
as an enzyme to cause breakdown of ATP into adenosine diphosphate (ADP), thus
from ATP. Myosin, one of the important contractile proteins of the muscle fiber, acts
ATP Energizes Muscle Contraction.
tion, which keeps the ammonia concentration of the body fluids at a low level.
the extreme toxicity of ammonia in the body fluids, one can see the value of this reac-
energy to form urea, which is simply discarded by the body. However, remembering
formation from ammonia. One might wonder about the advisability of expending
of the body. Even the urea excreted by the kidneys requires ATP to cause its
synthesis of cholesterol, phospholipids, the hormones, and almost all other substances
thesis of fatty acids from acetyl coenzyme A. In addition, ATP energy is used for the
ATP energy is also used in the synthesis of glucose from lactic acid and in the syn-
tually stored in each of the peptide linkages.
of 48,000 calories of energy, which is far more than the 500 to 5000 calories even-
the cascade of reactions required to form each peptide linkage. This provides a total
5000 calories of energy per mole. It will be recalled from the discussion of protein
linkages, depending on which types of amino acids are linked, require from 500 to
ages between amino acids during the synthesis of proteins. The different peptide
tant intracellular processes that require ATP energy is the formation of peptide link-
ATP Energizes the Synthesis of Most Important Cellular Components.
citric acid cycle and then to acetyl coenzyme A and carbon dioxide.
Combustion of proteins,
beta-oxidation.
citric acid (Krebs) cycle.
other sugars such as fructose; this occurs in the cytoplasm of the cell through
—mainly glucose, but also smaller amounts of
we discussed the transfer of energy from various foods to ATP. To summarize, ATP
In previous chapters,
ATP Is Generated by Combustion of Carbohydrates, Fats, and Proteins.
only a few hundred of the available 12,000 calories, and the remainder of this energy
energy transfer is achieved. Some chemical reactions that require ATP energy use
energy in each bond, when liberated by decomposition of ATP, is enough to cause
conditions) vested in each of its two high-energy phosphate bonds. The amount of
mole under standard conditions, but as much as 12,000 calories under physiologic
large quantity of free energy (about 7300 calories, or 7.3 Calories [kilocalories], per
An attribute of ATP that makes it highly valuable as an energy currency is the
triphosphate, GTP). Many of the attributes of ATP are presented in Chapter 2.
can be done only through this medium of ATP (or the similar nucleotide guanosine
Indeed, the transfer of energy from foodstuffs to most functional systems of the cells
tions. For this reason, ATP has been called an energy “currency” in cell metabolism.
(ATP), which can be used as an energy source for almost all other cellular func-
bohydrates, fats, and proteins can all be used by cells to
In the past few chapters, we have pointed out that car-
an “Energy Currency”
(ATP) Functions as
Adenosine Triphosphate
C
H
A
P
T
E
R
7
2
881
Energetics and Metabolic Rate
in Metabolism
synthesize large quantities of adenosine triphosphate
almost any step of any chemical reaction in the body to take place if appropriate
is lost in the form of heat.
is produced from
1. Combustion of carbohydrates
the anaerobic process of glycolysis and in the cell mitochondria through the
aerobic
2. Combustion of fatty acids in the cell mitochondria by
3.
which requires hydrolysis to their component amino
acids and degradation of the amino acids to intermediate compounds of the
Among the most impor-
synthesis in Chapter 3 that four high-energy phosphate bonds are expended during
Muscle contraction will not occur without energy

hypoxia.When a person stops breathing, there is already
the stored glycogen of the cells.
Thus,
state, without the additional expenditure of ATP.
using 1 mole of ATP before it can begin to be split; this
moles of ATP. The reason for this difference is that free
into pyruvic acid, 2 moles of ATP are formed. However,
gen to pyruvic acid. For each mole of glucose that is split
without the utilization of oxygen;
only significant foods that can be used to provide energy
cause synthesis of ATP. However,
carbohydrates, fats, and proteins can all be oxidized to
discussions in Chapters 67 through 69, it is noted that
derived from foods only by oxidative metabolism. In the
Anaerobic Versus Aerobic Energy
reactions in the body depend on this constancy.
constant, because the rates of almost all the metabolic
importance of keeping the concentration of ATP nearly
ATP “buffer” system. One can readily understand the
reason, we can call the ATP-phosphocreatine system an
level as long as any phosphocreatine remains. For this
the concentration of ATP at an almost constant high
phocreatine to synthesize new ATP. This effect keeps
ATP by the cells calls forth the energy from the phos-
its energy elsewhere. Therefore, the slightest usage of
every time even the slightest amount of ATP expends
to proceed rapidly toward the formation of new ATP
causes the reaction between phosphocreatine and ADP
to 1500 calories per mole greater than that in ATP)
ATP + Creatine
Phosphocreatine + ADP
between ATP and phosphocreatine is demonstrated by
tional systems of the cells. This reversible interrelation
transferred rapidly back to ATP and then to the func-
to be used up, the energy in the phosphocreatine is
this storehouse of energy. Then, when the ATP begins
used to synthesize phosphocreatine, thus building up
of ATP are available in the cell, much of its energy is
energy interchangeably with ATP. When extra amounts
and the functional cellular systems, but it can transfer
Unlike ATP, phosphocreatine cannot act as a direct
energy phosphate bonds of ATP. The formula for crea-
centrations of the reactants). This is slightly greater than
abundant. Also, the high-energy bond (
energy phosphate bonds, is three to eight times as
Phosphocreatine,
the cells.
pling agent for energy transfer, this substance is not the
Despite the paramount importance of ATP as a cou-
Energy and as an “ATP Buffer”
positions.
transport systems energized by ATP then retransport
during each of the action potentials. However, active
this energy storage, with small amounts of potassium
of energy. The energy needed to pass each action
of energy storage. Likewise, a high concentration
differences of ions across the membranes. That is, a
The energy used during
ATP Energizes Nerve Conduction.
