
are necessary to maintain and propagate life.
impulses; (5) cell division and growth; and (6) many other physiologic functions that
branes, and many other essential molecules of the body; (4) conduction of nerve
mechanical work; (3) various synthetic reactions that create hormones, cell mem-
cules across cell membranes; (2) contraction of muscles and performance of
convert adenosine diphosphate (ADP) to ATP, which is then consumed by the
Energy derived from the oxidation of carbohydrates, proteins, and fats is used to
repeatedly.
has been called the energy currency of the body, and it can be gained and spent
and energy-producing functions of the body (Figure 67–1). For this reason, ATP
Adenosine triphosphate (ATP) is an essential link between energy-utilizing
Role of Adenosine Triphosphate in Metabolism
of 1 mole (180 grams) of glucose is 686,000 calories.
substance. For instance, the amount of free energy liberated by complete oxidation
G. Free energy is usually expressed in terms of calories per mole of
free energy of oxidation of the food,
The amount of energy liberated by complete oxidation of a food is
“Free Energy.”
of which are explained in this and subsequent chapters.
pling is accomplished by special cellular enzyme and energy transfer systems, some
“coupled” with the systems responsible for these physiologic functions. This cou-
and to effect other functions. To provide this energy, the chemical reactions must be
case of muscle function, to concentrate solutes in the case of glandular secretion,
released suddenly, all in the form of heat. The energy needed by the physiologic
fire, also releasing large amounts of energy; in this case, however, the energy is
These same foods can also be burned with pure oxygen outside the body in an actual
oxidized in the cells, and during this process, large amounts of energy are released.
All the energy foods—carbohydrates, fats, and proteins—can be
functions.
in the cells, absorption of foods from the gastrointestinal tract, and many other
of membrane potentials by the nerve and muscle fibers, synthesis of substances
instance, energy is required for muscle activity, secretion by the glands, maintenance
the energy in foods available to the various physiologic systems of the cell. For
fit into the overall concept of homeostasis.
logic implications, especially the manner in which they
the discipline of biochemistry. Instead, these chapters
of all the various cellular reactions, because this lies in
possible for the cells to continue living. It is not the
body, which means the chemical processes that make it
The next few chapters deal with metabolism in the
Triphosphate
and Formation of Adenosine
Metabolism of Carbohydrates,
C
H
A
P
T
E
R
6
7
829
purpose of this textbook to present the chemical details
are devoted to (1) a review of the principal chemical
processes of the cell and (2) an analysis of their physio-
Release of Energy from Foods, and the Concept of
“Free Energy”
A great proportion of the chemical reactions in the cells is concerned with making
Coupled Reactions.
processes of the cells is not heat but energy to cause mechanical movement in the
called the
and this is generally represented by
the symbol
D
various reactions of the body that are necessary for (1) active transport of mole-
fore, glucose-6-phosphate can be degraded to glucose
There-
glucose phosphatase.
entirely glucose. The reason for this is that the liver cells
rides back into the blood, the final product is almost
Figure 67–3. Furthermore, the dynamics of the reactions
rides—glucose, fructose, and galactose—as shown in
In liver cells, appropriate enzymes are available to
transport of almost all carbohydrates to the tissue cells.
Glucose thus becomes the final common pathway for the
verted into glucose in the liver. Therefore, little fructose
After absorption from the intestinal tract, much of the
representing, on average, about 80 per cent of these.
entirely glucose, fructose, and galactose—with glucose
As explained in Chapter 65, the final products of car-
purpose.
ATP in the cells. Normally, 90 per cent or more of all
The principal purpose of this chapter is to explain
transfers take place by means of coupled reactions.
maintaining a supply of this substance. All these energy
released energy is used to form new ATP, thus always
turn, the food in the cells is gradually oxidized, and the
energy compound—guanosine triphosphate [GTP]). In
obtain it directly from ATP (or another similar high-
nucleoplasm of all cells, and essentially all the physio-
ATP is present everywhere in the cytoplasm and
The interconversions among ATP, ADP, and AMP are
radical, it becomes
becomes ADP, and after loss of the second phosphate
one phosphate radical from ATP, the compound
liberates about 12,000 calories of energy. After loss of
body, removal of each of the last two phosphate radicals
trations of the reactants in the body. Therefore, in the
energy bonds per mole of ATP is about 7300 calories
The amount of free energy in each of these high-
bonds, which are indicated by the symbol
radicals. The last two phosphate radicals are connected
combination of adenine, ribose, and three phosphate
67–2. From this formula, it can be seen that ATP is a
all cells. It has the chemical structure shown in Figure
ATP is a labile chemical compound that is present in
Metabolism and Temperature Regulation
830
Unit XIII
with the remainder of the molecule by high-energy
~.
under standard conditions and about 12,000 calories
under the usual conditions of temperature and concen-
adenosine monophosphate (AMP).
the following:
logic mechanisms that require energy for operation
how the energy from carbohydrates can be used to form
the carbohydrates utilized by the body are used for this
Central Role of Glucose in
Carbohydrate Metabolism
bohydrate digestion in the alimentary tract are almost
fructose and almost all the galactose are rapidly con-
and galactose are present in the circulating blood.
promote interconversions among the monosaccha-
are such that when the liver releases the monosaccha-
contain large amounts of
ATP
ADP
+
P
i
Oxidation
Energy production
•Proteins
•Carbohydrates
•Fats
Energy utilization
•Active ion transport
•Muscle contraction
•Synthesis of molecules
•Cell division and growth
producing and energy-utilizing systems of the body. ADP, adeno-
Adenosine triphosphate (ATP) as the central link between energy-
Figure 67–1
sine diphosphate; P
i
, inorganic phosphate.
OH
OH
P
O
O
O
-
O
-
O
-
H
NH
2
H
N
N
N
C
C
C
N
C
C
C
O
C
H
H
O
-
O
O
P
O
~
~
P
O
Triphosphate
Adenine
Ribose
CH
2
CH
HC
triphosphate (ATP).
