of dark brown
during thyroid operations because they often look like
quently resulted in removal of the parathyroid glands
as well.
Removal of half the parathyroid glands usually
removal of three of the four normal glands causes
tity of remaining parathyroid tissue is usually capable
When large quantities of PTH are injected, the
phate salts from the bone.
bone itself, not merely absorption of the calcium phos-
results from proliferation of the osteoclasts, followed
days or even weeks to become fully developed; it
second phase is a much slower one, requiring several
promote calcium and phosphate absorption. The
already existing bone cells (mainly the osteocytes) to
several hours. This phase results from activation of the
calcium and phosphate. One is a rapid phase that
Phosphate Absorption from the Bone
Parathyroid Hormone Increases Calcium and
increased phosphate absorption from the bone.
of PTH to increase renal phosphate excretion, an
excretion of calcium by the kidneys. The decline in
principally by two effects: (1) an effect of PTH to
2 hours. The rise in calcium concentration is caused
concentration, however, falls more rapidly than the
and reaches a plateau in about 4 hours. The phosphate
uing this for several hours. Note that at the onset of
Figure 79–10 shows the approximate effects on the
Concentrations in the
on Calcium and Phosphate
Effect of Parathyroid Hormone
by the fragments.
hours, a large share of the hormonal activity is caused
full PTH activity. In fact, because the kidneys rapidly
granules in the cytoplasm of the cells. The final
Golgi apparatus, and finally is packaged in secretory
mone with 90 amino acids, then to the hormone itself
of 110 amino acids. This is cleaved first to a prohor-
in the form of a preprohormone, a polypeptide chain
in a pure form. It is first synthesized on the ribosomes
Chemistry of Parathyroid Hormone.
hormone.
cells is not certain, but they are believed to be modi-
most, if not all, of the PTH. The function of the oxyphil
young humans. The chief cells are believed to secrete
, but
oxyphil cells
chief cells
shown in Figure 79–9, contains mainly
The parathyroid gland of the adult human being,
tion of all the glands.
986
Unit XIV
Endocrinology and Reproduction
of hypertrophying satisfactorily to perform the func-
and
a small to moderate number of
oxyphil cells are absent in many animals and in
fied or depleted chief cells that no longer secrete
PTH has been isolated
with 84 amino acids by the endoplasmic reticulum and
hormone has a molecular weight of about 9500.
Smaller compounds with as few as 34 amino acids
adjacent to the N terminus of the molecule have also
been isolated from the parathyroid glands that exhibit
remove the whole 84-amino acid hormone within
minutes but fail to remove many of the fragments for
Extracellular Fluid
blood calcium and phosphate concentrations caused
by suddenly infusing PTH into an animal and contin-
infusion the calcium ion concentration begins to rise
calcium rises and reaches a depressed level within 1 or
increase calcium and phosphate absorption from the
bone and (2) a rapid effect of PTH to decrease the
phosphate concentration is caused by a strong effect
effect that is usually great enough to override
PTH has two effects on bone in causing absorption of
begins in minutes and increases progressively for
by greatly increased osteoclastic reabsorption of the
Rapid Phase of Calcium and Phosphate Absorption—Osteoly-
sis.
calcium ion concentration in the blood begins to rise
Thyroid gland
Red blood cell
Oxyphil cell
Chief cell
Parathyroid gland
(located on posterior
side of the thyroid
gland)
no longer secrete PTH.
is uncertain, but they may be modified or depleted chief cells that
and secreted by the chief cells. The function of the oxyphil cells
gland. Almost all of the parathyroid hormone (PTH) is synthesized
The four parathyroid glands lie immediately behind the thyroid
Figure 79–9
0
1
2
3
4
Phosphate
Calcium
5
2.40
2.35
2.30
0.8
1.2
1.0
6
Hours
Calcium (mmol/L)
Phosphate (mmol/L)
Begin parathyroid hormone
during the first 5 hours of parathyroid hormone infusion at a mod-
Approximate changes in calcium and phosphate concentrations
Figure 79–10
erate rate.

tration, the concentration of cAMP increases in the
mechanism. Within a few minutes after PTH adminis-
on its target organs is mediated by the cyclic adeno-
vitamin D, as discussed earlier in the chapter.
At this point, we should be reminded again that PTH
Parathyroid Hormone Increases Intestinal
to increase calcium reabsorption, continual loss of
Were it not for the effect of PTH on the kidneys
, the early collecting ducts, and possibly the
, the
affects phosphate. The increased calcium absorption
it decreases the reabsorption of sodium, potassium,
reabsorption. Moreover, it increases the rate of reab-
ions.
Excretion and Increases Phosphate Excretion
Parathyroid Hormone Decreases Calcium
multinucleated osteoclasts.
even development of large cavities filled with large,
on the bones. Prolonged administration or secretion of
fluids, it is impossible to discern any immediate effect
osteoclastic activity. Still, even in the late stages, there
to correct the weakened state. Therefore, the late
After a few months of excess PTH, osteoclastic
osteoclastic system to become well developed, but it
clasts. Several days of excess PTH usually cause the
stages: (1) immediate activation of the osteoclasts that
up the bone over a period of weeks or months.
ondary but unknown “signal” to the osteoclasts,
the activated osteoblasts and osteocytes send a sec-
receptor proteins for PTH. Instead, it is believed that
is much clearer is its activation of the osteoclasts. Yet
Slow Phase of Bone Absorption and Calcium Phosphate
Then the calcium pump on the other side of the cell
osteocytic membrane, thus allowing calcium ions to
those amorphous bone crystals that lie near the cells.
PTH can activate the calcium pump strongly, thereby
osteocytes have receptor proteins for binding PTH.