secreted by the glandular cells. In addition, energy is
substances against concentration gradients, because
The same principles
ATP Energizes Glandular Secretion.
requires energy, which is provided by ATP.
direction. To oppose the electrochemical gradient
an electrochemical gradient, even though the natural
glucose, amino acids, and acetoacetate can occur against
blood is discussed. In each instance, we noted that active
Chapters 4, 27, and 65, active transport of electrolytes
ATP Energizes Active Transport Across Membranes.
postulated mechanism by which ATP energy is used to
level during short bursts of maximal contraction. The
of ATP usage can rise to at least 150 times the resting
when muscle contraction is not occurring, but this rate
a small amount of ATP is normally degraded in muscles
releasing the energy required to cause contraction. Only
Metabolism and Temperature Regulation
882
Unit XIII
cause muscle contraction is discussed in Chapter 6.
In
and various nutrients across cell membranes and from
the renal tubules and gastrointestinal tract into the
transport of most electrolytes and substances such as
diffusion of the substances would be in the opposite
apply to glandular secretion as to the absorption of
energy is required to concentrate substances as they are
required to synthesize the organic compounds to be
secreted.
propagation of a nerve impulse is derived from
the potential energy stored in the form of concentration
high concentration of potassium inside the fiber and a
low concentration outside the fiber constitute a type
of sodium on the outside of the membrane and a low
concentration on the inside represent another store
potential along the fiber membrane is derived from
transferring out of the cell and sodium into the cell
the ions back through the membrane to their former
Phosphocreatine Functions as an
Accessory Storage Depot for
most abundant store of high-energy phosphate bonds in
which also contains high-
~) of phospho-
creatine contains about 8500 calories per mole under
standard conditions and as much as 13,000 calories per
mole under conditions in the body (37°C and low con-
the 12,000 calories per mole in each of the two high-
tinine phosphate is the following:
coupling agent for energy transfer between the foods
the following equation:
Ø≠
Note particularly that the higher energy level of the
high-energy phosphate bond in phosphocreatine (1000
Anaerobic energy means energy that can be derived
from foods without the simultaneous utilization of
oxygen; aerobic energy means energy that can be
carbohydrates are the
this energy release
occurs during glycolytic breakdown of glucose or glyco-
when stored glycogen in a cell is split to pyruvic acid,
each mole of glucose in the glycogen gives rise to 3
glucose entering the cell must be phosphorylated by
is not true of glucose derived from glycogen because it
comes from the glycogen already in the phosphorylated
the best source of energy under anaerobic conditions is
Anaerobic Energy Utilization During Hypoxia.
One of the prime
examples of anaerobic energy utilization occurs in acute
a small amount of oxygen stored in the lungs and an
O
HOOC
CH
O
H
N
H
N
C
NH
~
~
2
CH
3
P
OH
glycogen follows rapidly. Thus, oxidative metabolism
house is used first, and then anaerobic breakdown of
by oxidative metabolism, the phosphocreatine store-
cellular activities. If greater amounts of energy are
nerve impulse conduction, active absorption, and other
and growth, muscle contraction, glandular secretion,
Energy from ATP can be used by the different func-
energy is in this energy storehouse.
present in the cells than ATP, much of the cells’ stored
equilibrium with phosphocreatine in the cells, and
to form additional ATP. In turn, ATP is in reversible
from carbohydrates, fats, proteins, and other substances
ATP and the aerobic utilization of compounds derived
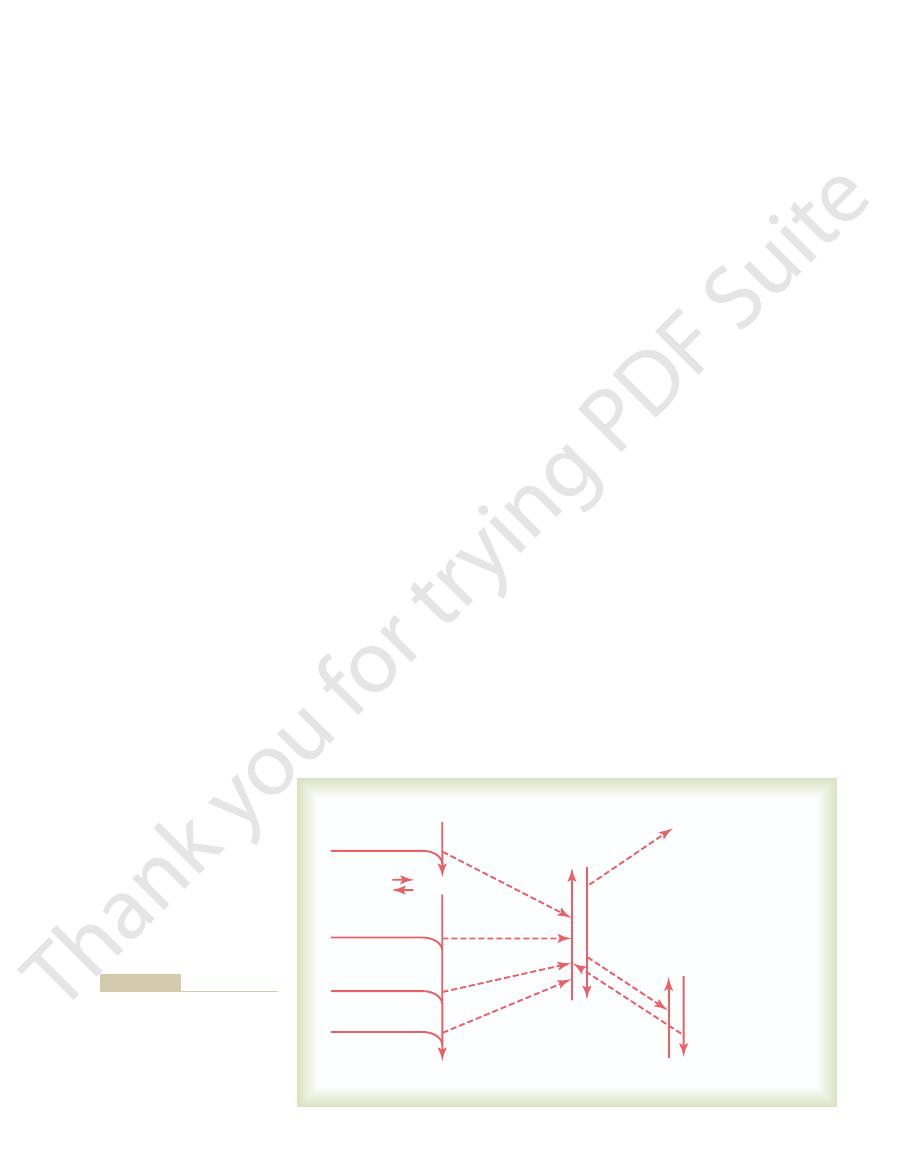
shown in Figure 72–1. This figure demonstrates the
ite picture of overall energy utilization by the cells, as
preceding discussion, we can now synthesize a compos-
With the background of the past few chapters and of the
important in many types of athletics.
