The chemical nature of this linkage is
peptide linkages.
In proteins, the amino acids are aggregated into
attached to the molecule, usually represented by the amino group (—NH
common: each amino acid has an acidic group (—COOH) and a nitrogen atom
las of these 20 amino acids, demonstrating that they all have two features in
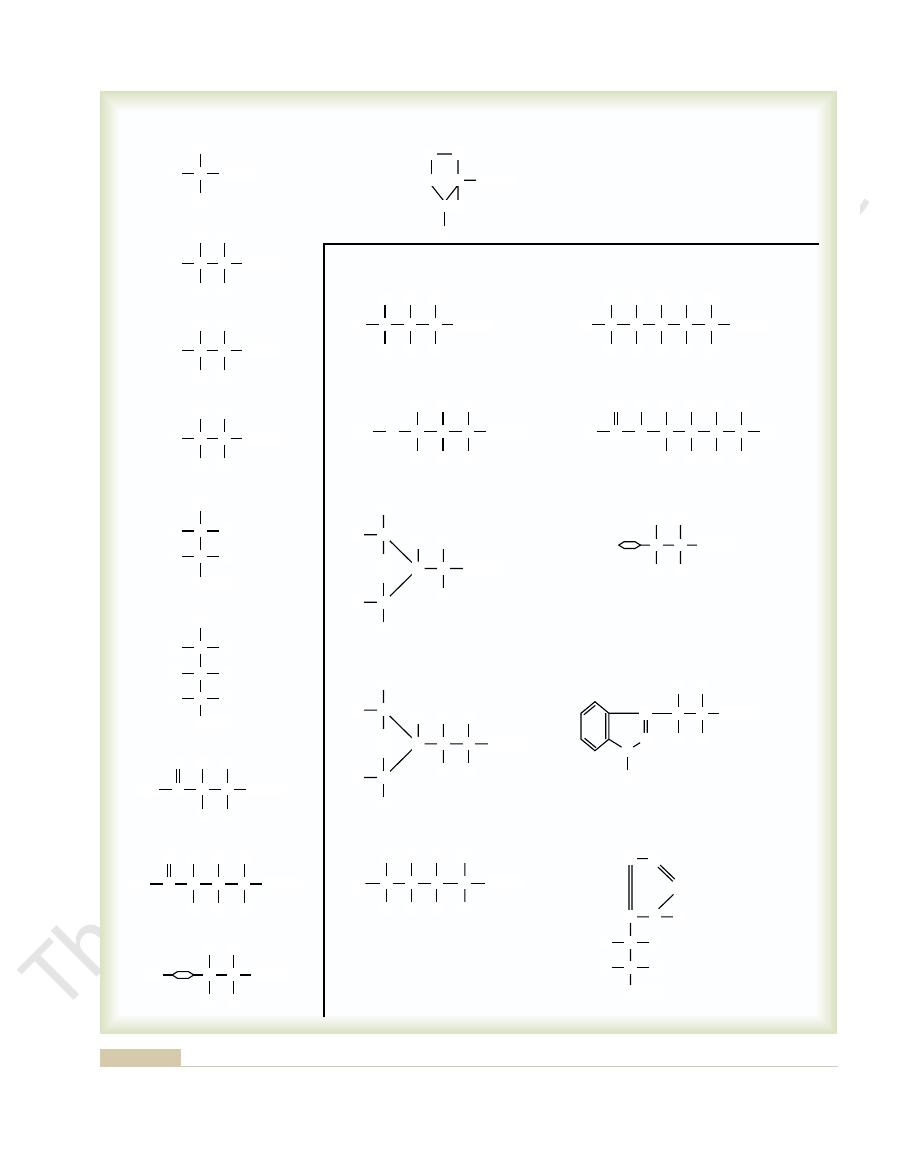
the body proteins in significant quantities. Figure 69–1 shows the chemical formu-
The principal constituents of proteins are amino acids, 20 of which are present in
Basic Properties
biochemistry. For this reason, the current discussion is confined to a few specific
The basic chemical properties that explain proteins’
lar functions throughout the body.
muscle that cause muscle contraction, and many other
teins, proteins that transport oxygen, proteins of the
These include structural proteins, enzymes, nucleopro-
About three quarters of the body solids are proteins.
C
H
A
P
T
E
R
6
9
852
Protein Metabolism
types that perform specific intracellular and extracellu-
diverse functions are so extensive that they
constitute a major portion of the entire discipline of
aspects of protein metabolism that are important as background for other discus-
sions in this text.
Amino Acids
2
).