But where does PTH fit into this picture? First,
matrix. When the pump is inactivated, the bone
without absorption of the bone’s fibrous and gel
, and it occurs
bone. This effect is called
fluid calcium concentration falls even lower, and
cytic pump becomes excessively activated, the bone
third that in the extracellular fluid. When the osteo-
bone fluid into the extracellular fluid, creating a
the osteocytic membrane pumps calcium ions from the
. Experiments suggest that
Between the osteocytic membrane and the bone is
, and it is believed to provide a membrane
This extensive system is called the
connect with the surface osteocytes and osteoblasts.
out the bone structure, and these processes also
processes extend from osteocyte to osteocyte through-
areas adjacent to the osteoclasts. In fact, long, filmy
the osteoblasts and osteocytes form a system of inter-
and its calcification. However, studies have shown that
osteocytes functioning to cause bone salt absorption,
surface.
vicinity of the osteocytes lying within the bone itself
two areas in the bone: (1) from the bone matrix in the
developed. Histological and physiologic studies have
within minutes, long before any new bone cells can be
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
987
shown that PTH causes removal of bone salts from
and (2) in the vicinity of the osteoblasts along the bone
One does not usually think of either osteoblasts or
because both these types of cells are osteoblastic in
nature and normally associated with bone deposition
connected cells that spreads all through the bone and
over all the bone surfaces except the small surface
osteocytic mem-
brane system
that separates the bone itself from the extracellular
fluid.
a small amount of bone fluid
calcium ion concentration in the bone fluid only one
calcium phosphate salts are then absorbed from the
osteolysis
fluid calcium concentration rises to a higher level,
and calcium phosphate salts are redeposited in the
matrix.
the cell membranes of both the osteoblasts and the
causing rapid removal of calcium phosphate salts from
PTH is believed to stimulate this pump by increasing
the calcium permeability of the bone fluid side of the
diffuse into the membrane cells from the bone fluid.
membrane transfers the calcium ions the rest of the
way into the extracellular fluid.
Release—Activation of the Osteoclasts.
A much better
known effect of PTH and one for which the evidence
the osteoclasts do not themselves have membrane
causing them to set about their usual task of gobbling
Activation of the osteoclastic system occurs in two
are already formed and (2) formation of new osteo-
can continue to grow for months under the influence
of strong PTH stimulation.
resorption of bone can lead to weakened bones and
secondary stimulation of the osteoblasts that attempt
effect is actually to enhance both osteoblastic and
is more bone absorption than bone deposition in the
presence of continued excess PTH.
Bone contains such great amounts of calcium in
comparison with the total amount in all the extracel-
lular fluids (about 1000 times as much) that even when
PTH causes a great rise in calcium concentration in the
PTH—over a period of many months or years—finally
results in very evident absorption in all the bones and
by the Kidneys
Administration of PTH causes rapid loss of phosphate
in the urine owing to the effect of the hormone to
diminish proximal tubular reabsorption of phosphate
PTH also increases renal tubular reabsorption of
calcium at the same time that it diminishes phosphate
sorption of magnesium ions and hydrogen ions while
and amino acid ions in much the same way that it
occurs mainly in the late distal tubules
collecting
tubules
ascending loop of Henle to a lesser extent.
calcium into the urine would eventually deplete both
the extracellular fluid and the bones of this mineral.
Absorption of Calcium and Phosphate
greatly enhances both calcium and phosphate absorp-
tion from the intestines by increasing the formation in
the kidneys of 1,25-dihydroxycholecalciferol from
Cyclic Adenosine Monophosphate Mediates the Effects of
Parathyroid Hormone.
A large share of the effect of PTH
sine monophosphate (cAMP) second messenger
exchangeable bone calcium salts. This effect is
throughout the bone, thus shifting the balance
osteolytic effect of the osteocytic membrane
1. The immediate effect is to decrease the absorptive
ways.
minutes after injection of the calcitonin, in at least two
calcium ion concentration rapidly, beginning within
some young animals, calcitonin decreases blood
concentration, but one that is relatively weak and
79–11. This provides a second hormonal feedback
citonin, which is shown by the blue line in Figure
and in humans, an increase in plasma calcium concen-
In young animals, but much less so in older animals
This contrasts with PTH secretion, which is stimulated
The primary stimulus for calcitonin secre-
ians, reptiles, and birds. Calcitonin is a 32-amino acid
of lower animals such as fish, amphib-
brachial glands
fluid between the follicles of the thyroid gland. These
, lying in the interstitial
, or
PTH. However, the quantitative role of calcitonin is
tion and, in general, has effects opposite to those of
gland, tends to
Calcitonin, a peptide hormone secreted by the thyroid
body’s extremely potent feedback system for long-
tion can double PTH secretion. This is the basis of the
hypertrophy greatly, is shown by the dashed red line;
of many weeks, thus allowing time for the glands to
The approximate chronic effect that one finds when
changed over a period of a few hours. This shows that
PTH concentration. The solid red curve shows the
Figure 79–11 shows the approximate relation
example, bone absorption caused by disuse of the
diet, (2) increased vitamin D in the diet, and (3) bone
and reduced size of the parathyroid glands. Such con-
Conversely, conditions that increase the calcium ion
the mother’s extracellular fluid is hardly measurable;
, even
calcium is usually depressed only a small amount; also,
, in which the level of
rickets
more. For instance, the parathyroid glands become
sists, the glands will hypertrophy, sometimes fivefold or
minutes; if the decreased calcium concentration per-
by Calcium Ion Concentration
Control of Parathyroid Secretion
dihydroxycholecalciferol in the kidneys. There are
osteocytes, osteoclasts, and other target cells. This
988
Unit XIV
Endocrinology and Reproduction
cAMP in turn is probably responsible for such func-
tions as osteoclastic secretion of enzymes and acids to
cause bone reabsorption and formation of 1,25-
probably other direct effects of PTH that function
independently of the second messenger mechanism.
Even the slightest decrease in calcium ion concentra-
tion in the extracellular fluid causes the parathyroid
glands to increase their rate of secretion within
greatly enlarged in
they become greatly enlarged in pregnancy
though the decrease in calcium ion concentration in
and they are greatly enlarged during lactation because
calcium is used for milk formation.
concentration above normal cause decreased activity
ditions include (1) excess quantities of calcium in the
absorption caused by factors other than PTH (for
bones).
between plasma calcium concentration and plasma
acute effect when the calcium concentration is
even small decreases in calcium concentration from
the normal value can double or triple the plasma PTH.
the calcium ion concentration changes over a period
this demonstrates that a decrease of only a fraction of
a milligram per deciliter in plasma calcium concentra-
term control of plasma calcium ion concentration.