Chemical structure of adenosine
Figure 67–2
ATP
-12,000 cal
+12,000 cal
-12,000 cal
+12,000 cal
ADP
PO
3
+
ADP
2PO
3
+

diately for release of energy to the cell, or it can be
After absorption into a cell, glucose can be used imme-
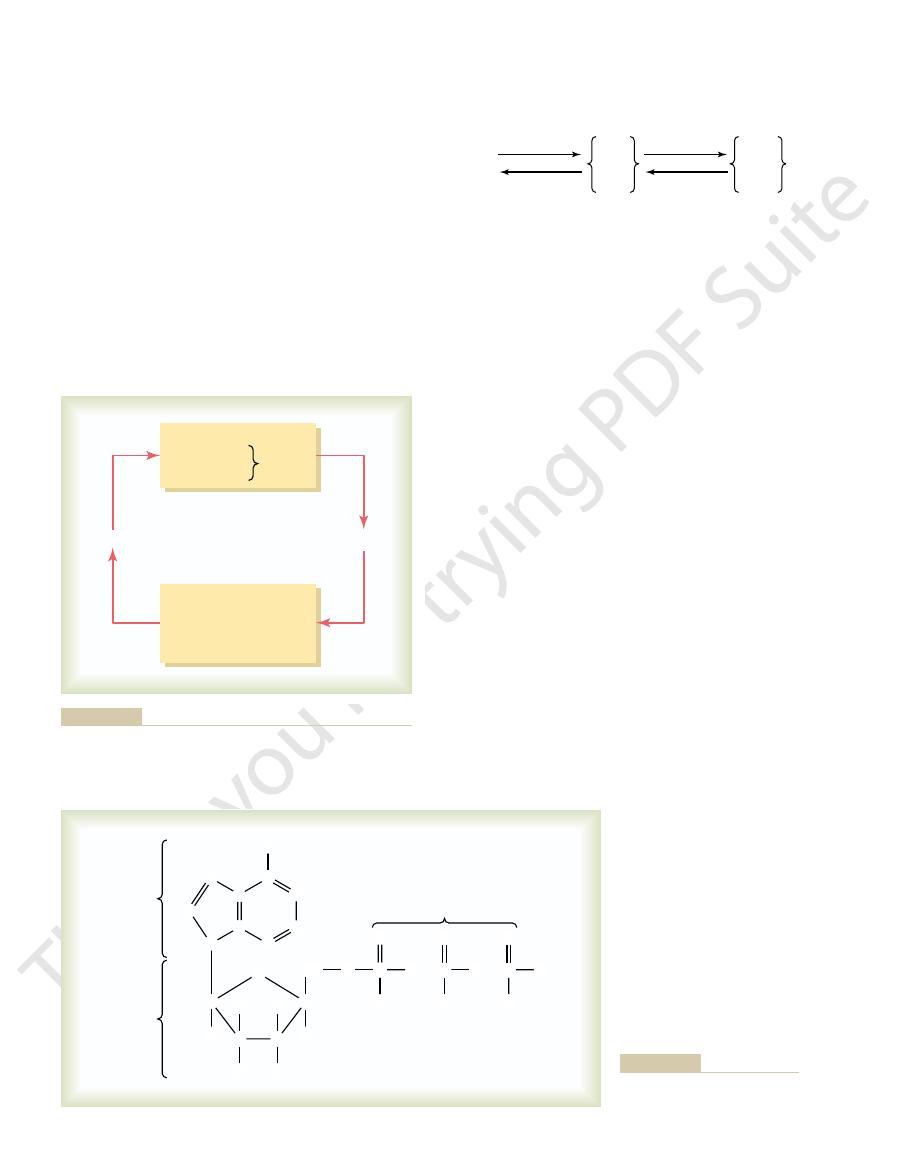
Glycogen Is Stored in Liver
have phosphatase.
except from those special cells, especially liver cells, that
with phosphate, the glucose will not diffuse back out,
cell. That is, because of its almost instantaneous binding
it can reverse the reaction. In most tissues of the body,
, is also available, and when this is activated,
epithelial cells; in these cells, another enzyme,
the renal tubular epithelial cells, and the intestinal
almost completely irreversible except in the liver cells,
most other cells. The phosphorylation of glucose is
This phosphorylation is promoted mainly by the
Immediately on entry into the cells, glucose combines
Phosphorylation of Glucose
Chapter 78.
the pancreas. The functions of insulin and its control of
In effect, the rate of carbohydrate utilization by most
of liver and brain cells, are far too little to supply the
the body in the absence of insulin, with the exception
when no insulin is secreted. Conversely, the amounts of
the pancreas, the rate of glucose transport into most
insulin. When large amounts of insulin are secreted by
The rate of glucose transport as well as transport of
Insulin Increases Facilitated
The details of
active absorption of glucose. At other cell membranes,
This sodium
against a concentration difference.
these cases, the glucose is transported by the mechanism
through the epithelium of the renal tubules. In both
The transport of glucose through the membranes of
the other side, more glucose will be transported from
and then released. Therefore, if the concentration of
this bound form, the glucose can be transported by the
molecules that can bind with glucose. In
cally, they are the following. Penetrating through the
of this type of transport are discussed in Chapter 4. Basi-
The principles
molecular weight of 180. Yet glucose does pass to the
that can diffuse readily is about 100, and glucose has a
into the cellular cytoplasm. However, glucose
Before glucose can be used by the body’s tissue cells, it
Through the Cell Membrane
Transport of Glucose
glucose.
Once again, it should be emphasized that usually
and phosphate, and the glucose can then be transported
Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Chapter 67
831
through the liver cell membrane back into the blood.
more than 95 per cent of all the monosaccharides that
circulate in the blood are the final conversion product,
must be transported through the tissue cell membrane
cannot
easily diffuse through the pores of the cell membrane
because the maximum molecular weight of particles
interior of the cells with a reasonable degree of freedom
by the mechanism of facilitated diffusion.
lipid matrix of the cell membrane are large numbers of
protein carrier
carrier from one side of the membrane to the other side
glucose is greater on one side of the membrane than on
the high-concentration area to the low-concentration
area than in the opposite direction.
most tissue cells is quite different from that which
occurs through the gastrointestinal membrane or
of active sodium-glucose co-transport, in which active
transport of sodium provides energy for absorbing
glucose
co-transport mechanism functions only in certain
special epithelial cells that are specifically adapted for
glucose is transported only from higher concentration
toward lower concentration by facilitated diffusion,
made possible by the special binding properties of mem-
brane glucose carrier protein.
facilitated
diffusion for cell membrane transport are presented in
Chapter 4.
Diffusion of Glucose
some other monosaccharides is greatly increased by
cells increases to 10 or more times the rate of transport
glucose that can diffuse to the insides of most cells of
amount of glucose normally required for energy metab-
olism.
cells is controlled by the rate of insulin secretion from
carbohydrate metabolism are discussed in detail in
with a phosphate radical in accordance with the follow-
ing reaction:
enzyme glucokinase in the liver and by hexokinase in
glucose
phosphatase
phosphorylation serves to capture the glucose in the
and Muscle
ææææææææ
Glucose
Glucose-6-phosphate
glucokinase or hexokinase
ATP
æ
Æ
+
Galactose
Galactose-1-phosphate
ATP
ATP
ATP
Cell membrane
Uridine diphosphate galactose
Uridine diphosphate glucose
Glucose-1-phosphate
Glucose-6-phosphate
Fructose-6-phosphate
Glucose
Fructose
Glycogen
Glycolysis
Interconversions of the three major monosaccharides—glucose,
Figure 67–3
fructose, and galactose—in liver cells.
ATP.