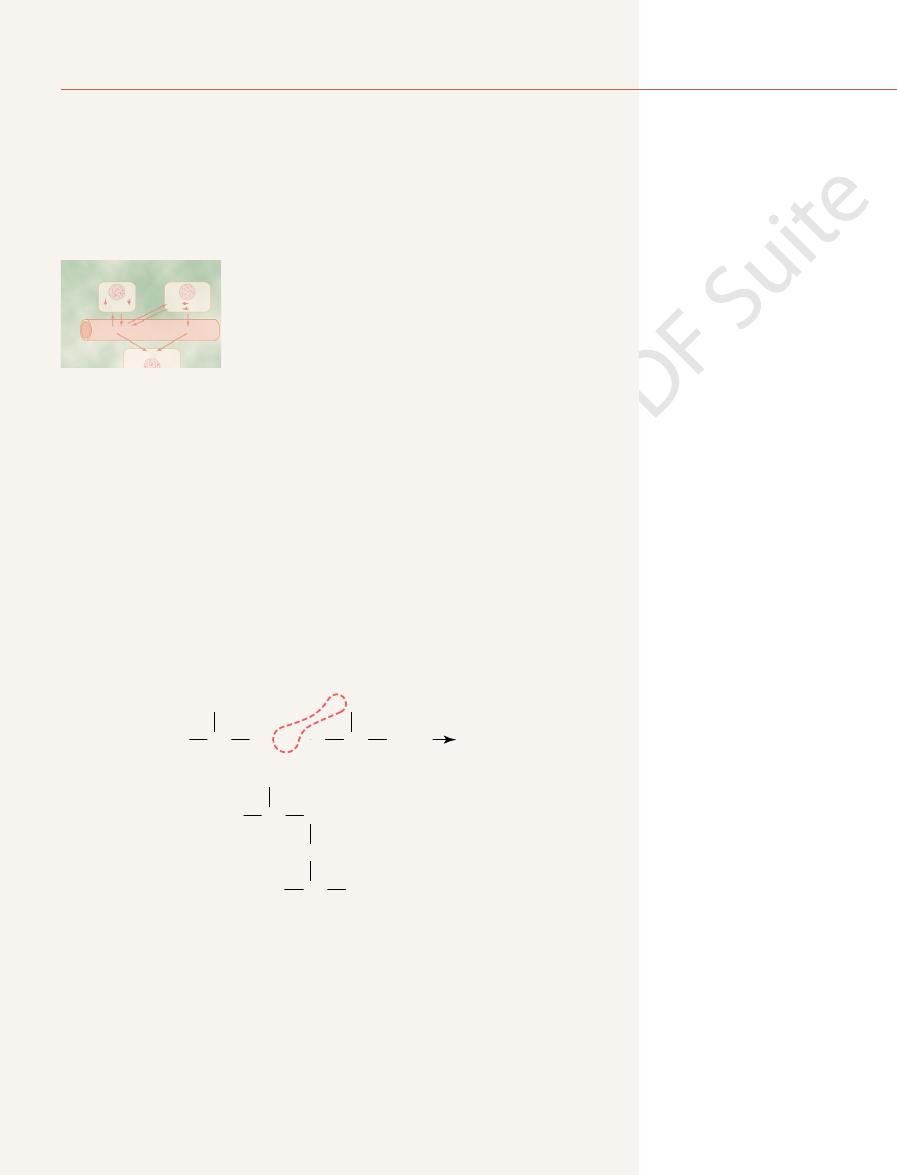
Peptide Linkages and Peptide Chains.
long chains by means of
demonstrated by the following reaction:
CO
CH
COOH
OH
+ R'
NH
2
CH
R
H NH
CO
NH
2
NH
+
H
2
O
CH
R
COOH
CH
R'
peptide chains rather than a single chain, and these chains are bound to one another
peptide linkages. The average is about 400 amino acids.
molecules have many thousand amino acids combined by peptide linkages, and even
peptide chain.
site ends of the new, longer molecule. Each of these radicals is capable of combin-
linkage has been formed, an amino radical and a carboxyl radical are still at oppo-
boxyl radical; these two combine to form a molecule of water. After the peptide
ion is released from the amino radical, and a hydroxyl ion is released from the car-
bonds with the carbon of the carboxyl radical of the other amino acid. A hydrogen
Note in this reaction that the nitrogen of the amino radical of one amino acid
ing with additional amino acids to form a
Some complicated protein
the smallest protein molecule usually has more than 20 amino acids combined by
Other Linkages in Protein Molecules.