Calcitonin
decrease plasma calcium concentra-
far less than that of PTH in regulating calcium ion
concentration.
Synthesis and secretion of calcitonin occur in the
parafollicular cells
C cells
cells constitute only about 0.1 per cent of the human
thyroid gland and are the remnants of the ultimo-
peptide with a molecular weight of about 3400.
Increased Plasma Calcium Concentration Stimulates Calcitonin
Secretion.
tion is increased plasma calcium ion concentration.
by decreased calcium concentration.
tration of about 10 per cent causes an immediate
twofold or more increase in the rate of secretion of cal-
mechanism for controlling the plasma calcium ion
works in a way opposite that of the PTH system.
Calcitonin Decreases Plasma Calcium Concentration.
In
activities of the osteoclasts and possibly the
in favor of deposition of calcium in the
especially significant in young animals because of
the rapid interchange of absorbed and deposited
calcium.
6
8
10 12 14
0
2
4
Normal levels
3
2
1
0
0
1000
800
600
400
200
16
Plasma calcium (mg/dL)
Parathyroid hormone
(ng/mL)
Plasma calcitonin
(pg/mL)
Parathyroid hormone
Acute
effect
Chronic
effect
Calcitonin
100 per cent change in parathyroid hormone concentration.
centration of only a few percentage points can cause as much as
Note especially that long-term, chronic changes in calcium con-
plasma concentrations of parathyroid hormone and calcitonin.
Approximate effect of plasma calcium concentration on the
Figure 79–11

deficiency, only the PTH mechanism seems to be really
mechanism alone.
Therefore, in very young animals, excess calcitonin can
bones, and perhaps in some cells of other tissues.
dren (but probably to a smaller extent in adults), the
increases. In young animals and possibly in young chil-
At the same time that PTH decreases, calcitonin
decreases. As already explained, this sets into play
calcium ion concentration, the rate of PTH secretion
act. Within 3 to 5 minutes after an acute increase in the
calcium in the extracellular fluid, both the parathyroid
able calcium mechanism in the bones is “buffering” the
Hormonal Control of Calcium Ion Concentration—the Second
buffer system for helping to maintain constancy of the
cially of the liver and intestine, contain a reasonable
of many of the tissues of the body, espe-
mitochondria
In addition to the buffer function of the bones, the
of the bones in about 70 minutes.
the extracellular fluid each minute. Therefore, about
the bones each minute—that is, about 1 per cent of all
Also, about 5 per cent of all the blood flows through
perhaps 1 acre or more.
exchangeable salt. This reaction is rapid because the
osition of exchangeable salt. Conversely, a decrease in
able salts and their ease of resolubility, an increase in
salts of the bone, a total of 5 to 10 grams of calcium.
The quantity of these salts that is available for
amorphous calcium phosphate compounds, probably
in the bones, discussed earlier in this chapter, are
The exchangeable calcium salts
Buffer Function of the Exchangeable Calcium in Bones—the
hypocalcemia. However, there is a first line of defense
The addition or subtraction of 0.3 gram to
gram in 1 hour. This figure compares with a
vitamin D activity, a person may absorb as much as 0.3
calcium, particularly when there is also an excess of
Conversely, after ingestion of large quantities of
day.
into the intestinal tract, and lost into the feces each
calcium can be secreted in the intestinal juices, passed
For instance, in cases of diarrhea, several grams of
from the body fluids is as much as 0.3 gram in 1 hour.
At times, the amount of calcium absorbed into or lost
Summary of Control of
in which osteoclastic activity is greatly accelerated, cal-
Paget’s disease
Also, in certain bone diseases, such as
great as 5 grams or more per day—equal to 5 to 10
children, with absorption and deposition of calcium as
tion. The effect of calcitonin in children is much
rate of absorption is slowed by calcitonin, this still has
and deposition of calcium are small, and even after the
Second, in the adult, the daily rates of absorption
bly altered, which again demonstrates the overriding
removed and calcitonin is no longer secreted, the long-
the calcitonin effect. When the thyroid gland is
stimulation of PTH secretion, which almost overrides
calcitonin on plasma calcium is twofold. First, any
The reason for the weak effect of
Calcitonin Has a Weak Effect on Plasma Calcium Concentration
the effects are opposite those of PTH, but they appear
dling in the kidney tubules and the intestines. Again,
effect on plasma calcium is mainly a transient one,
plasma calcium ion concentration. That is, the
consequently, very little prolonged effect on
over a long period, the net result is reduced
by decreased numbers of osteoblasts. Therefore,
of bone leads secondarily to osteoblastic activity,
osteoclasts. Also, because osteoclastic resorption
2. The second and more prolonged effect of
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
989
calcitonin is to decrease the formation of new
decreased numbers of osteoclasts are followed
osteoclastic and osteoblastic activity and,
lasting for a few hours to a few days at most.
Calcitonin also has minor effects on calcium han-
to be of such little import that they are seldom
considered.
in the Adult Human.
initial reduction of the calcium ion concentration
caused by calcitonin leads within hours to a powerful
term blood calcium ion concentration is not measura-
effect of the PTH system of control.
only a small effect on plasma calcium ion concentra-
greater because bone remodeling occurs rapidly in
times the total calcium in all the extracellular fluid.
,
citonin has a much more potent effect of reducing the
calcium absorption.
Calcium Ion Concentration
total quan-
tity of calcium in all the extracellular fluid of about
1 gram.
or from such a small amount of calcium in the extra-
cellular fluid would cause serious hypercalcemia or
to prevent this from occurring even before the
parathyroid and calcitonin hormonal feedback
systems have a chance to act.
First Line of Defense.
mainly CaHPO
4
or some similar compound loosely
bound in the bone and in reversible equilibrium with
the calcium and phosphate ions in the extracellular
fluid.
exchange is about 0.5 to 1 per cent of the total calcium
Because of the ease of deposition of these exchange-
the concentrations of extracellular fluid calcium and
phosphate ions above normal causes immediate dep-
these concentrations causes immediate absorption of
amorphous bone crystals are extremely small and their
total surface area exposed to the fluids of the bone is
one half of any excess calcium that appears in the
extracellular fluid is removed by this buffer function
amount of exchangeable calcium (a total of about 10
grams in the whole body) that provides an additional
extracellular fluid calcium ion concentration.