The next sections describe the basic principles of
ATP for each mole of glucose metabolized by the cells.
cule of ATP at a time, forming a total of 38 moles of
a little at a time in many successive steps, so that its
cule. Fortunately, all cells of the body contain special
carbon dioxide while forming only a single ATP mole-
gram-molecule of ATP, energy would be wasted if
glucose releases 686,000 calories of energy and
Release of Energy from the
glucose concentration. The function of glucagon in
release into the blood, thereby elevating the blood
mainly in the liver cells, and this in turn promotes
falls too low. It stimulates formation of cyclic AMP
pathetic stimulation, to preparing the body for action,
thereby contributing, along with other effects of sym-
rine occurs markedly in both liver cells and muscle,
for rapid energy metabolism. This function of epineph-
Therefore, one of the functions of the sympathetic
the phosphorylase. This is discussed in detail in Chapter
in the cells, which then
phorylase and thereby cause rapid glycogenolysis. The
hormones,
Two
Activation of Phosphorylase by Epinephrine or by Glucagon.
two.
accomplished in several ways, including the following
rst be activated. This can be
it is necessary to re-form glucose from glycogen, the
inactive form, so that glycogen will remain stored. When
Under resting conditions, the phosphorylase is in an
phosphorylase.
reactions that form glycogen; instead, each succeeding
glucose can then be used to provide energy. Glycogenol-
stored glycogen to re-form glucose in the cells. The
deaminated amino acids,
lactic acid, glycerol, pyruvic acid,
pounds, including
can enter into the reactions. Certain smaller com-
enzymes are required to cause these conversions, and
nally converted into glycogen. Several speci
uridine diphosphate glucose,
gure, it can be seen that
4. From this
Figure 67
The chemical reactions for glycogenesis are shown in
of Glycogen Formation
uids.
low-molecular-weight soluble monosaccharides would
uids. High concentrations of
molecular-weight precipitated compound (glycogen)
This conversion of the monosaccharides into a high-
the form of solid granules.
million or greater; most of the glycogen precipitates in
weight, with the average molecular weight being 5
can store up to 1 to 3 per cent glycogen. The glycogen
muscle cells,
cent of their weight as glycogen, and
liver cells,
some glycogen, but certain cells can store large amounts,
of glucose.
Metabolism and Temperature Regulation
832
Unit XIII
stored in the form of glycogen, which is a large polymer
All cells of the body are capable of storing at least
especially
which can store up to 5 to 8 per
which
molecules can be polymerized to almost any molecular
makes it possible to store large quantities of carbohy-
drates without significantly altering the osmotic pres-
sure of the intracellular fl
play havoc with the osmotic relations between intracel-
lular and extracellular fl
Glycogenesis
—
The Process
–
fi
glucose-
6-phosphate can become glucose-1-phosphate; this is
converted to
which is
fi
fic
any monosaccharide that can be converted into glucose
and
some
can also be converted
into glucose or closely allied compounds and then con-
verted into glycogen.
Removal of Stored Glycogen—
Glycogenolysis
Glycogenolysis means the breakdown of the cell’s
ysis does not occur by reversal of the same chemical
glucose molecule on each branch of the glycogen
polymer is split away by phosphorylation, catalyzed by
the enzyme
phosphorylase must fi
epinephrine and glucagon, can activate phos-
initial effect of each of these hormones is to promote
the formation of cyclic AMP
initiates a cascade of chemical reactions that activates
78.
Epinephrine is released by the adrenal medullae
when the sympathetic nervous system is stimulated.
nervous system is to increase the availability of glucose
as discussed fully in Chapter 60.
Glucagon is a hormone secreted by the alpha cells
of the pancreas when the blood glucose concentration
conversion of liver glycogen into glucose and its
blood glucose regulation is discussed more fully in
Chapter 78.
Glucose Molecule by the
Glycolytic Pathway
Because complete oxidation of 1 gram-molecule of
only 12,000 calories of energy are required to form 1
glucose were decomposed all at once into water and
protein enzymes that cause the glucose molecule to split
energy is released in small packets to form one mole-
the processes by which the glucose molecule is pro-
gressively dissected and its energy released to form
Cell membrane
Uridine diphosphate glucose
Glycogen
Glycolysis
Glucose-1-phosphate
Glucose-6-phosphate
Glucose
Blood
glucose
(glucokinase)
(phosphatase)
(phosphorylase)
is present in liver cells but not in most other cells.)
(The phosphatase required for the release of glucose from the cell
also interconversions between blood glucose and liver glycogen.
Chemical reactions of glycogenesis and glycogenolysis, showing
Figure 67–4
more quantities of acetyl-CoA from pyruvic acid. The
coenzyme A portion of the acetyl-CoA is released and
The
oxaloacetic acid
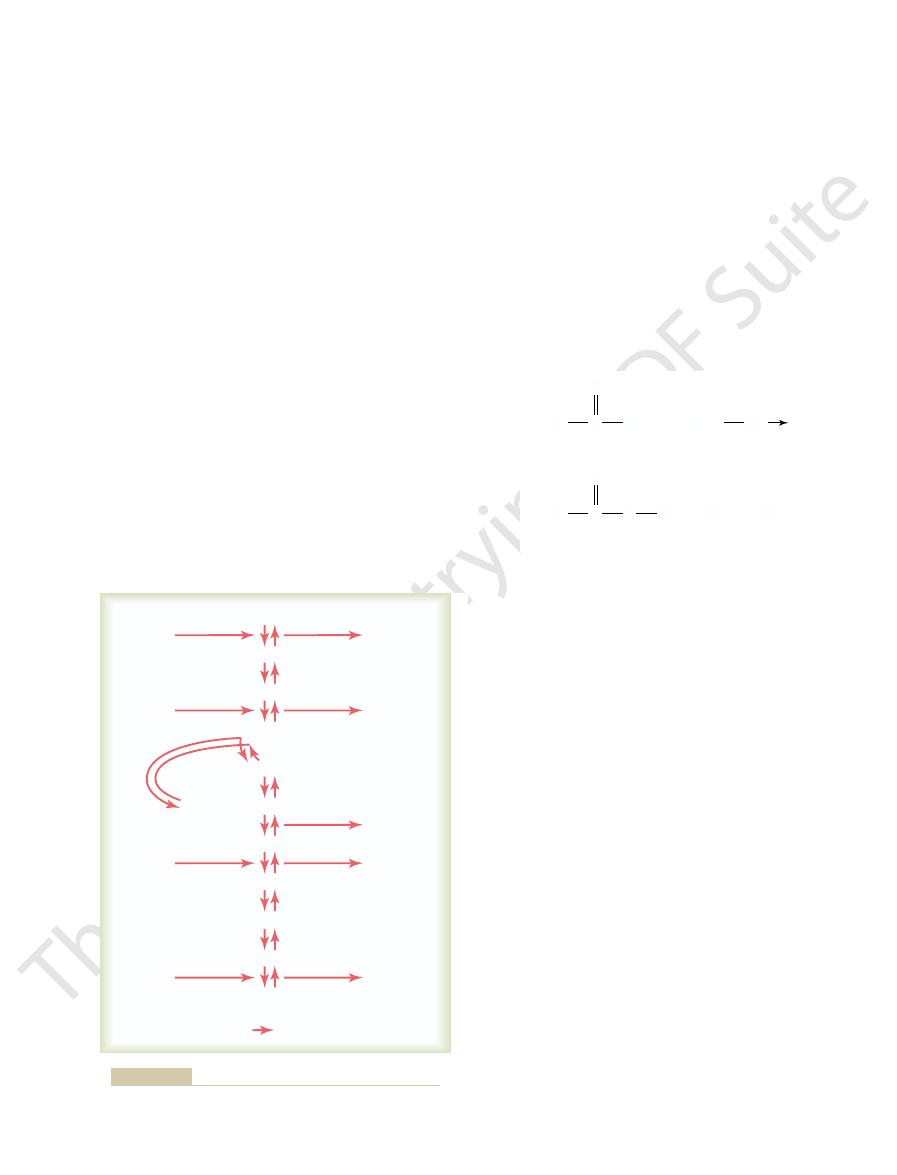
In the initial stage of the citric acid cycle,
cycle can continue over and over.