Some protein molecules are composed of several
Chapter 69
Protein Metabolism
853
VALINE
PHENYLALANINE
LEUCINE
TRYPTOPHAN
Glycine
Proline
NH H
C
C
C
C
HC
N
H
COOH
H
COOH
H
COOH
H
COOH
C
H
NH
2
C
H
H
N
COOH
CH
2
H
2
C
H
2
C
C
C
H
C
O
COOH
H
NH
2
NH
2
C
H
H
C
H
NH
2
C
H
OH
C
H
NH
2
C
H
SH
C
H
NH
2
H
C
H
COOH
COOH
C
NH
2
H
H
C
H
COOH
COOH
C
NH
2
H
H
C
H
H
C
COOH
CH
C
N
H
H
H
C
H
C
C
C
H
H
C
O
COOH
H
NH
2
H
NH
2
H
H
H
H
H
H
C
C
H
COOH
H
HO
H
NH
2
C
C
H
COOH
H
H
NH
2
H
CH
3
COOH
H
H
H
H
H
H
C
C
H
H
C
H
H
C
C
NH
2
COOH
H
H
H
H
C
C
H
H
C
H
H
C
COOH
NH
2
COOH
CH
3
S
C
C
H
H
H
C
H
H
COOH
H
C
C
H
H
OH
C
H
H
NH
2
COOH
H
C
C
H
H
C
H
H
H
NH
2
NH
2
NH
2
C
C
H
H
H
N
C
C
H
H
C
C
H
H
COOH
NH
2
NH
2
C
H
H
H
H
2
N
H
C
C
H
COOH
H
NH
2
NH
2
H
C
CH
N
H
Alanine
Glutamine
Asparagine
Serine
Cysteine
Aspartic Acid
Glutamic Acid
HISTIDINE
ISOLEUCINE
Tyrosine
METHIONINE
THREONINE
AMINO ACIDS
LYSINE
ESSENTIAL AMINO ACIDS
ARGININE
be obtained, already formed, from food.
amino acids cannot be synthesized in sufficient quantities in the body; these essential amino acids must
Figure 69–1
Amino acids. The 10 essential

nisms is still poorly understood, but a few are discussed
mechanisms. The nature of some of the carrier mecha-
fore, significant quantities of amino acids can move
readily through the pores of the cell membranes. There-
The molecules
Active Transport of Amino Acids into the Cells.
form of amino acids each hour.
and tissue fluids. Nevertheless, the turnover rate of the
especially by the liver. Therefore, almost never do large
within 5 to 10 minutes by cells throughout the body,
entering the blood, the excess amino acids are absorbed
amino acids to be absorbed at a time. Second, after
over 2 to 3 hours, which allows only small quantities of
few milligrams per deciliter, for two reasons: First,
person’s blood rises, but the increase is usually only a
diately after a meal, the amino acid concentration in a
absorbed from the digestive tract into the blood. Imme-
The products of protein digestion and absorption in the
Fate of Amino Acids Absorbed from the Gastrointestinal Tract.
ent cells.
to some extent on the types of proteins eaten, but
of the negative ions in the blood. The precise distribu-
radical. They actually account for 2 to 3 milliequivalents
in the blood principally in the ionized state, resulting
the amino acids are relatively strong acids, they exist
are present in far greater amounts than others. Because
2 mg/dl for each of the 20 amino acids, although some
is between 35 and 65 mg/dl. This is an average of about
The normal concentration of amino acids in the blood
Transport and Storage
shapes by similar hydrogen bonding and other forces.
Many peptide chains are coiled or folded, and the suc-
the CO and NH radicals of the peptides, as follows:
by other linkages, often by
Metabolism and Temperature Regulation
854
Unit XIII
hydrogen bonding between
cessive coils or folds are held in a tight spiral or in other
of Amino Acids
Blood Amino Acids
from the removal of one hydrogen atom from the NH
2
tion of the different amino acids in the blood depends
the concentrations of at least some individual amino
acids are regulated by selective synthesis in the differ-
gastrointestinal tract are almost entirely amino acids;
only rarely are polypeptides or whole protein molecules
protein digestion and absorption are usually extended
concentrations of amino acids accumulate in the blood
amino acids is so rapid that many grams of proteins can
be carried from one part of the body to another in the
of all the amino acids are much too large to diffuse
either inward or outward through the membranes only
by facilitated transport or active transport using carrier
in Chapter 4.