Line of Defense.
At the same time that the exchange-
and the calcitonin hormonal systems are beginning to
multiple mechanisms for reducing the calcium ion con-
centration back toward normal.
calcitonin causes rapid deposition of calcium in the
cause a high calcium ion concentration to return to
normal perhaps considerably more rapidly than can
be achieved by the exchangeable calcium-buffering
In prolonged calcium excess or prolonged calcium

only a few days.
concurrent elevation of phosphate, death can occur in
poisoning, but once such elevation develops along with
above 17 mg/dl before there is danger of parathyroid
Ordinarily, the level of calcium in the blood must rise
days.
ies throughout the body. This extensive
area of the stomach mucosa, and the walls of the arter-
of the kidneys, the thyroid gland, the acid-producing
begin to deposit in the alveoli of the lungs, the tubules
rated, so that calcium phosphate (CaHPO
absorbed from the bone. Therefore, the calcium and
usually the case, probably because the kidneys cannot
centration often rises markedly instead of falling, as is
high values. Even the extracellular fluid phosphate con-
rare occasions, extreme quantities of PTH are secreted,
When, on
depressed relaxation of the heart during diastole.
tion, abdominal pain, peptic ulcer, lack of appetite, and
peripheral nervous systems, muscle weakness, constipa-
earlier in the chapter, are depression of the central and
The effects of such elevated calcium levels, as detailed
level to rise to 12 to 15 mg/dl and, rarely, even higher.
phosphatase.
fore, one of the important diagnostic findings in hyper-
There-
alkaline phosphatase.
activity. When the osteoblasts become active, they
The cystic bone disease of hyperparathyroidism is called
from only slight trauma, especially where cysts develop.
in the form of so-called giant cell osteoclast “tumors.”
cystic areas of the bone that are filled with osteoclasts
decalcification and, occasionally, large punched-out
broken bone. Radiographs of the bone show extensive
away almost entirely. Indeed, the reason a hyper-
osteoblastic deposition, and the bone may be eaten
clastic reabsorption of bone, in severe hyperparathy-
of increased renal excretion of phosphate.
activity in the bones. This elevates the calcium ion con-
predispose to the development of such a tumor.
than in men or children, mainly because pregnancy and
secretion. The cause of primary hyperparathyroidism
parathyroid glands causes inappropriate, excess PTH
In primary hyperparathyroidism, an abnormality of the
prevent overactivity by this activated form of vitamin D.
unwanted effects, because it is sometimes difficult to
potent and much more rapid action. This can also cause
normal range. At times, it might be necessary to admin-
of calcium, keeps the calcium ion concentration in a
as 100,000 units per day, along with intake of 1 to 2 grams
tion of extremely large quantities of vitamin D, to as high
In most patients with hypothyroidism, the administra-
effective, hypoparathyroidism is usually not treated
because the tendency of the body to develop anti-
because its effect lasts for a few hours at most, and
However, because of the expense of this hormone,
Treatment of Hypoparathyroidism with PTH and Vitamin D.
of these muscles obstructs respiration, which is the usual
sitive to tetanic spasm are the laryngeal muscles. Spasm
develop. Among the muscles of the body especially sen-
low calcium level is reached, the usual signs of tetany
blood phosphate concentration may double. When this
9.4 mg/dl to 6 to 7 mg/dl within 2 to 3 days, and the
When the parathyroid glands are suddenly removed,
usually remains strong.
phates are not being absorbed from the bone, the bone
body fluids decreases. Yet, because calcium and phos-
totally inactive. As a result, calcium reabsorption from
PTH, the osteocytic reabsorption of exchangeable
When the parathyroid glands do not secrete sufficient
Vitamin D, and
Parathyroid Hormone,
tion from the gut and calcium excretion in the urine.
calcium, the long-term control of extracellular calcium
of calcium or, oppositely, becomes saturated with
by PTH. Yet, when the bone reservoir either runs out
buffer-reservoir of calcium that can be manipulated
out of calcium. Thus, in effect, the bones are a large
year or more, but eventually, even the bones will run
ciency of calcium in the diet, PTH often can stimulate
concentration. When a person has a continuing defi-
990
Unit XIV
Endocrinology and Reproduction
important in maintaining a normal plasma calcium ion
enough calcium absorption from the bones to main-
tain a normal plasma calcium ion concentration for 1
ion concentration resides almost entirely in the roles
of PTH and vitamin D in controlling calcium absorp-
Pathophysiology of
Bone Disease
Hypoparathyroidism
calcium decreases and the osteoclasts become almost
the bones is so depressed that the level of calcium in the
the calcium level in the blood falls from the normal of
cause of death in tetany unless appropriate treatment is
applied.
PTH
is occasionally used for treating hypoparathyroidism.
bodies against it makes it progressively less and less
with PTH administration.
ister 1,25-dihydroxycholecalciferol instead of the nonac-
tivated form of vitamin D because of its much more
Primary Hyperparathyroidism
ordinarily is a tumor of one of the parathyroid glands;
such tumors occur much more frequently in women
lactation stimulate the parathyroid glands and therefore
Hyperparathyroidism causes extreme osteoclastic
centration in the extracellular fluid while usually
depressing the concentration of phosphate ions because
Bone Disease in Hyperparathyroidism.
Although in mild
hyperparathyroidism new bone can be deposited
rapidly enough to compensate for the increased osteo-
roidism the osteoclastic absorption soon far outstrips
parathyroid person seeks medical attention is often a
Multiple fractures of the weakened bones can result
osteitis fibrosa cystica.
Osteoblastic activity in the bones also increases
greatly in a vain attempt to form enough new bone to
make up for the old bone absorbed by the osteoclastic
secrete large quantities of
parathyroidism is a high level of plasma alkaline
Effects of Hypercalcemia in Hyperparathyroidism.