Chapter 84 in relation to sports physiology; the ability
The principle of oxygen debt is discussed further in
oxygen debt.
its normal level. This extra consumption of oxygen after
of oxygen bound with hemoglobin and myoglobin, and
phocreatine, (4) to re-establish normal concentrations
ATP, (3) to reconvert creatine and phosphate to phos-
to reconvert adenosine monophosphate and ADP to
has accumulated during exercise back into glucose, (2)
sometimes for as long as 1 hour thereafter. This addi-
cise, a person continues to breathe hard and to consume
muscles, where it is stored once more in the form of
to glucose occurs principally in the liver cells, and
and oxidized in the citric acid cycle. The reconversion
exercise is over, oxidative metabolism is used to recon-
the lactic acid concentration of the blood rises. After the
during strenuous bouts of exercise is reduced, whereas
colysis. As a result, the glycogen content of muscles
quently, most of the extra energy required during stren-
rapidly than can oxidative release of energy. Conse-
can be maintained for only 5 to 10 seconds.
using all the phosphocreatine, maximum contraction
the cells is three to eight times this amount, but even by
than a second or so. The amount of phosphocreatine in
5 mmol/L of intracellular fluid, and this amount can
The maximum amount of ATP in muscle is only about
in the muscle cells, (2) phosphocreatine in the cells, and
comes from anaerobic sources: (1) ATP already present
they are too slow to respond. Instead, the extra energy
are much less capable during prolonged activity. Most
lactic acid, which diffuses out of the cells, as described
ting into pyruvic acid, and the pyruvic acid becoming
from glycolysis—that is, the glycogen of the cells split-
of energy. This can be derived for another minute or so
processes functioning for only about 2 minutes. Contin-
blood. This oxygen is sufficient to keep the metabolic
Chapter 72
Energetics and Metabolic Rate
883
additional amount stored in the hemoglobin of the
ued life beyond this time requires an additional source
in Chapter 67.
Anaerobic Energy Utilization During Strenuous Bursts of Activity
Is Derived Mainly from Glycolysis.
Skeletal muscles can
perform extreme feats of strength for a few seconds but
of the extra energy required during these bursts of activ-
ity cannot come from the oxidative processes because
(3) anaerobic energy released by glycolytic breakdown
of glycogen to lactic acid.
maintain maximum muscle contraction for no more
Release of energy by glycolysis can occur much more
uous activity that lasts for more than 5 to 10 seconds but
less than 1 to 2 minutes is derived from anaerobic gly-
vert about four fifths of the lactic acid into glucose;
the remainder becomes pyruvic acid and is degraded
the glucose is then transported in the blood back to the
glycogen.
Oxygen Debt Is the Extra Consumption of Oxygen After Completion
of Strenuous Exercise.
After a period of strenuous exer-
large amounts of oxygen for at least a few minutes and
tional oxygen is used (1) to reconvert the lactic acid that
(5) to raise the concentration of oxygen in the lungs to
exercise is over is called repaying the
of a person to build up an oxygen debt is especially
Summary of Energy Utilization
by the Cells
anaerobic utilization of glycogen and glucose to form
because larger quantities of phosphocreatine are
tioning systems of the cells to provide for synthesis
demanded for cellular activities than can be provided
O
Creatine + PO
5. Active absorption
ATP
Acetyl-CoA
Deaminated
amino acids
Other substrates
Glucose
Glycogen
AMP
Phosphocreatine
(Anaerobic)
Energy for
1. Synthesis and growth
2. Muscular contraction
3. Glandular secretion
4. Nerve conduction
6. Etc.
CO
2
+
H
2
4
Lactic acid Pyruvic acid
Press, 1946, 1952.)
(Modified from Soskin S, Levine
fer from foods to the adenylic
Figure 72–1
Overall schema of energy trans-
acid system and then to the
functional elements of the cells.
R: Carbohydrate Metabolism.
Chicago: University of Chicago

person with diabetes mellitus—that is, the substrate
by the rising levels of the curves. As an example, when
the reaction increases proportionately, as demonstrated
from an arbitrary value of 1 up to 2, 4, or 8, the rate of
enzyme. Thus, as the enzyme concentration increases
of the figure, the rate of a chemical reaction is deter-
Figure 72–2 shows that
Role of Enzyme Concentration in Regulation of Metabolic
Figure
Michaelis-Menten equation.