R
N
H
O
C
C
O
H
N
HC
R'
CH
instance, if any particular tissue requires proteins, it can
labile proteins in virtually all cells of the body. For
plasma almost as rapidly, there is constant interchange
rapidly from plasma amino acids, and because many of
a much less extent, in other tissues) can be synthesized
Because cellular proteins in the liver (and, to
the Body.
mones increase the concentration of plasma amino acids.
proteins, whereas adrenocortical glucocorticoid hor-
teins and circulating amino acids. For instance, growth
tained at a reasonably constant value. Later, it is noted
replenish their supply in the plasma. In this way, the
acid concentrations fall below normal levels, the
Whenever plasma amino
quantities of rapidly exchangeable proteins; this is also
systems for processing amino acids, can store large
instance, the liver, which is a large organ and has special
of amino acids to a greater extent than others. For
transport back out of the cells.
and muscle contractile proteins; these proteins do not
cell into the blood. Special exceptions to this reversal
of intracellular lysosomal digestive enzymes; these
they are stored mainly in the form of actual proteins.
of free amino acids does not occur in the cells; instead,
usually remains low. Thus, storage of large quantities
ribosomal system, to form cellular proteins. Therefore,
under the direction of the cell’s messenger RNA and
acids combine with one another by peptide linkages,
Almost immediately after entry into tissue cells, amino
in the Cells
Storage of Amino Acids as Proteins
reabsorbed is lost into the urine.
glomerular filtrate, the excess that cannot be actively
reason, when the concentration of a particular type
each type of amino acid can be transported. For this
renal tubules, there is an upper limit to the rate at which
tubules through the glomerular membranes. However,
through the proximal tubular epithelium, which
In the kidneys, the
Renal Threshold for Amino Acids.
different amino acids can be actively reabsorbed
removes them from the glomerular filtrate and returns
them to the blood if they should filter into the renal
as is true of other active transport mechanisms in the
of amino acid becomes too high in the plasma and
the concentration of free amino acids inside the cells
But many of these intracellular proteins can be rapidly
decomposed again into amino acids under the influence
amino acids can then be transported back out of the
process are the proteins in the chromosomes of the
nucleus and the structural proteins such as collagen
participate significantly in this reverse digestion and
Some tissues of the body participate in the storage
true to a lesser extent of the kidneys and the intestinal
mucosa.
Release of Amino Acids from the Cells as a Means of Regulating
Plasma Amino Acid Concentration.
required amino acids are transported out of the cells to
plasma concentration of each type of amino acid is main-
that some of the hormones secreted by the endocrine
glands are able to alter the balance between tissue pro-
hormone and insulin increase the formation of tissue
Reversible Equilibrium Between the Proteins in Different Parts of
these proteins can be degraded and returned to the
and equilibrium between the plasma amino acids and
synthesize new proteins from the amino acids of the

-keto acids,
because they can be synthesized in the body.
proteins, but only that the others are
tial” amino acids are not required for the formation of
“essential” does not mean that the other 10 “nonessen-
essential amino acids.
quantities too small to supply the body’s needs. This
teins can be synthesized in the cells, whereas the other
Ten of the amino acids normally present in animal pro-
hours, the amino acids of the administered protein are
plasma protein. Within a few days, or sometimes within
body protein deficiency is intravenous transfusion of
of the most effective therapies for severe, acute whole-
plasma proteins and the other proteins of the body, one
starvation or severe debilitating diseases, the ratio of
among the different proteins of the body. Even during
state of flux of amino acids. This demonstrates the
amino acids of the plasma, and the tissue proteins. It has
as shown in Figure 69–2, among the plasma proteins, the
There is a constant state of equilibrium,
Tissue Proteins.
needed. In this way, the plasma proteins function as a
pinocytosis; once in these cells, they are split into amino
ment. Indeed, whole plasma proteins can be imbibed in
When the tissues become depleted of proteins, the
Plasma Proteins as a Source of Amino Acids for the Tissues.
plasma colloid osmotic pressure, which causes general-
As discussed in Chapter 25, this leads to decreased
reduction in their ability to synthesize plasma proteins.
develop among the liver parenchymal cells, causing a
cirrhosis of the liver,
proteins.
the urine each day for months, and it is continually
such states. Occasionally, a person with severe renal
denuded areas each day. The rapid production of plasma
conditions cause rapid loss of plasma proteins; severe
be extremely high, as much as 30 g/day. Certain disease
The rate of plasma protein formation by the liver can
entirely in the lymphoid tissues. They are mainly the
liver. The remainder of the globulins are formed almost
as 50 to 80 per cent of the globulins, are formed in the
albumin and fibrinogen of the plasma proteins, as well
that help repair leaks in the circulatory system, dis-
during blood coagulation, thereby
against invading organisms, dis-
cipally responsible for the body’s both natural and
in the plasma, but equally important, they are prin-
The
loss from the capillaries, as discussed in Chapter 16.
in the plasma, which prevents plasma
albumin, globulin,
The major types of protein present in the plasma are
Plasma Proteins
or glycogen and stored in these forms.
as discussed subsequently, or they are converted to fat
are degraded into other products and used for energy,
their limits, the excess amino acids still in the circulation
proteins it can store. After all the cells have reached
therefore, the proteins of the other cells can become
cells. Cancer cells are often prolific users of amino acids;
especially from the liver cells. These effects are particu-
degradation of proteins from other cells of the body,
blood; in turn, the blood amino acids are replenished by
Chapter 69
Protein Metabolism
855
larly noticeable in relation to protein synthesis in cancer
markedly depleted.