Hyper-
parathyroidism can at times cause the plasma calcium
Parathyroid Poisoning and Metastatic Calcification
the level of calcium in the body fluids rises rapidly to
excrete rapidly enough all the phosphate being
phosphate in the body fluids become greatly supersatu-
4
) crystals
metastatic dep-
osition of calcium phosphate can develop within a few

in adults, especially in old age. It is different from osteo-
D–resistant rickets
of calcium and vitamin D, and it is called
phosphates by the renal tubules. This type of rickets
severe one.
hemodialysis, the problem of renal rickets is often a
vitamin D. In patients whose kidneys have been
form 1,25-dihydroxycholecalciferol, the active form of
prolonged kidney damage.
The cause of this
rickets” is a type of osteomalacia that results from
disability.
rickets can occur, although this almost never proceeds
feces. Under these conditions, an adult occasionally has
rhea, both vitamin D and calcium tend to pass into the
form insoluble soaps with fat; consequently, in steator-
bone growth as in children. However, serious deficien-
deficiency of vitamin D or calcium
of vitamin D. If vitamin D is not administered, little
and, equally important, on administering large amounts
The treatment of rickets depends on
Treatment of Rickets.
relieves the tetany immediately.
unless intravenous calcium is administered, which
falls below 7 mg/dl, the usual signs of tetany develop,
calcium may fall rapidly. As the blood level of calcium
bones finally become exhausted of calcium, the level of
calcium in the extracellular fluid. However, when the
and, therefore, maintain an almost normal level of
In the early stages of rickets, tetany
Tetany in Rickets
cified, and weak osteoid gradually takes the place of the
phosphate ions. Consequently, the newly formed, uncal-
down large quantities of osteoid, which does not
rapid osteoblastic activity as well. The osteoblasts lay
imposes marked physical stress on the bone, resulting in
extreme osteoclastic absorption of the bone; this in turn
During prolonged rickets, the
Rickets Weakens the Bones
excretion of phosphates in the urine.
system for preventing a falling level of phosphate, and
begins to fall. However, there is no good regulatory
greatly depressed. This is because the parathyroid
is only slightly depressed, but the level of phosphate is
The plasma calcium concentration in rickets
first few months of vitamin D deficiency.
months. Also, calcium and phosphate absorption from
without some supplementation in the diet. Rickets tends
intestines, as discussed earlier in the chapter.
, which prevents rickets by
adequately exposed to sunlight, the 7-dehydrocholes-
fluid, usually caused by lack of vitamin D. If the child is
calcium or phosphate deficiency in the extracellular
Rickets occurs mainly in children. It results from
Vitamin D De
high levels of PTH cause absorption of the bones.
(inadequate mineralization of the bones), and
the next section, the vitamin D deficiency leads to
dihydroxycholecalciferol. As discussed in more detail in
amounts of the active form of vitamin D, 1,25-
vitamin D deficiency or chronic renal disease in which
contrasts with primary hyperparathyroidism, which is
as a primary abnormality of the parathyroid glands. This
In secondary hyperparathyroidism, high levels of PTH
acid urine. For this reason, acidotic diets and acidic
alkaline media, the tendency for formation of renal
cause calcium precipitation at high calcium levels.
forming calcium phosphate stones.Also, calcium oxalate
calcium phosphate tend to precipitate in the kidney,
of these substances in the urine. As a result, crystals of
roidism must eventually be excreted by the kidneys,
tendency to form kidney stones. The reason is that the
result of elevated calcium, but they do have an extreme
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
991
Formation of Kidney Stones in Hyperparathyroidism
Most
patients with mild hyperparathyroidism show few signs
of bone disease and few general abnormalities as a
excess calcium and phosphate absorbed from the intes-
tines or mobilized from the bones in hyperparathy-
causing a proportionate increase in the concentrations
stones develop because even normal levels of oxalate
Because the solubility of most renal stones is slight in
calculi is considerably greater in alkaline urine than in
drugs are frequently used for treating renal calculi.
Secondary Hyperparathyroidism
occur as a compensation for hypocalcemia rather than
associated with hypercalcemia.
Secondary hyperparathyroidism can be caused by
the damaged kidneys are unable to produce sufficient
osteo-
malacia
Rickets—
ficiency
terol in the skin becomes activated by the ultraviolet
rays and forms vitamin D
3
promoting calcium and phosphate absorption from the
Children who remain indoors through the winter in
general do not receive adequate quantities of vitamin D
to occur especially in the spring months because vitamin
D formed during the preceding summer is stored in the
liver and available for use during the early winter
the bones can prevent clinical signs of rickets for the
Plasma Concentrations of Calcium and Phosphate Decrease
in Rickets
glands prevent the calcium level from falling by pro-
moting bone absorption every time the calcium level
the increased parathyroid activity actually increases the
marked compensatory increase in PTH secretion causes
causes the bone to become progressively weaker and
become calcified because of insufficient calcium and
older bone that is being reabsorbed.
almost never occurs because the parathyroid glands
continually stimulate osteoclastic absorption of bone
and the child may die of tetanic respiratory spasm
supplying adequate calcium and phosphate in the diet
calcium and phosphate are absorbed from the gut.
Osteomalacia—“Adult Rickets”.
Adults seldom have a
serious dietary
because large quantities of calcium are not needed for
cies of both vitamin D and calcium occasionally
occur as a result of steatorrhea (failure to absorb fat)
because vitamin D is fat-soluble and calcium tends to
such poor calcium and phosphate absorption that adult
to the stage of tetany but often is a cause of severe bone
Osteomalacia and Rickets Caused by Renal Disease.
“Renal
condition is mainly failure of the damaged kidneys to
removed or destroyed and who are being treated by
Another type of renal disease that leads to rickets
and osteomalacia is congenital hypophosphatemia,
resulting from congenitally reduced reabsorption of
must be treated with phosphate compounds instead
vitamin
.
Osteoporosis—Decreased
Bone Matrix
Osteoporosis is the most common of all bone diseases
Also, it increases in thickness and strength with age,
layer of cementum becomes thicker and stronger.
When the teeth are exposed to excessive strain, the
gen fibers and the cementum hold the tooth in place.
membrane, and then into the cementum. These colla-
from the bone of the jaw, through the periodontal
the tooth socket. Many collagen fibers pass directly
, which lines
might result when the teeth are struck by solid objects.
ant to compressional forces, and the collagen fibers
The calcium salts in dentin make it extremely resist-
of the pulp cavity.