This is called the
tration of the substrate that binds with the enzyme. The
that it can react with other substances. Therefore, the
loosely with one of the substrates of the reaction. This
The mechanism by which an enzyme catalyzes a
throughout the body.
enzymatically catalyzed chemical reactions, which are
cussing the control of energy release in the cell, it is nec-
in the Cell
Control of Energy Release
slower rates of usage, the oxidative processes can con-
nearly as rapidly as the anaerobic processes can, but at
Metabolism and Temperature Regulation
884
Unit XIII
cannot deliver bursts of extreme energy to the cells
tinue as long as energy stores (mainly fat) exist.
Rate Control of Enzyme-Catalyzed Reactions.
Before dis-
essary to consider the basic principles of rate control of
the types of reactions that occur almost universally
chemical reaction is for the enzyme first to combine
alters the bonding forces on the substrate sufficiently so
rate of the overall chemical reaction is determined by
both the concentration of the enzyme and the concen-
basic equation expressing this concept is as follows:
72–2 shows the application of this equation.
Reactions.
when the substrate is
present in high concentration, as shown in the right half
mined almost entirely by the concentration of the
large quantities of glucose enter the renal tubules in a
glucose is in great excess in the tubules—further
increases in tubular glucose have little effect on glucose
K
Substrate
K
Enzyme
Substrate
Rate of reaction
1
2
=
¥
[
]
¥
[
]
+
[
]
peptide linkages, and this stores energy in these link-
thesized, large portions of ATP are used to form the
becomes heat. For example, when proteins are syn-
tional systems of the cells, most of this eventually
the functional systems.
so that even under optimal conditions, no more than 27
ferred from ATP to the functional systems of the cells,
tion. Then, still more energy becomes heat as it is trans-
the energy in foods becomes heat during ATP forma-
of this energy becomes heat. On average, 35 per cent of
in foods is transferred to ATP; instead, a large portion
the preceding chapters, we noted that not all the energy
Body.
heat liberation during chemical reactions.
ical reactions in all the cells of the body, and the
The
because all the ADP soon becomes ATP.
absence of cellular activity, the release of energy stops
trolled by the degree of activity of the cell. In the
process, the amount of energy released in the cell is con-
release of energy from food. Thus, by this simple
of activity of the cell. This ADP then automatically
concentration of ADP in direct proportion to the degree
of activity, ATP is converted into ADP, increasing the
When the cells become active, regardless of the type
for almost all energy metabolism of the body.
energy in the body. Thus,
metabolic pathways that release energy from food, as
strates are quite slow. They include all the oxidative
cal reactions that depend on ADP as one of the sub-
ADP in the cells is extremely slight, so that the chemi-
conditions, the concentration of
in the entire series.
rate of reaction of the slowest step in the series. This is
tion, and so on. Therefore, the overall rate of a complex
reactions of the body occur in series, with the product
and renal tubules when their concentrations are low.
enzyme concentration. This is the relationship seen in
tion, the rate of the reaction becomes directly propor-
Note also in Figure 72
Role of Substrate Concentration in Regulation of Meta-
the concentration of the glucose itself.
transport enzymes in the proximal tubular cells, not by
rated. Under these conditions, the rate of reabsorption
reabsorption, because the transport enzymes are satu-
of the glucose is limited by the concentration of the
bolic Reactions.
–2 that when the
substrate concentration becomes low enough that only
a small portion of the enzyme is required in the reac-
tional to the substrate concentration as well as the
the absorption of substances from the intestinal tract
Rate Limitation in a Series of Reactions.
Almost all chemical
of one reaction acting as a substrate for the next reac-
series of chemical reactions is determined mainly by the
called the rate-limiting step
ADP Concentration as a Rate-Controlling Factor in Energy
Release.
Under resting
well as essentially all other pathways for the release of
ADP is a major rate-limiting
factor
increases the rates of all the reactions for the metabolic
Metabolic Rate
metabolism of the body simply means all the chem-
meta-
bolic rate is normally expressed in terms of the rate of
Heat Is the End Product of Almost All the Energy Released in the
In discussing many of the metabolic reactions in
per cent of all the energy from food is finally used by
Even when 27 per cent of the energy reaches the func-
8
4
Enzyme concentration
2
1
Rate of reaction
Substrate concentration
enzyme-catalyzed reaction.
Effect of substrate and enzyme concentrations on the rate of
Figure 72–2

In general, over a 24-hour period, a person performing
about 17 times as much energy as lying in bed asleep.
physical activity. For example, walking up stairs requires
ent individuals, depending on the type and amount of
energy expenditure, but it can vary markedly in differ-
The amount of energy used to perform daily physical
ing only essential functions is 2000 Calories.
2000 to 2250 Calories. Therefore, the approximate daily
without exercising, his total energy requirement reaches
about 1850 Calories per day. If he sits in a chair all day
or more Calories, so that the same man lying in bed and
amount of energy used each day by an additional 200
bed all day uses about 1650 Calories of energy. The
for Daily Activities
processing food; and (4) maintaining body temperature.
various physical activities; (3) digesting, absorbing, and
metabolic rate); (2) performing
into several measurable components, including energy
American diet. Energy output can also be partitioned
from fats, and 15 per cent from proteins in the average
intake is derived from carbohydrates, 40 per cent
stable body weight. About 45 per cent of daily energy
As discussed in Chapter 71, energy intake is balanced
Energy Output
Energy Metabolism—
versely, if the person obtains most energy from fat, the
Calories/L), would be about 4 per cent too little. Con-
for the average energy equivalent of oxygen (4.825
culated quantity of energy liberated, based on the value
the period of the metabolic rate determination, the cal-
given period of time.