Upper Limit for the Storage of Proteins.
Each particular type
of cell has an upper limit with regard to the amount of
Functional Roles of the
and fibrinogen.
A major function of albumin is to provide colloid
osmotic pressure
globulins perform a number of enzymatic func-
tions
acquired immunity
cussed in Chapter 34.
Fibrinogen polymerizes into long fibrin threads
forming blood clots
cussed in Chapter 36.
Formation of the Plasma Proteins.
Essentially all the
gamma globulins that constitute the antibodies used in
the immune system.
burns that denude large surface areas of the skin can
cause the loss of several liters of plasma through the
proteins by the liver is valuable in preventing death in
disease loses as much as 20 grams of plasma protein in
replaced mainly by liver production of the required
In
large amounts of fibrous tissue
ized edema.
plasma proteins can act as a source of rapid replace-
toto by tissue macrophages through the process of
acids that are transported back into the blood and used
throughout the body to build cellular proteins wherever
labile protein storage medium and represent a readily
available source of amino acids whenever a particular
tissue requires them.
Reversible Equilibrium Between the Plasma Proteins and the
been estimated from radioactive tracer studies that
normally about 400 grams of body protein are synthe-
sized and degraded each day as part of the continual
general principle of reversible exchange of amino acids
total tissue proteins to total plasma proteins in the body
remains relatively constant at about 33:1.
Because of this reversible equilibrium between
distributed throughout the cells of the body to form new
proteins where they are needed.
Essential and Nonessential
Amino Acids
10 either cannot be synthesized or are synthesized in
second group of amino acids that cannot be synthesized
is called the
Use of the word
not essential in the
diet
Synthesis of the nonessential amino acids depends
mainly on the formation of appropriate
a
Amino acids
Blood
Tissue cells
Liver cells
Tissue cells
Liver cells
Proteins
Amino acids
Proteins
Amino acids
Amino
acids
Amino
acids
Proteins
Proteins
Plasma proteins
Plasma proteins
Imbibed plasma protein
Imbibed plasma protein
Reticuloendothelial cell
Reticuloendothelial cell
Amino acids
Blood
Reversible equilibrium among the tissue proteins, plasma pro-
Figure 69–2
teins, and plasma amino acids.
cycle, and (2) this substance is degraded by the cycle and
processes: (1) the keto acid is changed into an appro-
bolic purposes. This usually involves two successive
most instances, be oxidized to release energy for meta-
have been deaminated, the resulting keto acids can, in
into the body fluids and is excreted by the kidneys.
After its formation, the urea diffuses from the liver cells
The stages in the formation of urea are essentially the
blood. This is extremely toxic, especially to the brain,
serious liver disease, ammonia accumulates in the
thesized in the liver. In the absence of the liver or in
blood almost entirely by conversion into urea; two mol-
The ammonia released
Urea Formation by the Liver.
ferases,
excess amino acids in the cells, especially in the liver,
repeated again and again. To initiate this process, the
-ketoglutaric acid, so that the cycle can be
of losing the amino group, the glutamic acid once again
). In the process
then becomes glutamic acid. The glutamic acid can then
-ketoglutaric acid, which
The greatest amount of deamination occurs by the
synthesis of amino acids.
group to some acceptor substance, which is the reverse
transamination, which means transfer of the amino
groups from the amino acids. This occurs mainly by
almost entirely in the liver, and it begins with
or secondarily as glycogen. This degradation occurs
protein, any additional amino acids in the body fluids are
and protein formation cannot proceed normally.
this vitamin, the amino acids are synthesized only poorly,
). Without
atives of pyridoxine, one of the B vitamins (B
aminotransferases,
Transamination is promoted by several enzymes,
asparagine, glutamic
serve as an amino radical storehouse. In addition, amino
large quantities, and one of its principal functions is to
glutamine.