is formed again. Thus, the
oxaloacetic acid
reactions,
oxaloacetic acid,
right. Note at the top of the column that the cycle begins
the left are added during the chemical reactions, and the
cal reactions in the citric acid cycle. The substances to
Figure 67
amounts of energy to form ATP.
oxidized (as discussed later), releasing tremendous
The released hydrogen atoms add to
mitochondrion.
atoms. These reactions all occur in the
). This is a sequence of
boxylic acid cycle
The next stage in the degradation of the glucose mole-
Citric Acid Cycle (Krebs Cycle)
dized, as discussed later.
formed, but up to six molecules of ATP are formed
molecules of acetyl-CoA. In this conversion, no ATP is
derivative of the vitamin pantothenic acid, to form two
pyruvic acid molecules combine with coenzyme A, a
released, while the remaining portions of the two
From this reaction, it can be seen that two carbon
(acetyl-CoA), in accordance with the following
Figure 67
The next stage in the degradation of glucose is a two-
to Acetyl Coenzyme A
Conversion of Pyruvic Acid
The remaining 57 per cent of the energy is lost in the
for ATP formation of only 43 per cent.
energy were lost from the original glucose, giving an
ATP, but during glycolysis, a total of 56,000 calories of
This amounts to
moles for each mole of glucose utilized.
ATP molecules by the entire glycolytic process is only 2
before glycolysis could begin. Therefore,
Yet 2 moles of ATP were required to phosphorylate
of 4 moles of ATP were formed for each mole of fruc-
coupled in such a way that ATP is formed. Thus, a total
amount required to form ATP, and the reactions are
released are greater than 12,000 calories per mole, the
and the pyruvic acid stages, the packets of energy
stages, and again between the phosphoenolpyruvic acid
released at most steps. However, between the 1,3-
ical reactions in the glycolytic series, only a small
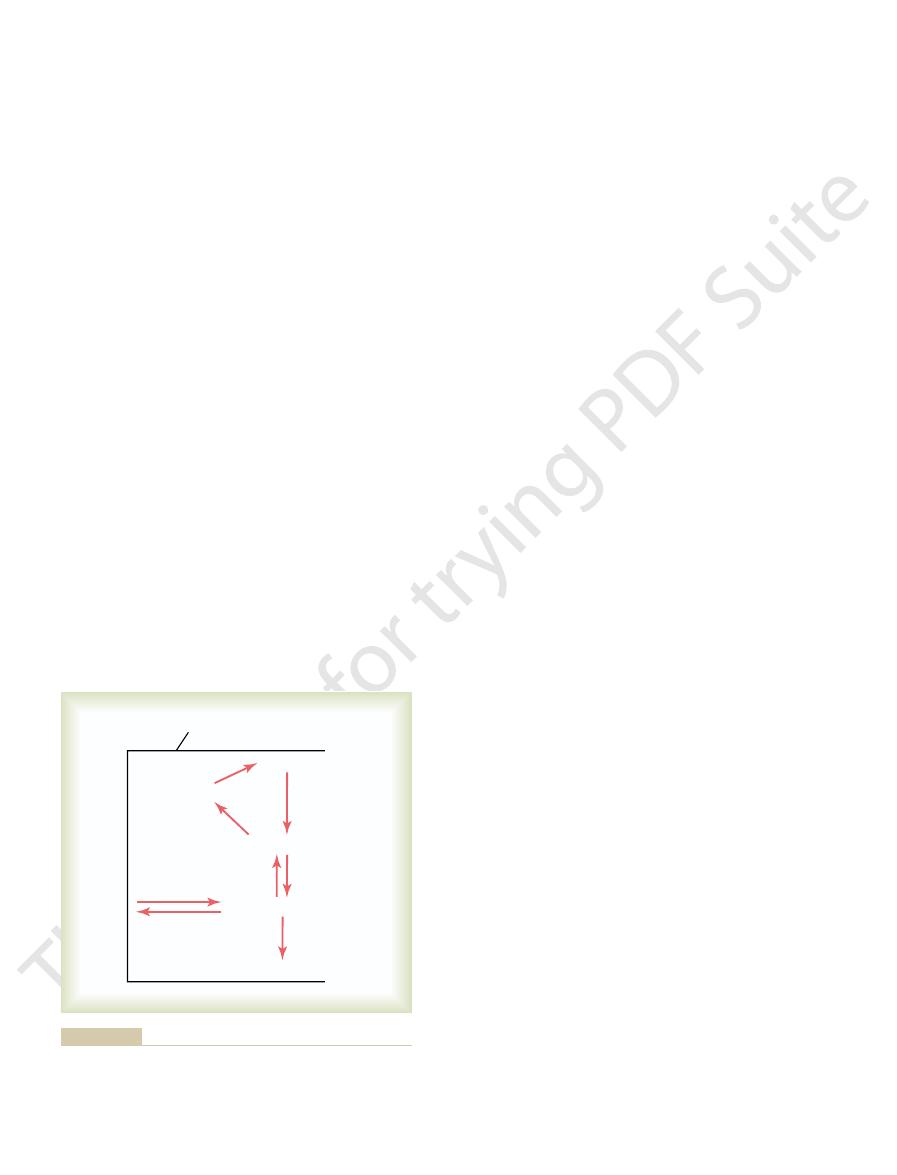
Formation of ATP During Glycolysis.
3-phosphate, each of which is then converted through
into two three-carbon-atom molecules, glyceraldehyde-
c protein enzyme. Note that glucose is
5. Each step is catalyzed by at least
shown in Figure 67
Glycolysis occurs by 10 successive chemical reactions,
two molecules of pyruvic acid.
energy. Glycolysis means splitting of the glucose mole-
The
glycolysis.
of Pyruvic Acid
Glycolysis and the Formation
Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Chapter 67
833
By far the most important means of releasing energy
from the glucose molecule is initiated by
end products of glycolysis are then oxidized to provide
cule to form
–
one specifi
first
converted into fructose-1,6-diphosphate and then split
five additional steps into pyruvic acid.
Despite the many chem-
portion of the free energy in the glucose molecule is
diphosphoglyceric acid and the 3-phosphoglyceric acid
tose-1,6-diphosphate that is split into pyruvic acid.
the original glucose to form fructose-1,6-diphosphate
the net gain in
24,000 calories of energy that becomes transferred to
overall efficiency
form of heat.
step conversion of the two pyruvic acid molecules from
–5 into two molecules of acetyl coenzyme
A
reaction:
dioxide molecules and four hydrogen atoms are
when the four released hydrogen atoms are later oxi-
cule is called the citric acid cycle (also called the tricar-
or Krebs cycle
chemical reactions in which the acetyl portion of acetyl-
CoA is degraded to carbon dioxide and hydrogen
matrix of the
the number of these atoms that will subsequently be
–6 shows the different stages of the chemi-
products of the chemical reactions are shown to the
with
and at the bottom of the chain of
acetyl-CoA
combines with
to form citric acid.
can be used again and again for the formation of still
C
S
CoA
(Pyruvic acid)
(Coenzyme A)
COOH
2CoA
C
SH
+
2CH
3
O
2CH
3
+ 2CO
2
+ 4H
O
(Acetyl-CoA)
2 Pyruvic acid + 2ATP + 4H
Glucose + 2ADP + 2PO
2ADP
2ATP
2ATP
ATP
ADP
ATP
ADP
2ADP
+
Net reaction per molecule of glucose:
4
-
4H
Glucose
Glucose-6-phosphate
Fructose-6-phosphate
Fructose-1,6-diphosphate
2 (1,3-Diphosphoglyceric acid)
2 (3-Phosphoglyceric acid)
2 (2-Phosphoglyceric acid)
Dihydroxyacetone phosphate
2 (Glyceraldehyde-3-phosphate)
2 (Phosphoenolpyruvic acid)
2 (Pyruvic acid)
-
-
Sequence of chemical reactions responsible for glycolysis.