oxygen; using this energy equivalent, one can calculate
This is called the
4.825 Calories.
ated per liter of oxygen used in the body averages about
lized. For the average diet, the
oxygen, regardless of the type of food being metabo-
gures, it is striking how nearly equiva-
4.60 Calories.
are released; with fat, 4.70 Calories; and with protein,
released; when metabolized with starches, 5.06 Calories
metabolized with glucose, 5.01 Calories of energy are
rate of oxygen utilization. When 1 liter of oxygen is
be calculated with a high degree of accuracy from the
different foods, the whole-body metabolic rate can also
Indirect Calorimetry—The “Energy Equivalent” of Oxygen.
and is used only for research purposes.
s body.
an accurate thermometer, is equal to the rate at which
heat gain by the water bath, which can be measured with
the air through pipes in a cool water bath. The rate of
air of the chamber. However, the air temperature within
chamber. Heat formed by the subject
The
calorimeter.
body in a large, specially constructed
try, one measures the quantity of heat liberated from the
heat liberated from the body in a given time.
external work, the whole-body metabolic rate can be
Direct Calorimetry Measures Heat Liberated from the Body.
Metabolic Rate
Measurement of the Whole-Body
kilocalorie,
body. Consequently, the Calorie
C. The calorie is
is the unit used for this purpose. It will be recalled that
functional processes of the body. Most often, the
and related subjects intelligently, it is necessary to use
To discuss the metabolic rate of the body
diture of energy is not taking place, all the energy
raising a mass against gravity. But when external expen-
body up steps, a type of potential energy is created by
some form of work outside the body. For instance, when
eventually converted into heat. The only signi
vessels, the friction of the different layers of blood
tial energy. As the blood
pumping blood. The blood distends the arterial system,
within the tissues, which generates heat.
limbs can move. This viscous movement causes friction
ity. Much of this energy simply overcomes the viscosity
body.
When proteins are degraded, the energy stored in the
ages. But there is also continuous turnover of proteins
Chapter 72
Energetics and Metabolic Rate
885
—
some being degraded while others are being formed.
peptide linkages is released in the form of heat into the
Another example is the energy used for muscle activ-
of the muscles themselves or of the tissues so that the
Consider also the energy expended by the heart in
and this distention itself represents a reservoir of poten-
flows through the peripheral
flowing over one another and the friction of the blood
against the walls of the vessels turn all this energy into
heat.
Essentially all the energy expended by the body is
ficant
exception occurs when the muscles are used to perform
the muscles elevate an object to a height or propel the
released by the metabolic processes eventually becomes
body heat.
The Calorie.
some unit for expressing the quantity of energy released
from the different foods or expended by the different
Calorie
1 calorie—spelled with a small “c” and often called a
gram calorie—is the quantity of heat required to raise
the temperature of 1 gram of water 1°
much too small a unit when referring to energy in the
—sometimes spelled
with a capital “C” and often called a
which
is equivalent to 1000 calories—is the unit ordinarily
used in discussing energy metabolism.
Because a person ordinarily is not performing any
determined by simply measuring the total quantity of
In determining the metabolic rate by direct calorime-
subject is placed in an air chamber that is so well insu-
lated that no heat can leak through the walls of the
’s body warms the
the chamber is maintained at a constant level by forcing
heat is liberated by the subject’
Direct calorimetry is physically difficult to perform
Because more than 95 per cent of the energy expended
in the body is derived from reactions of oxygen with the
Using these fi
lent are the quantities of energy liberated per liter of
quantity of energy liber-
energy equivalent of
with a high degree of precision the rate of heat libera-
tion in the body from the quantity of oxygen used in a
If a person metabolizes only carbohydrates during
calculated value would be about 4 per cent too great.
Factors That Influence
with energy output in healthy adults who maintain a
used for (1) performing essential metabolic functions of
the body (the “basal”
Overall Energy Requirements
An average man who weighs 70 kilograms and lies in
process of eating and digesting food increases the
eating a reasonable diet requires a dietary intake of
energy requirement for a very sedentary man perform-
activities is normally about 25 per cent of the total
heavy labor can achieve a maximal rate of energy uti-
skeletal muscle mass.
cant. Much of this effect of the male
increase the BMR a small amount, but usually not
about 10 to 15 per cent. The female sex hormones may
The male sex
regions.
living in different geographical zones; for example,
body and therefore increases metabolic rate. Adapta-
normal. As discussed in Chapter 76, thyroxine increases
normal. Conversely, total loss of thyroid secretion
gland secretes maximal amounts of thyroxine, the meta-
When the thyroid
uence the BMR, as discussed next.