acids,
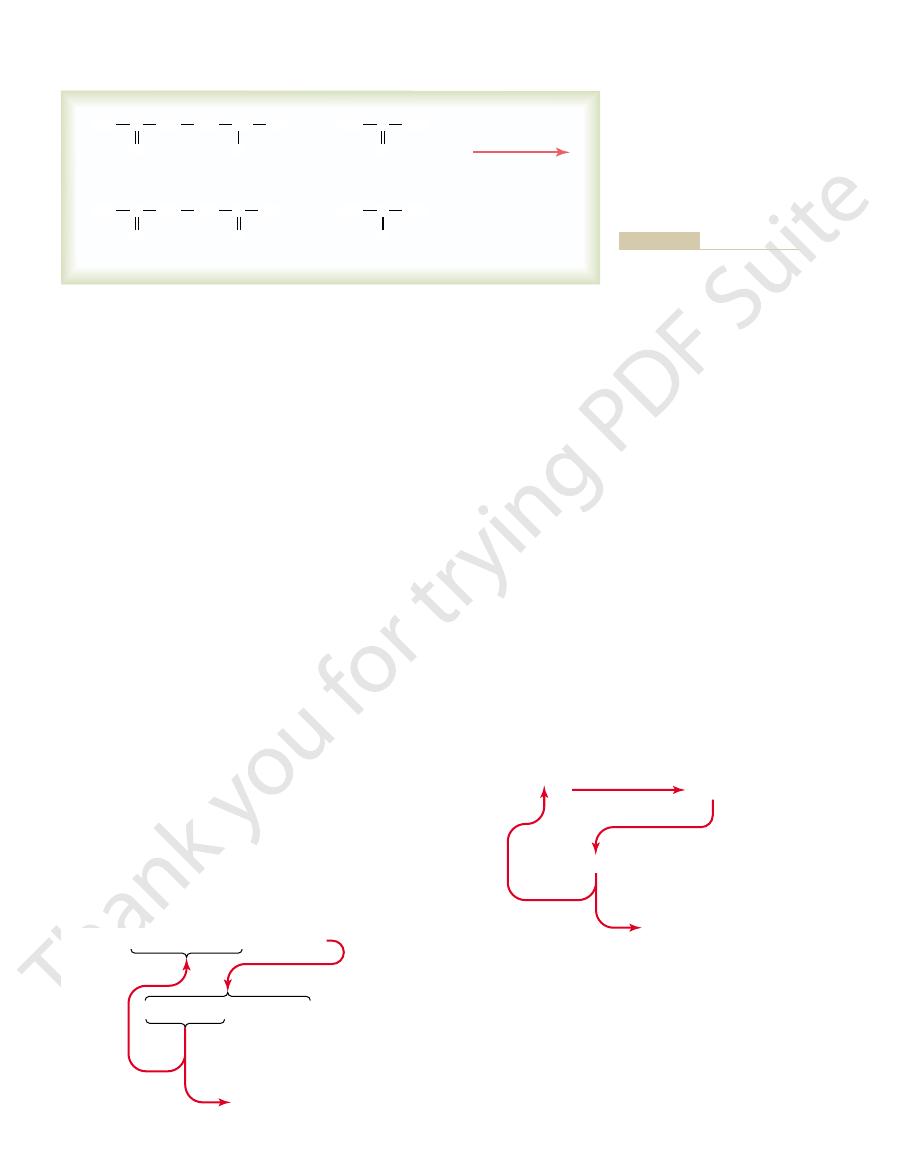
This reaction is shown in Figure 69–3. Note in this figure
-keto acid, and the keto
Then, by the process of
alanine.
quantities during the glycolytic breakdown of glucose,
pyruvic acid,
For instance,
which are the precursors of the respective amino acids.
Metabolism and Temperature Regulation
856
Unit XIII
which is formed in large
is the keto acid precursor of the amino acid
transamination, an amino
radical is transferred to the
a
oxygen is transferred to the donor of the amino radical.
that the amino radical is transferred to the pyruvic acid
from another chemical that is closely allied to the amino
Glutamine is present in the tissues in
radicals can be transferred from
acid, and aspartic acid.
among which are the
which are deriv-
6
Use of Proteins for Energy
Once the cells are filled to their limits with stored
degraded and used for energy or are stored mainly as fat
deamina-
tion, which is explained in the following section.
Deamination.
Deamination means removal of the amino
of the transamination explained earlier in relation to the
following transamination schema:
Note from this schema that the amino group from the
amino acid is transferred to
a
transfer the amino group to still other substances or
release it in the form of ammonia (NH
3
becomes
a
induce the activation of large quantities of aminotrans-
the enzymes responsible for initiating most
deamination.
during deamination of amino acids is removed from the
ecules of ammonia and one molecule of carbon dioxide
combine in accordance with the following net reaction:
2 NH
3
+ CO
2
Æ H
2
N—C—NH
2
+ H
2
O
||
O
Essentially all urea formed in the human body is syn-
often leading to a state called hepatic coma.
following:
Oxidation of Deaminated Amino Acids.