Figure 67–5
citric acid cycle, as well as to those for the formation of
process.
. Instead, they pass
not subsequently released to NAD
citric acid cycle between the succinic and fumaric acid
The remaining four hydrogen atoms released during
later.
that form tremendous quantities of ATP, as discussed
gen ion and the hydrogen bound with NAD
to act as a hydrogen carrier. Both the free hydro-
NAD
This reaction will not occur without intermediation of
the vitamin niacin, in accordance with the following
), a derivative of
namide adenine dinucleotide (NAD
Twenty of the
dehydrogenase.
in each instance, the release is catalyzed by a speci
uid. Instead, they are released in packets of two, and
However, the hydrogen
original molecule of glucose.
this makes a total of 24 hydrogen atoms released for each
CoA from pyruvic acid, and 16 in the citric acid cycle;
atoms during glycolysis, 4 during formation of acetyl-
chemical reactions of the citric acid cycle
sion, hydrogen atoms are released during different
Function of Dehydrogenases and Nicotinamide Adenine Dinu-
a total of two molecules of ATP formed.
the citric acid cycle, each forming a molecule of ATP, or
metabolized, two acetyl-CoA molecules pass through
of ATP formed. Thus, for each molecule of glucose
of energy is released during the citric acid cycle itself; in
Formation of ATP in the Citric Acid Cycle.
molecules of ATP are formed, as follows.
hydrogen atoms, and 2 molecules of coenzyme A. Two
are then degraded into 4 carbon dioxide molecules, 16
citric acid cycle, along with 6 molecules of water. These
metabolized, 2 acetyl-CoA molecules enter into the
6, demon-
in the explanation at the bottom of Figure 67
The net results of the entire citric acid cycle are given
gure.
cycle, as shown on the right in the
carbon dioxide
gure, and
citric acid cycle, several molecules of water are added,
citric acid molecule. During the successive stages of the
acetyl portion, however, becomes an integral part of the
Metabolism and Temperature Regulation
834
Unit XIII
as shown on the left in the fi
and hydrogen atoms are released at other stages in the
fi
–
strating that for each molecule of glucose originally
Not a great amount
only one of the chemical reactions—during the change
from
a-ketoglutaric acid to succinic acid—is a molecule
cleotide in Causing Release of Hydrogen Atoms in the Citric Acid
Cycle.
As already noted at several points in this discus-
—4 hydrogen
atoms are not simply turned loose in the intracellular
fl
fic
protein enzyme called a
24 hydrogen atoms immediately combine with nicoti-
+
reaction:
the specific dehydrogenase or without the availability of
+
+
subse-
quently enter into multiple oxidative chemical reactions
the breakdown of glucose—the four released during the
stages—combine with a specific dehydrogenase but are
+
directly from the dehydrogenase into the oxidative
Function of Decarboxylases in Causing Release of Carbon Dioxide.
Referring again to the chemical reactions of the
dehydrogenase
H
H
NADH
+ H
+
+ Substrate
+ NAD
+
Substrate
+ 16H + 2CoA + 2ATP
2 Acetyl-CoA + 6H
CO
CoA
(Acetyl coenzyme A)
C
COOH
C
COOH
C
COOH
HC
COOH
HC
COOH
C
COOH
C
COOH
C
COOH
HC
COOH
C
COOH
HC
COOH
C
COOH
C
COOH
C
COOH
ATP
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
ADP
H
2
H
2
H
2
C
H
COOH
H
2
(Citric acid)
HOC
COOH
HOC
COOH
H
2
H
2
C
CO
2
CO
2
H
2
C
COOH
H
2
H
2
CH
H
HOOC
C
O
COOH
H
2
H
2
2H
2H
2H
2H
C
O
COOH
C
O
COOH
H
2
(Oxaloacetic acid)
(cis-Aconitic acid)
(Isocitric acid)
(Oxalosuccinic acid)
C
HO
COOH
(Malic acid)
(Oxaloacetic acid)
(
a
-Ketoglutaric acid)
(Succinic acid)
(Fumaric acid)
C
O
COOH
CH
3
CoA
Net reaction per molecule of glucose:
2
O + 2ADP
4CO
2
carbon dioxide and a number of hydrogen atoms during the cycle.
Chemical reactions of the citric acid cycle, showing the release of
Figure 67–6
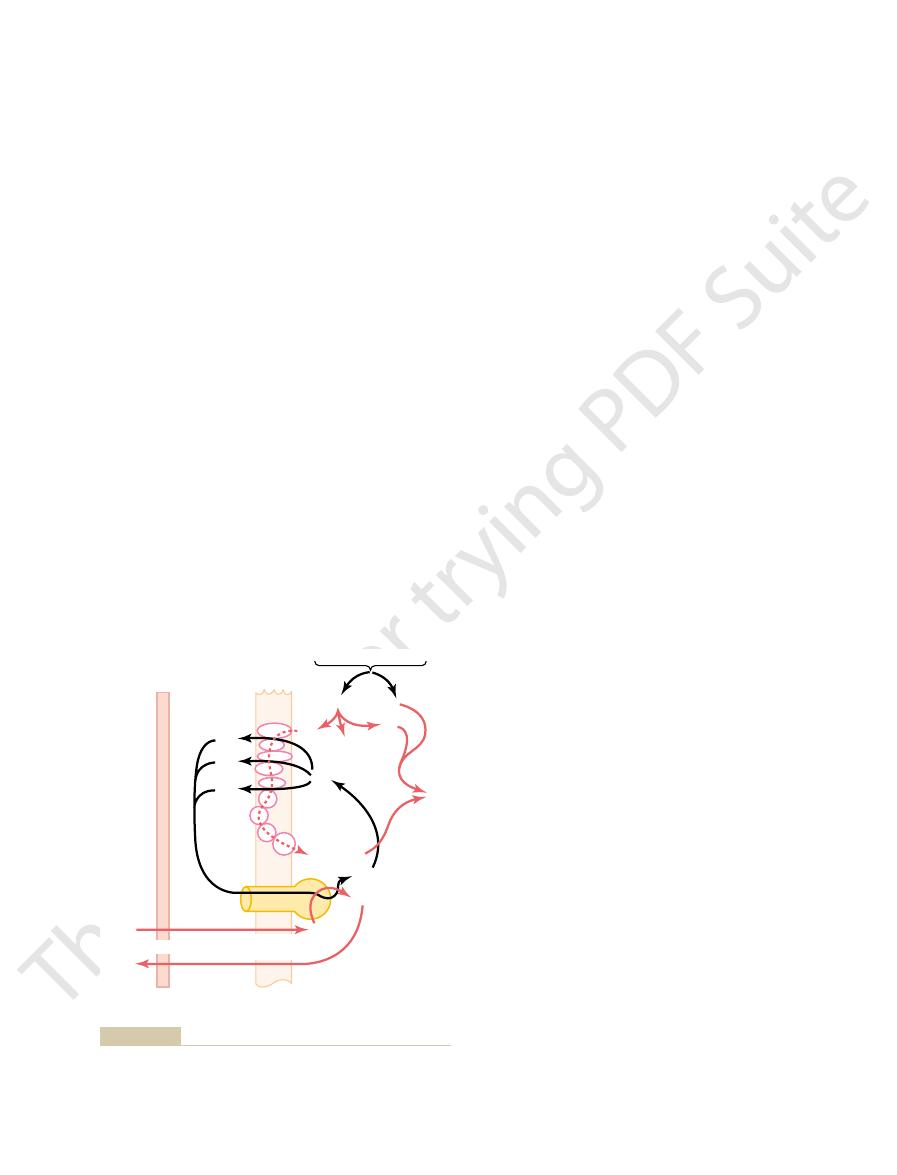
ATP synthetase.