of adipose tissue. However, there are other factors that
women, compared with men, are due partly to their
rate of metabolism. Likewise, slightly lower BMRs in
ment of muscle with adipose tissue, which has a lower
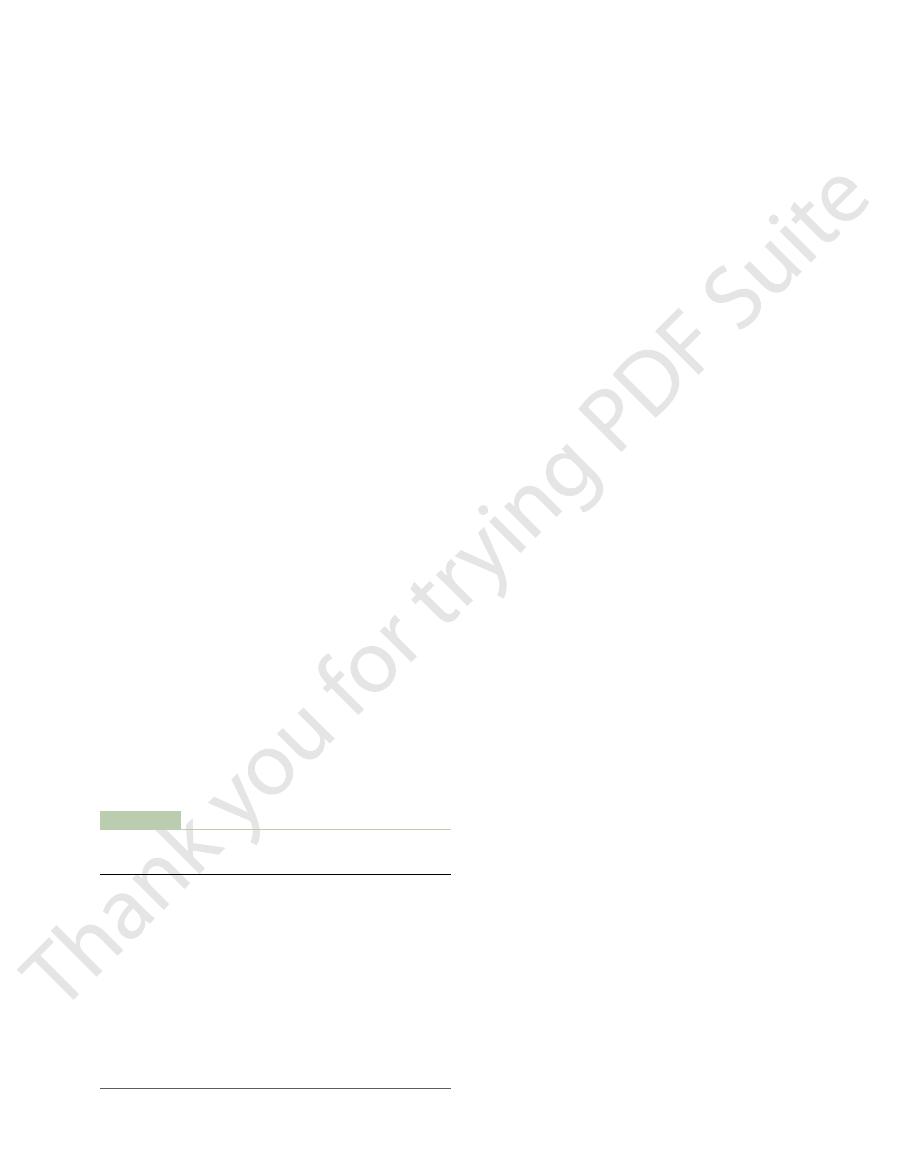
females of different ages are shown in Figure 72
height and weight. The average values for males and
square meter of body surface area, calculated from
reason, BMR is usually corrected for differences in
accounts for 20 to 30 per cent of the BMR. For this
Skeletal muscle, even under resting conditions,
amount of skeletal muscle and body size.
other organs, the
ties of the central nervous system, heart, kidneys, and
per hour in an average 70-kilogram man. Although
The BMR normally averages about 65 to 70 Calories
6. No physical activity is permitted during the test.
5. The temperature of the air must be comfortable
4. All psychic and physical factors that cause
3. No strenuous activity is performed for at least 1
sleep.
2. The BMR is determined after a night of restful
hours.
1. The person must not have eaten food for at least 12
s metabolic rate with that of another. The usual
able among different individuals, measurement of
expenditure in most sedentary individuals (Figure 72
of the body. This minimum level of energy required
Even when a person is at complete rest, considerable
for the Body to Exist
The Minimum Energy Expenditure
ical activity.
lization as great as 6000 to 7000 Calories, or as much as
Metabolism and Temperature Regulation
886
Unit XIII
3.5 times the energy used under conditions of no phys-
Basal Metabolic Rate (BMR)—
energy is required to perform all the chemical reactions
to exist is called the basal metabolic rate (BMR) and
accounts for about 50 to 70 per cent of the daily energy
–3).
Because the level of physical activity is highly vari-
the BMR provides a useful means of comparing one
person’
method for determining BMR is to measure the rate of
oxygen utilization over a given period of time under the
following conditions:
hour before the test.
excitement must be eliminated.
and between 68° and 80°F.
much of the BMR is accounted for by essential activi-
variations in BMR among different
individuals are related mainly to differences in the
body size by expressing it as Calories per hour per
–4.
Much of the decline in BMR with increasing age is
probably related to loss of muscle mass and replace-
lower percentage of muscle mass and higher percentage
can infl
Thyroid Hormone Increases Metabolic Rate.
bolic rate sometimes rises 50 to 100 per cent above
decreases the metabolic rate to 40 to 60 per cent of
the rates of the chemical reactions of many cells in the
tion of the thyroid gland—with increased secretion in
cold climates and decreased secretion in hot climates—
contributes to the differences in BMRs among people
people living in arctic regions have BMRs 10 to 20 per
cent higher than those of persons living in tropical
Male Sex Hormone Increases Metabolic Rate.
hormone testosterone can increase the metabolic rate
enough to be signifi
sex hormone is related to its anabolic effect to increase
Growth Hormone Increases Metabolic Rate.
Growth hormone
can increase the metabolic rate 15 to 20 per cent as a
result of direct stimulation of cellular metabolism.