, which line its inner surface along the wall
clasts, or spaces for blood vessels or nerves. Instead, it
does not contain any osteoblasts, osteocytes, osteo-
ference is its histological organization, because dentin
very much the same as those of bone. The major dif-
In other words, the principal constituents of dentin are
are imbedded in a strong meshwork of collagen fibers.
to those in bone but much more dense. These crystals
dentin, which has a strong, bony structure. Dentin is
The main body of the tooth is composed of
makes enamel resistant to acids, enzymes, and other
constituting only about 1 per cent of the enamel mass,
Also, the special protein fiber meshwork, although
The crystalline structure of the salts makes the
keratin of hair.
carbonate, magnesium, sodium, potassium, and other
formed. Enamel is composed of very large and very
Once the tooth has erupted, no more enamel is
ameloblasts.
The outer surface of the tooth is covered by a
neck
The collar between the crown and the root where the
which is the portion within the bony socket of the jaw.
trudes out from the gum into the mouth, and the
, which is the portion that pro-
crown
. The tooth can also be
, and
demonstrating its major functional parts: the
Figure 79–12 shows a sagittal section of a tooth,
of the Teeth
Function of the Different Parts
between the tooth surfaces.
the lower. This fitting is called
that interdigitate, so that the upper set of teeth fits with
the jaw teeth, 150 to 200 pounds. Also, the upper and
perform these functions, the jaws have powerful
The teeth cut, grind, and mix the food eaten. To
Physiology of the Teeth
cause osteoporosis.
many diseases of deficiency of protein metabolism can
specific effect of depressing osteoblastic activity. Thus,
, because massive
Cushing’s syndrome
torily; and (6)
age, so that bone matrix cannot be deposited satisfac-
growth factors diminish greatly, plus the fact that many
, in which growth hormone and other
clasts; (5)
postmenopausal lack of estrogen secretion
cells, including formation of osteoid by the osteoblasts;
, which is nec-
lack of vitamin C
cannot be formed; (3)
because of inactivity; (2)
lack
The many common causes of osteoporosis are (1)
diminished bone is excess osteoclastic activity.
sionally, as in hyperparathyroidism, the cause of the
rate of bone osteoid deposition is depressed. But occa-
bone usually is less than normal, and consequently the
fication. In osteoporosis the osteoblastic activity in the
992
Unit XIV
Endocrinology and Reproduction
malacia and rickets because it results from diminished
organic bone matrix rather than from poor bone calci-
of physical stress on the bones
malnutrition to the extent that sufficient protein matrix
essary for the secretion of intercellular substances by all
(4)
because
estrogens decrease the number and activity of osteo-
old age
of the protein anabolic functions also deteriorate with
quantities of glucocorticoids secreted in this disease
cause decreased deposition of protein throughout the
body and increased catabolism of protein and have the
muscles capable of providing an occlusive force
between the front teeth of 50 to 100 pounds and for
lower teeth are provided with projections and facets
occlusion, and it allows
even small particles of food to be caught and ground
enamel,
dentin, cementum
pulp
divided into the
root,
tooth is surrounded by the gum is called the
.
Enamel.
layer of enamel that is formed before eruption of the
tooth by special epithelial cells called
dense crystals of hydroxyapatite with adsorbed
ions imbedded in a fine meshwork of strong and
almost insoluble protein fibers that are similar in phys-
ical characteristics (but not chemically identical) to the
enamel extremely hard-much harder than the dentin.
corrosive agents because this protein is one of the
most insoluble and resistant proteins known.
Dentin.
made up principally of hydroxyapatite crystals similar
is deposited and nourished by a layer of cells called
odontoblasts
make it tough and resistant to tensional forces that
Cementum.
Cementum is a bony substance secreted
by cells of the periodontal membrane
Cementum
Dentin
Pulp chamber
Enamel
Crown
Root
Neck
Functional parts of a tooth.
Figure 79–12
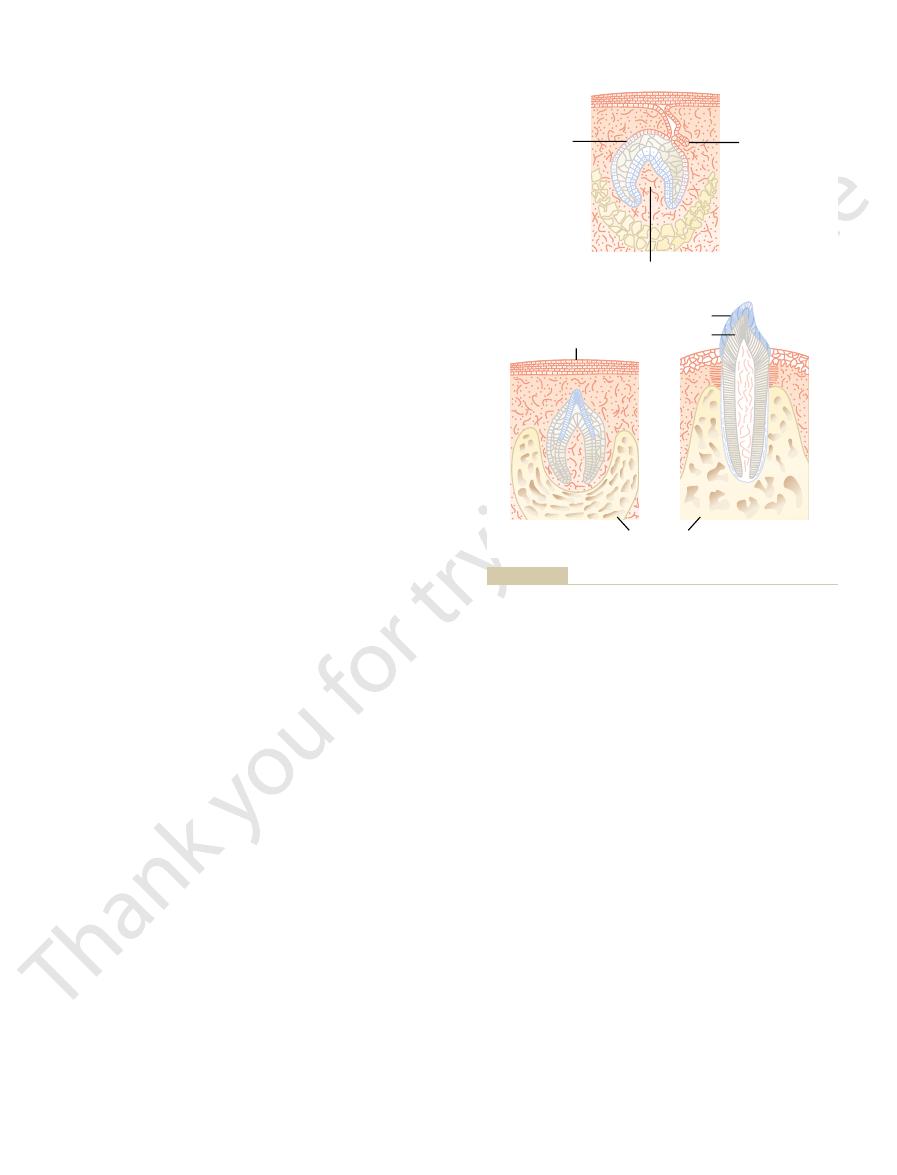
in bone. Deposition and reabsorption occur mainly in
old salts are being reabsorbed from the teeth, as occurs
Also, new salts are constantly being deposited while
cations bound together in a hard crystalline substance.