7. It is called
ical nature of which is shown in Figure 67
chondrial matrix. This molecule is an ATPase, the phys-
lation is to convert ADP into ATP. This occurs in con-
The next step in oxidative phosphory-
Formation of ATP.
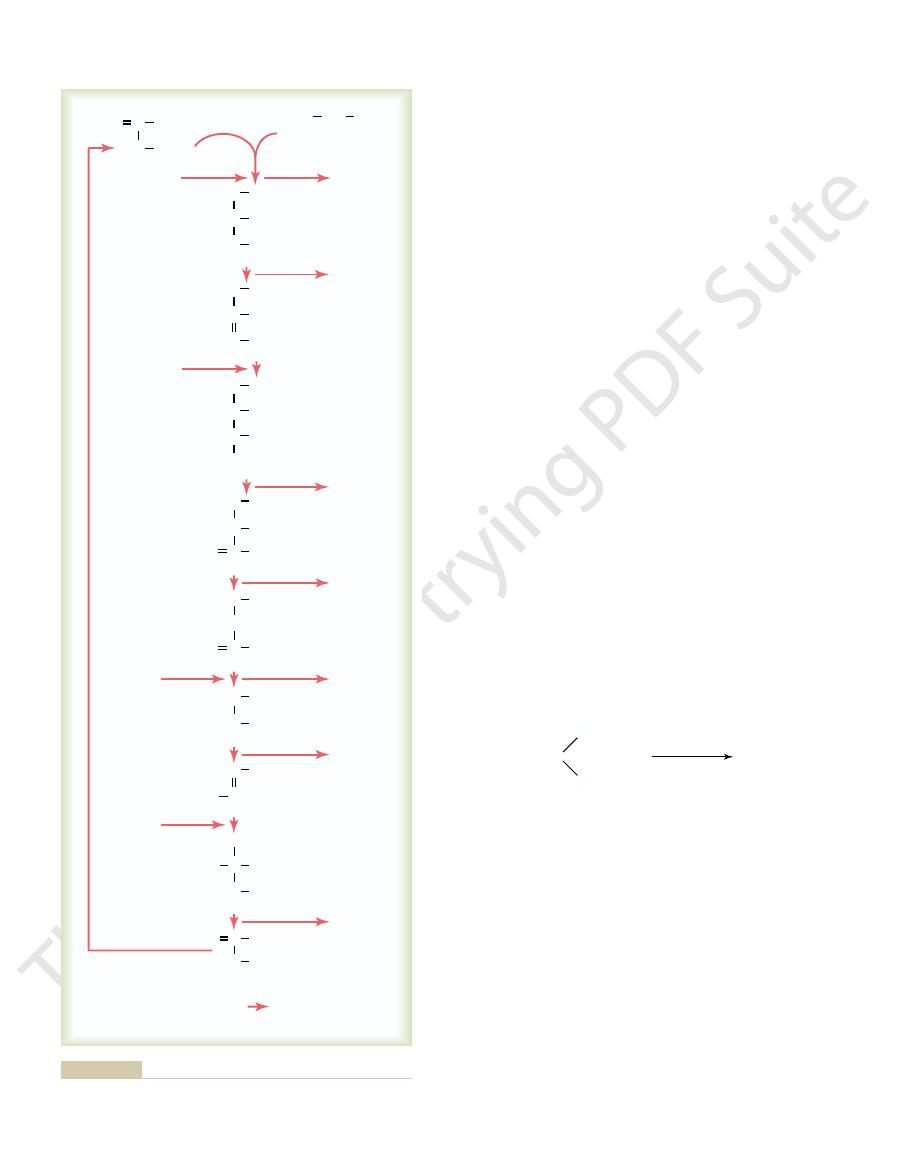
chamber; it also creates a strong negative electrical
drial membranes (to the left). This creates a high con-
the mitochondrion (to the right in Figure 67
large amounts of energy are released. This energy is
chondrion, Caused by the Electron Transport Chain.
follows.
released that is used to cause the synthesis of ATP, as
trons through the electron transport chain, energy is
of water molecules. During the transport of these elec-
use by cytochrome oxidase to cause the formation
Thus, Figure 67
ions to form water.
form ionic oxygen, which then combines with hydrogen
cytochrome oxidase
nally reaches cytochrome A3, which is called
3. Each electron is shut-
, and
cytochromes B, C
iron sulfide proteins, ubiquinone,
accepting or giving up electrons. The important
shelf membrane) of the mitochondrion. The electron
electron transport chain of electron acceptors
The electrons that are removed from the hydrogen
; this process also reconstitutes NAD
gen ion, H
hydrogen atom from the NADH to form another hydro-
. The initial effect is to release the other
NADH and H
portion of Figure 67
to form NADH. The upper
other combines with NAD
; the
pairs: one immediately becomes a hydrogen ion, H
described earlier, these hydrogen atoms are removed in
have been removed from the food substrates. As
The
tion of Water.
Ionization of Hydrogen, the Electron Transport Chain, and Forma-
Mitochondria to Form ATP
Chemiosmotic Mechanism of the
mechanism.
chemiosmotic
This occurs entirely in the mitochondria by a
oxidative phospho-
tion of ATP in this manner is called
quantities of energy are released to form ATP. Forma-
this sequence of oxidative reactions, tremendous
combine with each other to form water. During
hydroxyl ions. Then the hydrogen and hydroxyl ions
These reactions (1) split
in the mitochondria.
7, by a series of enzymatically catalyzed
in Figure 67
Oxidation of hydrogen is accomplished, as illustrated
early stages of glucose degradation. Indeed, the princi-
almost 90 per cent of the total ATP created through
cycle for each molecule of glucose metabolized. Instead,
only two ATP molecules in
boxylation, pitifully small amounts of ATP are formed
citric acid cycle, (3) dehydrogenation, and (4) decar-
Despite all the complexities of (1) glycolysis, (2) the
(the Process of Oxidative
of ATP by Oxidation of Hydrogen
Formation of Large Quantities
lungs, where it is expired from the body (see Chapter
dioxide away from the substrate. The carbon dioxide is
decarboxylases,
protein enzymes, called
cause the release of carbon dioxide, other speci
three stages in which carbon dioxide is released. To
acetyl-CoA from pyruvic acid, we
Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Chapter 67
835
find that there are
fic
split the carbon
then dissolved in the body fluids and transported to the
40).