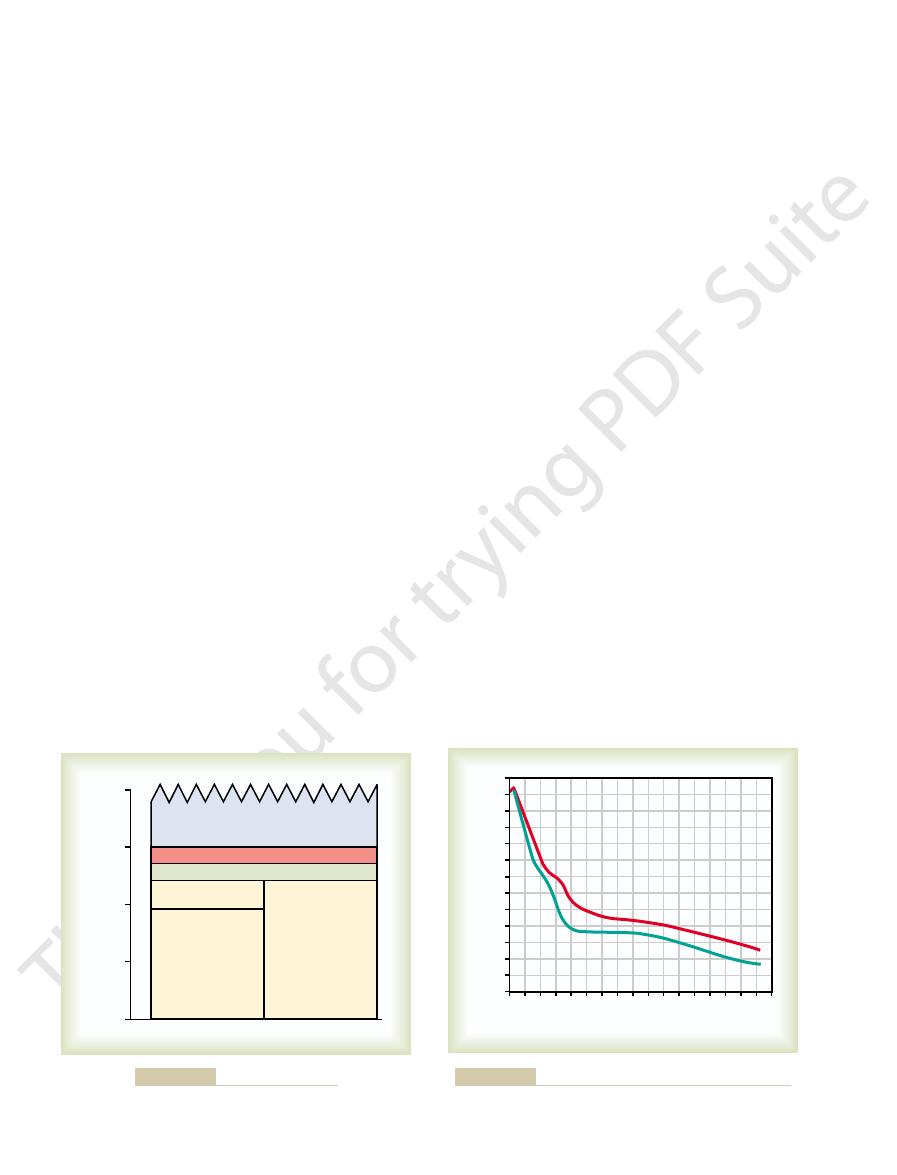
Thermic effect of food (8%)
100
Purposeful physical activity (25%)
Nonexercise activity (7%)
Arousal
Sleeping
metabolic
rate
Basal
metabolic
rate (60%)
75
50
25
0
% Daily energy usage
Components of energy expenditure.
Figure 72–3
50
70
80
20
40
0
10
30
54
52
50
48
46
44
42
40
38
Males
Females
36
34
32
30
Age (years)
60
Basal metabolism (Calories/m
2
/hour)
Normal basal metabolic rates at different ages for each sex.
Figure 72–4
intake. The mechanism responsible for sympathetic acti-
buffer against obesity. Recent studies indicate that
per cent, although this might increase signi
who has virtually no brown fat, is probably less than 15
s metabolism more than 100 per cent.The mag-
cells, and maximal sympathetic stimulation can increase
large amount of heat but almost no ATP, so that almost
the sympathetic nerves, the mitochondria produce a
That is, when the cells are stimulated by
of one large fat globule. In these cells, the process of
amounts of heat. This type of fat contains large numbers
brown fat,
In certain types of fat tissue, called
which releases norepinephrine and epinephrine, which
response to cold stress. This type of thermogenesis is
nonshivering thermogenesis,
as discussed in Chapter 73. Another mechanism,
by increasing muscle activity in response to cold stress,
aimed primarily at regulation of body temperature.
food cause liberation of heat, these mechanisms are not
in many persons.
The thermogenic effect of food accounts for
and this lasts for 3 to 12 hours. This effect of protein on
the metabolic rate usually begins rising within an hour,
about 4 per cent. However, after a high-protein meal,
hydrates or fats, the metabolic rate usually increases
body. This is called the
with digestion, absorption, and storage of food in the
After a meal is ingested, the metabolic rate increases as
Thermogenic Effect of Food
s daily energy usage.
Together,
dgeting.
no daily exercise or physical work, signi
physical activity to prevent excess fat stores and obesity.
and the excess energy is stored mainly as fat.This under-
caloric intake periodically exceeds energy expenditure,
food supplies are plentiful, such as the United States,
balance. However, in industrialized countries where
cal activity among individuals, this component of energy
Table 72
body for a few seconds to about 50 times normal, or to
a few seconds. For the entire body, maximal muscle
rate is strenuous exercise. Short bursts of maximal
The factor that most dramatically increases metabolic
Energy Used for Physical Activities
decrease in metabolic rate, to the extent that the body
nal stages of many disease conditions, the
cells. In the
This fall is due to two principal factors: (1) decreased
decreases 10 to 15 per cent below normal during sleep.
The metabolic rate
in temperature. This is discussed in more detail in
an average of about 120 per cent for every 10
cause, increases the chemical reactions of the body by
Fever, regardless of its
Chapter 72
Energetics and Metabolic Rate
887
Fever Increases Metabolic Rate.
°C rise
Chapter 73.
Sleep Decreases Metabolic Rate.
tone of the skeletal musculature during sleep and (2)
decreased activity of the central nervous system.
Malnutrition Decreases Metabolic Rate.