Once amino acids
priate chemical substance that can enter the citric acid
(Arginase)
Arginine
Citrulline
Urea
Ornithine
+ CO
2
+ NH
3
NH
3
-H
2
O
+ H
2
O
-H
2
O
-Ketoglutamic acid)
(Alanine)
(Glutamine)
(Pyruvic acid)
(
a
Transaminase
+
+
O
O
NH
2
NH
2
CH
2
CH
2
CH
C
COOH
CH
3
C
COOH
O
O
NH
2
CH
2
CH
2
C
C
COOH
NH
CH
3
C
COOH
Synthesis of alanine from pyruvic
Figure 69–3
acid by transamination.
Amino acid
a-Ketoglutaric acid +
Glutamic acid
+
a-Keto acid
+ NAD
+
+ H
2
O
NADH
+ H
+
+ NH
3

reactions.
essence, it is believed that thyroxine has little specific
inhibited because of lack of protein synthesis. In
deficiency of thyroxine causes growth to be greatly
protein synthesis. In growing animals or human beings,
cellular fluid, thyroxine can actually increase the rate of
proteins and uses them for energy. Conversely, if ade-
able for energy, thyroxine causes rapid degradation of
olism. If insufficient carbohydrates and fats are avail-
all cells and, as a result, indirectly affects protein metab-
Thyroxine increases the rate of metabolism of
testosterone.
also causes some deposition of protein, but its effect
Estrogen, the principal female sex hormone,
tion ceases.
administration of testosterone, further protein deposi-
tissues have reached a maximum, despite continued
several months. Once the muscles and other protein
extent, some other protein tissues to enlarge for only
testosterone causes the muscles and, to a much lesser
tissues to continue growing almost indefinitely, whereas
hormone, in the following way: Growth hormone causes
increase). The mechanism of this effect is unknown, but
tion of protein in tissues throughout the body, especially
terone, the male sex hormone, causes increased deposi-
Testos-
Testosterone Increases Protein Deposition in Tissues.
cellular proteins and plasma proteins.
acids available in the body fluids. This supposedly allows
teins, thereby making increased quantities of amino
teins.
The glucocorticoids secreted by the adrenal cortex
Glucocorticoids Increase Breakdown of Most Tissue Proteins.
glucose to the cells, so that the need for amino acids for
synthesis. Also, insulin increases the availability of
acids into cells, which could be the stimulus to protein
mechanism by which this occurs is also unknown, but
insulin reduces protein synthesis to almost zero. The
Total lack of
Insulin Is Necessary for Protein Synthesis.
translation processes for protein synthesis.
acceleration of the DNA and RNA transcription and
known, but it is believed to result mainly from increased
The precise mechanism by which this occurs is not
Growth hormone causes the tissue proteins to increase.
of Protein Metabolism
Hormonal Regulation
sparers.
utilization, carbohydrates and fats are called
precipitously. Because carbohydrate and fat utilization
daily—and, as a result, cellular functions deteriorate
oxidized for energy. From this point on, the proteins
of stored carbohydrates and fats begin to run out, the
after several weeks of starvation, when the quantities
for energy, as long as they are available. However,
to 30 grams of obligatory protein degradation each day,
Effect of Starvation on Protein Degradation.
acids are deaminated and oxidized. A protein that has
in relation to protein synthesis. The unusable amino
whole proteins or none at all, as explained in Chapter 3
of essential amino acid is low in concentration, the
to form new proteins in the tissues. If one particular type
The ratios of the different amino acids in the dietary
safe side, a minimum of 60 to 75 grams is usually
of 20 to 30 grams of protein each day; to be on the
protein from the body, one must ingest a minimum
of proteins. Therefore, to prevent net loss of
of protein each day, which is called the
deaminated and oxidized. This involves 20 to 30 grams
When a person eats no proteins, a certain proportion of
Obligatory Degradation of Proteins
acids.
glucose, and 19 of them can be converted into fatty
Of the 20 deaminated amino acids, 18 have
genesis.
gluconeogenesis,
The conversion of amino acids into glucose or glyco-
bodies, as explained in Chapter 68.
to form acetoacetic acid, which is one of the ketone
acids. Also, two molecules of acetyl-CoA can condense
acetyl-CoA, which can then be polymerized into fatty
or glycogen. Alternatively, it can be converted into
pyruvic acid. This can be converted into either glucose
or fatty acids. For instance, deaminated alanine is
by the cells, mainly the liver cells, to synthesize glucose
phate (ATP) formed for each gram of protein that is
and 68. In general, the amount of adenosine triphos-
lipid metabolism is used, as explained in Chapters 67
zyme A (acetyl-CoA) derived from carbohydrate and
Chapter 69
Protein Metabolism
857
used for energy in the same manner that acetyl coen-
oxidized is slightly less than that formed for each gram
of glucose oxidized.