Phosphorylation)
during all these processes—
the glycolysis scheme and another two in the citric acid
glucose metabolism is formed during subsequent oxi-
dation of the hydrogen atoms that were released at
pal function of all these earlier stages is to make the
hydrogen of the glucose molecule available in forms
that can be oxidized.
–
reactions
each hydrogen atom into a hydrogen ion and an elec-
tron and (2) use the electrons eventually to combine dis-
solved oxygen of the fluids with water molecules to form
rylation.
highly specialized process called the
first step in oxidative phosphorylation
in the mitochondria is to ionize the hydrogen atoms that
+
+
–7 shows the subsequent fate of the
+
+
+
that
will be reused again and again.
atoms to cause the hydrogen ionization immediately
enter an
that are an integral part of the inner membrane (the
acceptors can be reversibly reduced or oxidized by
members of this electron transport chain include flavo-
protein, several
and
1, C, A
A
tled from one of these acceptors to the next until it
fi
because it is capable of giving up
two electrons and thus reducing elemental oxygen to
–7 shows the transport of electrons
through the electron chain and then their ultimate
Pumping of Hydrogen Ions into the Outer Chamber of the Mito-
As the
electrons pass through the electron transport chain,
used to pump hydrogen ions from the inner matrix of
–7) into the
outer chamber between the inner and outer mitochon-
centration of positively charged hydrogen ions in this
potential in the inner matrix.
junction with a large protein molecule that protrudes all
the way through the inner mitochondrial membrane
and projects with a knoblike head into the inner mito-
–
ATPase
ATPase
diffusion
diffusion
Diffusion
Diffusion
3 ATP
3 ATP
ATP
ATP
3 ADP
3 ADP
Food substrate
Food substrate
6H
+
6H
+
6H
+
6H
+
2H
+
2H
+
2H
+
2H
+
2H
+
2H
+
Outer
membrane
Outer
membrane
Inner
membrane
Inner
membrane
Facilitated
Facilitated
FMN
FMN
FeS
FeS
FeS
FeS
Q
Q
b
b
C
1
C
1
C
1
C
1
a
3
a
3
a
a
-
2e
-
2e
NAD
+
NAD
+
H
+
H
+
H
+
H
+
NADH +
NADH +
2e
+
1/2 O
2
2e
+
1/2 O
2
H
2
O
H
2
O
ADP
ADP
relationship of the oxidative and phosphorylation steps at the outer
lation for forming large quantities of ATP. This figure shows the
Mitochondrial chemiosmotic mechanism of oxidative phosphory-
Figure 67–7
and inner membranes of the mitochondrion.

glycolysis stage of carbohydrate degradation, because
take place. Yet even under these conditions, a small
cient, so that oxidative phosphorylation cannot
Occasionally, oxygen becomes either unavailable or
as very strenuous exercise.
maintained, except during extreme cellular activity, such
this way, essentially a full store of ATP is automatically
AMP are almost instantly returned to the ATP state. In
AMP turn on the energy processes again, and ADP and
siologic functions in the cell, the newly formed ADP and
ATP is used by the cell to energize the different phy-
to form ATP is stopped. Then, when
fats, and proteins
glucose,
ATP simply cannot be formed. As a result, the entire
cell has already been converted into ATP, additional
for energy release, we see that if all the ADP in the
lowing: Referring back to the various chemical reactions
ling energy release from fats and proteins, is the fol-
controls carbohydrate metabolism, as well as control-
A third way by which the ATP-ADP-AMP system
glycolysis.
acid cycle
inhibits phosphofructokinase,
the citric acid cycle. An excess of this ion also
store of ATP is replenished.
glycolytic process is set in motion, and the total cellular
activity as a result of the excess ADP formed. Thus, the
reactions, this reduces the ATP inhibition of the enzyme
ity. Whenever ATP is used by the tissues for energizing
site change in this enzyme, greatly increasing its activ-
Conversely, ADP (and AMP as well) causes the oppo-
excess cellular ATP is to slow or even stop glycolysis,
in the glycolytic series of reactions, the net effect of
tion of fructose-1,6-diphosphate, one of the initial steps
fructokinase.
energy metabolism is to inhibit the enzyme
One important way in which ATP helps control
reactions in the energy metabolism sequence.
both ADP and ATP in controlling the rates of chemical
within the chemical schemata. Among the more impor-
need for ATP. This control is
ful process. Instead, glycolysis and the subsequent oxi-
in Controlling the Rate of Glycolysis
of ATP and ADP Cell Concentrations
Needs Additional Energy: Effect
c functions.
and, therefore, cannot be used by the cells to perform
The remaining 34 per cent of the energy becomes heat
molecule of glucose. This represents an overall
in the form of ATP, whereas 686,000 calories are
and water. Thus, 456,000 calories of energy can be stored
38 ATP molecules
Now, adding all the ATP molecules formed, we
molecules.
four more ATP
oxidized, thus giving a total of
7. Two ATP molecules are
rst stage of Figure 67
4. The remaining four hydrogen atoms are released
30 ATP molecules.
atoms of hydrogen metabolized. This gives an
with the release of three ATP molecules per two
chemiosmotic mechanism shown in Figure 67
glycolysis and during the citric acid cycle. Twenty of
3. During the entire schema of glucose breakdown, a
ATP.
for each molecule of glucose metabolized, giving
molecules, there are two revolutions of the cycle
one molecule of ATP is formed. However, because
2. During each revolution of the citric acid cycle,
ATP.
going. This gives a net gain of
formed, and two are expended to cause the initial
1. During glycolysis, four molecules of ATP are
the energy from one molecule of glucose.
cules that, under optimal conditions, can be formed by
We can now determine the total number of ATP mole-
the Breakdown of Glucose
Summary of ATP Formation During
two hydrogen atoms), up to three ATP molecules are
For each two electrons that pass through the entire elec-
the other direction for continual conversion into ATP.
membrane. In turn, ADP is continually transferred in
plasm. This occurs by facilitated diffusion outward
the inside of the mitochondrion back to the cell cyto-
nal step in the process is transfer of ATP from
The
molecule.
ing ADP with a free ionic phosphate radical (Pi), thus
used by ATPase to convert ADP into ATP by combin-
doing so, energy derived from this hydrogen ion
through the substance of the ATPase molecule.