The salts of teeth, like those of bone, are composed of
Mineral Exchange in Teeth
tive, so that the teeth will be abnormal throughout life.
cient, the calcification of the teeth also may be defec-
be correspondingly healthy, but when they are defi-
all these factors are normal, the dentin and enamel will
vitamin D present, and the rate of PTH secretion.When
calcium and phosphate in the diet, the amount of
factors of metabolism, such as the availability of
hormones. Also, the deposition of salts in the early-
The rate of development and the speed of eruption of
uence Development of the Teeth.
Metabolic Factors In
the place of the original one.
Soon thereafter, the permanent tooth erupts to take
bone. In so doing, it erodes the root of the deciduous
like the deciduous tooth, pushes outward through the
When each permanent tooth becomes fully formed, it,
nent teeth throughout the first 6 to 20 years of life.
These tooth-producing organs slowly form the perma-
will be needed after the deciduous teeth are gone.
onic life, a tooth-forming organ also develops in the
Development of the Permanent Teeth.
offered in an attempt to explain this phenomenon. The
tion” is unknown, although several theories have been
oral epithelium into the mouth. The cause of “erup-
During early childhood, the teeth
Eruption of Teeth.
shown in Figure 79–13
formed on the inside, giving rise to an early tooth, as
is formed on the outside of the tooth, and dentin is
and the odontoblasts that secrete dentin. Thus, enamel
the tooth. The epithelial cells below invaginate upward
ameloblasts, which form the enamel on the outside of
producing organ. The epithelial cells above form
; this is followed by the development of a tooth-
tion and eruption of teeth. Figure 79–13
Figure 79–13 shows the forma-
Formation of the Teeth.
does not occur in everyone.
finally appear, which
wisdom teeth
number of permanent teeth 28 to 32, depending on
appear posteriorly in the jaws, making the total
tooth replaces it, and an additional 8 to 12 molars
year. After each deciduous tooth is lost, a permanent
2nd year of life, and they last until the 6th to the 13th
humans. They erupt between the 7th month and the
, and they number 20 in
milk teeth
, or
teeth during a lifetime. The first teeth are called the
for exchange of calcium, phosphate, and other miner-
all the way through the dentin; they are of importance
However, the odontoblasts are still viable and send
the pulp cavity remains essentially constant in size.
it smaller. In later life, the dentin stops growing and
encroach more and more on the pulp cavity, making
of the tooth, lay down the dentin but at the same time
are the odontoblasts, which, during the formative years
phatics. The cells lining the surface of the pulp cavity
dant supply of nerve fibers, blood vessels, and lym-
The pulp cavity of each tooth is filled with
jaws by adulthood and later.
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
993
causing the teeth to become more firmly seated in the
Pulp.
pulp,
which is composed of connective tissue with an abun-
projections into small dentinal tubules that penetrate
als with the dentin.
Dentition
Humans and most other mammals develop two sets of
deciduous teeth
whether the four
A shows
invagination of the oral epithelium into the dental
lamina
into the middle of the tooth to form the pulp cavity
B.
begin to protrude outward from the bone through the
most likely theory is that growth of the tooth root as
well as of the bone underneath the tooth progressively
shoves the tooth forward.
During embry-
deeper dental lamina for each permanent tooth that
tooth and eventually causes it to loosen and fall out.
fl
teeth can be accelerated by both thyroid and growth
forming teeth is affected considerably by various
hydroxyapatite with adsorbed carbonates and various
the dentin and cementum and to a very limited extent
Dentin
Enamel
Primordium of
enamel organ of
permanent tooth
Enamel organ
of milk tooth
Mesenchymal primordium of pulp
Oral epithelium
Alveolar bone
A
B
C
, Primordial tooth organ.
Figure 79–13
A
B, Developing tooth. C, Erupting tooth.

differentiation. Endocr Rev 23:763, 2002.
dihydroxyvitamin D(3): implications in cell growth and
factor/cytokine synthesis and signaling by 1alpha,25-
Gurlek A, Pittelkow MR, Kumar R: Modulation of growth
new PTH assays in renal osteodystrophy. Kidney Int 63:1,
Parathyroid hormone (PTH), PTH-derived peptides, and
Goodman WG, Juppner H, Salusky IB, Sherrard DJ:
Lancet 359:2018, 2002.
Delmas PD: Treatment of postmenopausal osteoporosis.
Compston JE: Sex steroids and bone. Physiol Rev 81:419,
Renal Physiol 286:F1005, 2004.
receptor in parathyroid gland physiology. Am J Physiol
Chen RA, Goodman WG: Role of the calcium-sensing
primary hyperparathyroidism. N Engl J Med 350:1746,
Bilezikian JP, Silverberg SJ: Clinical practice. Asymptomatic
porosis. JAMA 285:1415, 2001.