Gluconeogenesis and Ketogenesis.
Certain deaminated
amino acids are similar to the substrates normally used
gen is called
and the conversion of
amino acids into keto acids or fatty acids is called keto-
chemical structures that allow them to be converted into
body proteins is degraded into amino acids and then
obligatory
loss
recommended.
protein must be about the same as the ratios in the body
tissues if the entire dietary protein is to be fully usable
others become unusable because cells synthesize either
a ratio of amino acids different from that of the average
body protein is called a partial protein or incomplete
protein, and such a protein is less valuable for nutrition
than is a complete protein.
Except for the 20
the body uses almost entirely carbohydrates or fats
amino acids of the blood are rapidly deaminated and
of the tissues degrade rapidly—as much as 125 grams
for energy normally occurs in preference to protein
protein
Growth Hormone Increases the Synthesis of Cellular Proteins.
transport of amino acids through the cell membranes or
insulin does accelerate the transport of some amino
energy is correspondingly reduced.
decrease the quantity of protein in most tissues while
increasing the amino acid concentration in the plasma,
as well as increasing both liver proteins and plasma pro-
It is believed that the glucocorticoids act by
increasing the rate of breakdown of extrahepatic pro-
the liver to synthesize increased quantities of hepatic
the contractile proteins of the muscles (30 to 50 per cent
it is definitely different from the effect of growth
Estrogen.
is relatively insignificant in comparison with that of
Thyroxine.
quate quantities of carbohydrates and fats are available
and excess amino acids are also available in the extra-
effect on protein metabolism but does have an
important general effect by increasing the rates of
both normal anabolic and normal catabolic protein

exchange. Am J Clin Nutr 79:185, 2004.
olism of amino acids: its role in interorgan amino acid
van de Poll MC, Soeters PB, Deutz NE, et al: Renal metab-
6:79, 2003.
to muscle myofibrils. Curr Opin Clin Nutr Metab Care
Tessari P: Protein metabolism in liver cirrhosis: from albumin
metabolism. Curr Opin Clin Nutr Metab Care 7:71, 2004.
Prod’homme M, Rieu I, Balage M, et al: Insulin and amino
and development. J Nutr 134(6 Suppl):1566S, 2004.
Pencharz PB, Ball RO: Amino acid needs for early growth
Biochem Biophys Res Commun 313:405, 2004.
acids as a protein- and energy-source in liver cirrhosis.
Moriwaki H, Miwa Y, Tajika M, et al: Branched-chain amino
nonproteinogenic pathways. J Nutr 133(6 Suppl 1):2057S,
Meijer AJ: Amino acids as regulators and components of
muscle cells. Physiol Rev 83:183, 2003.
Mann GE, Yudilevich DL, Sobrevia L: Regulation of amino
control during weight loss. J Nutr 134:968S, 2004.
Layman DK, Baum JI: Dietary protein impact on glycemic
Kuhn CM: Anabolic steroids. Recent Prog Horm Res 57:411,
313:423, 2004.
chain amino acids. Biochem Biophys Res Commun
mRNA translation by oral administration of branched-
Kimball SR, Jefferson LS: Regulation of global and specific
teolysis. J Nutr 133(6 Suppl 1):2052S, 2003.
Kadowaki M, Kanazawa T: Amino acids as regulators of pro-
76:651, 1996.
nucleus: central role of phosphorylation. Physiol Rev
Jans DA, Hubner S: Regulation of protein transport to the
requirements and toxicity. J Nutr 134(6 Suppl):1569S,
Fukagawa NK, Galbraith RA: Advancing age and other
Rev 78:487, 1998.
animal cells: discovery, structure, and function. Physiol
Deves R, Boyd CA: Transporters for cationic amino acids in
peptide transport. Annu Rev Physiol 66:361, 2004.
Daniel H: Molecular and integrative physiology of intestinal
J Nutr 134(6 Suppl):1600S, 2004.
the range of amino acid adequacy: the biological aspects.
Caldwell J: Pharmacogenetics and individual variation in
Sci 18:191, 2003.
Altenberg GA: The engine of ABC proteins. News Physiol
Metabolism and Temperature Regulation
858
Unit XIII
References
factors influencing the balance between amino acid
2004.
2002.
acid and glucose transporters in endothelial and smooth
2003.
acids both strongly participate to the regulation of protein