Prolonged malnutri-
tion can decrease the metabolic rate 20 to 30 per cent,
presumably due to the paucity of food substances in the
fi
inanition that accompanies the disease causes a marked
temperature may fall several degrees shortly before
death.
muscle contraction in a single muscle can liberate as
much as 100 times its normal resting amount of heat for
exercise can increase the overall heat production of the
about 20 times normal for more sustained exercise in a
well-trained individual.
–1 shows the energy expenditure during dif-
ferent types of physical activity for a 70-kilogram man.
Because of the great variation in the amount of physi-
expenditure is the most important reason for the dif-
ferences in caloric intake required to maintain energy
scores the importance of maintaining a proper level of
Even in sedentary individuals who perform little or
ficant energy
is spent on spontaneous physical activity required
to maintain muscle tone and body posture and on other
nonexercise activities such as “fi
”
these nonexercise activities account for about 7 per cent
of a person’
Energy Used for Processing Food—
a result of the different chemical reactions associated
thermogenic effect of food,
because these processes require energy and generate
heat.
After a meal that contains a large quantity of carbo-
reaching a maximum of about 30 per cent above normal,
the metabolic rate is called the specific dynamic action
of protein.
about 8 per cent of the total daily energy expenditure
Energy Used for Nonshivering
Thermogenesis—Role of
Sympathetic Stimulation
Although physical work and the thermogenic effect of
Shivering provides a regulated means of producing heat
can also produce heat in
stimulated by sympathetic nervous system activation,
in turn increase metabolic activity and heat generation.
sym-
pathetic nervous stimulation causes liberation of large
of mitochondria and many small globules of fat instead
oxidative phosphorylation in the mitochondria is mainly
“uncoupled.”
all the released oxidative energy immediately becomes
heat.
A neonate has a considerable number of brown fat
the child’
nitude of this type of thermogenesis in an adult human,
ficantly after
cold adaptation.
Nonshivering thermogenesis may also serve as a
sympathetic nervous system activity is increased in
obese persons who have a persistent excess caloric
Energy Expenditure During Different Types of Activity for a
Table 72–1
Walking up stairs rapidly
1100
Running (5.3 miles per hour)
570
Swimming
500
Sawing wood
480
Carpentry, metalworking, industrial painting
240
Walking slowly (2.6 miles per hour)
200
Typewriting rapidly
140
Dressing and undressing
118
Standing relaxed
105
Sitting at rest
100
Awake lying still
77
Sleeping
65
Form of Activity
Calories per Hour
70-Kilogram Man
Extracted from data compiled by Professor M. S. Rose.

hormone action. Physiol Rev 81:1097, 2001.
Yen PM: Physiological and molecular basis of thyroid
91:1017, 2001.
vated protein kinase in skeletal muscle. J Appl Physiol
Winder WW: Energy-sensing and signaling by AMP-acti-
Physiol 95:1728, 2003.
Wilson MM, Morley JE: Aging and energy balance. J Appl
ity on daily energy expenditure. Proc Nutr Soc 62:645,
Westerterp KR: Impacts of vigorous and non-vigorous activ-
J Exp Biol 204:3183, 2001.
Westerterp KR: Limits to sustainable human metabolic rate.
metabolism. Curr Opin Clin Nutr Metab Care 6:469, 2003.
van Marken Lichtenbelt WD, Daanen HA: Cold-induced
clinical implications. Ann Intern Med 139:205, 2003.
Silva JE: The thermogenic effect of thyroid hormone and its
53:276, 2004.
Seals DR, Bell C: Chronic sympathetic activation: conse-
1):S130, 2004.
mitochondrial uncoupling proteins. Diabetes 53(Suppl
Rousset S, Alves-Guerra MC, Mozo J, et al: The biology of
Annu Rev Pharmacol Toxicol 44:297, 2004.
and regulation of energy expenditure: a family affair.
Robidoux J, Martin TL, Collins S: Beta-adrenergic receptors
Diseases. 1998. Available at: http://www.nhlbi.nih.gov/
MD: National Heart, Lung, and Blood Institute and
and Obesity in Adults: The Evidence Report. Bethesda,
cation, Evaluation, and Treatment of Overweight
National Institutes of Health: Clinical Guidelines on the
tissue thermogenesis. News Physiol Sci 19:67, 2004.
Morrison SF: Central pathways controlling brown adipose
278:29385, 2003.
induced thermogenesis, and obesity. J Biol Chem
Lowell BB, Bachman ES: Beta-adrenergic receptors, diet-
reported energy intake. J Nutr 133(Suppl 3):895S, 2003.
Livingstone MB, Black AE: Markers of the validity of
286:E675, 2004.
environment and biology. Am J Physiol Endocrinol Metab
Levine JA: Nonexercise activity thermogenesis (NEAT):
journey to obesity. Nat Med 10:355, 2004.
Evans RM, Barish GD, Wang YX: PPARs and the complex
96:3, 2004.
understanding of modern chronic diseases. J Appl Physiol
genotypes: connecting the dots toward an evolutionary
Chakravarthy MV, Booth FW: Eating, exercise, and
cance. Physiol Rev 84:277, 2004.
Cannon B, Nedergaard J: Brown adipose tissue: function and
moregulation. J Appl Physiol 92:2187, 2002.
Argyropoulos G, Harper ME: Uncoupling proteins and ther-
mogenesis, helps to limit excess weight gain.
thalamus. Sympathetic stimulation, by increasing ther-
ated partly through the effects of increased leptin, which
vation in obese persons is uncertain, but it may be medi-
Metabolism and Temperature Regulation
888
Unit XIII
activates pro-opiomelanocortin neurons in the hypo-
References
physiological signifi
“thrifty”
Identifi
National Institute of Diabetes and Digestive and Kidney
guidelines/index.htm
quence and cause of age-associated obesity? Diabetes
2003.