Altkorn D, Vokes T: Treatment of postmenopausal osteo-
applied pressure.
on the tensional side of the tooth. In this way, the tooth
teeth with appropriate braces. The gentle pressure
The orthodontist can usually correct malocclusion
the upper jaw, causing such undesirable effects as pain
adequately. Malocclusion occasionally also results in
jaw to grow to abnormal positions. In malocclusion,
caries as teeth without fluorine.
phosphate to “heal” the enamel surface. Regardless of
Finally, when small pits do develop in the enamel, flu-
soluble. Fluorine may also be toxic to the bacteria.
tals, which in turn makes the enamel several times less
enamel harder than usual, but fluorine ions replace
not contain fluorine. Fluorine does not make the
Teeth formed in chil-
metabolic substrate for many hours of the day, and the
candy, the bacteria are supplied with their preferential
parcels throughout the day, such as in the form of
is important. If carbohydrates are eaten in many small
ingested but the frequency with which it is eaten that
However, it is not the quantity of carbohydrate
content will lead to excessive development of caries.
carbohydrates for their nutrition, it has frequently
the high degree of solubility of the dentin salts.
dentin, it proceeds many times as rapidly because of
in volume as each dentin crystal. Once the carious
because the crystals of enamel are dense, but also
demineralization by acids than is dentin, primarily
development of caries. Enamel is far more resistant to
The enamel of the tooth is the primary barrier to the
olytic enzymes.
And once the salts have become absorbed, the remain-
and proteolytic enzymes. The acids are the major
In addition, they form acids (particularly lactic acid)
bolic systems are strongly activated and they multiply.
food. When carbohydrates are available, their meta-
are readily available to cause caries. These bacteria
cipitated products of saliva and food, on the teeth.
, a film of pre-
. The first event in the develop-
of bacteria on the teeth, the most common of which is
the upper and lower teeth to interdigitate properly.
. Caries refers to erosion of the teeth,
The two most common dental abnormalities are
Dental Abnormalities
ment throughout life.
enamel exhibits extremely slow mineral exchange, so
anism of this exchange in dentin is unclear. However,
the dentin and cementum of teeth, although the mech-
In summary, continual mineral exchange occurs in
mineral exchange.
have these characteristics, as explained earlier. This
of osteoblasts and osteoclasts, whereas dentin does not
identical to those of usual bone, including the presence
that of bone. The cementum has characteristics almost
ing bone of the jaw, whereas the rate of deposition and
The rate of absorption and deposition of minerals in
saliva instead of with the fluids of the pulp cavity.
in the enamel. In the enamel, these processes occur
994
Unit XIV
Endocrinology and Reproduction
mostly by diffusional exchange of minerals with the
the cementum is about equal to that in the surround-
absorption of minerals in the dentin is only one third
difference undoubtedly explains the different rates of
that it maintains most of its original mineral comple-
caries
and malocclusion
whereas malocclusion is failure of the projections of
Caries and the Role of Bacteria and Ingested Carbohydrates.
It is generally agreed that caries result from the action
Streptococcus mutans
ment of caries is the deposit of plaque
Large numbers of bacteria inhabit this plaque and
depend to a great extent on carbohydrates for their
culprit in causing caries because the calcium salts of
teeth are slowly dissolved in a highly acidic medium.
ing organic matrix is rapidly digested by the prote-
because each enamel crystal is about 200 times as large
process has penetrated through the enamel to the
Because of the dependence of the caries bacteria on
been taught that eating a diet high in carbohydrate
development of caries is greatly increased.
Role of Fluorine in Preventing Caries.
dren who drink water that contains small amounts of
fluorine develop enamel that is more resistant to caries
than the enamel in children who drink water that does
many of the hydroxyl ions in the hydroxyapatite crys-
orine is believed to promote deposition of calcium
the precise means by which fluorine protects the teeth,
it is known that small amounts of fluorine deposited in
enamel make teeth about three times as resistant to
Malocclusion.
Malocclusion is usually caused by a
hereditary abnormality that causes the teeth of one
the teeth do not interdigitate properly and therefore
cannot perform their normal grinding or cutting action
abnormal displacement of the lower jaw in relation to
in the mandibular joint and deterioration of the teeth.
by applying prolonged gentle pressure against the
causes absorption of alveolar jaw bone on the com-
pressed side of the tooth and deposition of new bone
gradually moves to a new position as directed by the
References
2004.
2001.
2003.

people. BMJ 327:89, 2003.
Woolf AD, Akesson K: Preventing fractures in elderly
Wharton B, Bishop N: Rickets. Lancet 362:1389, 2003.
Rev 81:1567, 2001.
Tordoff MG: Calcium: taste, intake, and appetite. Physiol
283:F367, 2002.
hyperparathyroidism. Am J Physiol Renal Physiol
Silver J, Kilav R, Naveh-Many T: Mechanisms of secondary
calcium-transporting epithelia, News Physiol Sci 18:158,
Peng JB, Brown EM, Hediger MA: Apical entry channels in
diagnosis, and therapy. JAMA 285:785, 2001.
vention, Diagnosis, and Therapy. Osteoporosis prevention,
N Engl J Med 343:1863, 2000.
Marx SJ: Hyperparathyroid and hypoparathyroid disorders.
22:477, 2001.
fractures and therapeutic implications. Endocr Rev
roidism in the elderly: consequences for bone loss and
Lips P: Vitamin D deficiency and secondary hyperparathy-
phia: WB Saunders Co, 2003.
Williams Textbook of Endocrinology, 10th ed. Philadel-
Larsen PR, Kronenberg HM, Melmed S, Polonsky KS:
78:1193, 1998.
of the molecular actions of vitamin D. Physiol Rev
Jones G, Strugnell SA, DeLuca HF: Current understanding
signalling. Nat Rev Mol Cell Biol 4:530, 2003.
Hofer AM, Brown EM: Extracellular calcium sensing and
Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
Chapter 79
995
NIH Consensus Development Panel on Osteoporosis Pre-
2003.