The high concentration of positively charged hydro-
Metabolism and Temperature Regulation
836
Unit XIII
gen ions in the outer chamber and the large electrical
potential difference across the inner membrane cause
the hydrogen ions to flow into the inner mitochondrial
matrix
In
flow is
adding another high-energy phosphate bond to the
fi
through the inner membrane and then by simple dif-
fusion through the permeable outer mitochondrial
tron transport chain (representing the ionization of
synthesized.
phosphorylation of glucose to get the process
two molecules of
each glucose molecule splits into two pyruvic acid
a net production of two more molecules of
total of 24 hydrogen atoms are released during
these atoms are oxidized in conjunction with the
–7,
additional
by their dehydrogenase into the chemiosmotic
oxidative schema in the mitochondrion beyond the
fi
–
usually released for every two hydrogen atoms
find a maximum of
formed for each
molecule of glucose degraded to carbon dioxide
released during the complete oxidation of each gram-
maximum efficiency of energy transfer of 66 per cent.
specifi
Control of Energy Release from
Stored Glycogen When the Body
Continual release of energy from glucose when energy
is not needed by the cells would be an extremely waste-
dation of hydrogen atoms are continually controlled in
accordance with the cells’
accomplished by multiple feedback control mechanisms
tant of these are the effects of cell concentrations of
phospho-
Because this enzyme promotes the forma-
which in turn stops most carbohydrate metabolism.
a major fraction of almost all intracellular chemical
phosphofructokinase and at the same time increases its
Another control linkage is the citrate ion formed in
strongly
thus preventing the
glycolytic process from getting ahead of the citric
’s ability to use the pyruvic acid formed during
sequence involved in the use of foodstuffs—
—
Anaerobic Release of Energy—
“Anaerobic Glycolysis”
insuffi
amount of energy can still be released to the cells by the
the chemical reactions for the breakdown of glucose to
pyruvic acid do not require oxygen.

energy. By far the greatest portion of this reconversion
when oxygen is available again, the lactic acid can be
anaerobic glycolysis is not lost from the body because,
Thus, the large amount of lactic acid that forms during
glucose.
excess ATP then causes as much as three fourths of the
ately oxidized to form large quantities of ATP. This
. Large portions of these are immedi-
NADH plus H
When a person begins to breathe
Becomes Available Again.
quantities of ATP, even in the absence of respiratory
minutes, supplying the body with considerable extra
this conversion. Instead, it can proceed for several
longer than would otherwise be possible. Indeed, gly-
can disappear, thus allowing glycolysis to proceed far
less active cells. Therefore, lactic acid represents a type
of the pyruvic acid is converted into lactic acid, which
Thus, under anaerobic conditions, the major portion
other to form lactic acid, in accordance with the fol-
to be excessive, these two end products react with each
further formation of ATP. When their quantities begin
. The buildup of either or both of
form NADH and H
acid and (2) hydrogen atoms combined with NAD
5) are (1) pyruvic
the glycolytic reactions (see Figure 67
decreases, approaching zero. The two end products of
build up in a reacting medium, the rate of the reaction
The
Release of Extra Anaerobic Energy.
oxygen becomes unavailable.
anaerobic energy,
energy to the cells, which is called
glucose molecule. Nevertheless, this release of glycolytic
each molecule of glucose metabolized, which represents
only 24,000 calories of energy are used to form ATP for
This process is extremely wasteful of glucose, because
Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Chapter 67
837
only a little over 3 per cent of the total energy in the
can
be a lifesaving measure for up to a few minutes when
Formation of Lactic Acid During Anaerobic Glycolysis Allows
law of mass action
states that as the end products of a chemical reaction
–
+
to
+
these would stop the glycolytic process and prevent
lowing equation:
diffuses readily out of the cells into the extracellular
fluids and even into the intracellular fluids of other
of “sinkhole” into which the glycolytic end products
colysis could proceed for only a few seconds without
oxygen.
Reconversion of Lactic Acid to Pyruvic Acid When Oxygen
oxygen again after a period of anaerobic metabolism,
the lactic acid is rapidly reconverted to pyruvic acid and
+
remaining excess pyruvic acid to be converted back into
either reconverted to glucose or used directly for
C
COOH
NAD
C
COOH
NADH
H
dehydrogenase
lactic
+
+
CH
3
+
OH
(Pyruvic acid)
(Lactic acid)
+
CH
3
+
OH
H
substances.
in the form of NADPH can be used for
with NADP
cant, because only hydrogen bound
for an extra phosphate radical, P. This difference is
), which is almost identical to NAD
(NADP
with NAD
during the pentose phosphate cycle does not combine
The hydrogen released
stances, as follows.
however, it is used for the synthesis of fat or other sub-
phosphorylation pathway to form ATP; more often,
and hydrogen, and the hydrogen can enter the oxidative
Thus, by repeating the cycle again and again, all the
glucose is metabolized for each revolution of the cycle.
pathway is a cyclical process in which one molecule of
That is, the pentose phosphate
into the reactions.
sized for every six molecules of glucose that initially enter
However,
nations of these sugars can resynthesize glucose.
seven-, and three-carbon sugars. Finally, various combi-
ve-, four-,
-ribulose. This substance can
ve-carbon sugar,
four atoms of hydrogen, with the resultant formation
version, can release one molecule of carbon dioxide and
demonstrates that glucose, during several stages of con-
chemical reactions in the pentose phosphate pathway. It
Figure 67
Phosphate Pathway.
viding energy to multiple cellular synthetic processes.
malities occur in cells. It has a special capacity for pro-
citric acid cycle and therefore is an alternative pathway
This pathway is especially important because it can
liver and even more than this in fat cells.
), which is responsible for as
phosphogluconate pathway
pentose phosphate pathway
can be degraded and used to provide energy. A second
acid by glycolysis and then oxidized. However, this gly-
s muscles, essentially all the car-
Release of Energy from
occurs to a great extent during heavy exercise, when
acid and then using the pyruvic acid for energy. This
Use of Lactic Acid by the Heart for Energy.
other tissues.
occurs in the liver, but a small amount can also occur in
Heart muscle
is especially capable of converting lactic acid to pyruvic
large amounts of lactic acid are released into the blood
from the skeletal muscles and consumed as an extra
energy source by the heart.
Glucose by the Pentose
Phosphate Pathway
In almost all the body’
bohydrates utilized for energy are degraded to pyruvic
colytic scheme is not the only means by which glucose
important mechanism for the breakdown and oxidation
of glucose is called the
(or
much as 30 per cent of the glucose breakdown in the
provide energy independently of all the enzymes of the
for energy metabolism when certain enzymatic abnor-
Release of Carbon Dioxide and Hydrogen by Means of the Pentose
–8 shows most of the basic
of a fi
D
change progressively into several other fi
only five molecules of glucose are resynthe-
glucose can eventually be converted into carbon dioxide
Use of Hydrogen to Synthesize Fat; the Function of Nicotinamide
Adenine Dinucleotide Phosphate.
+
as in the glycolytic pathway but combines
with nicotinamide adenine dinucleotide phosphate
+
+
except
extremely signifi
+
the synthesis of fats from carbohydrates (as discussed
in Chapter 68) and for the synthesis of some other

into glucose. Thus, one of the most important means
uids. A high
essentially all cells of the body, making these available
In turn, cortisol mobilizes proteins from
glucocorticoid hormones,
This stimulates the adrenal cortex to produce
for reasons not completely understood, begins to
are not available to the cells, the adenohypophysis,
When normal quantities of carbohydrates
Effect of Corticotropin and Glucocorticoids on Gluco-
follows.
is especially important in this regulation, as
glycerol into carbohydrates. In addition, the hormone
glycolytic and phosphogluconate reactions, thus allow-
stimuli that increase the rate of gluconeogenesis. Dimin-
become glucose. Similar interconversions can change
simple interconversions, many of the amino acids can
glucose. Thus, by means of deamination plus several
ve-, or seven-carbon atoms; they can then enter
four-,
into pyruvic acid simply by deamination; the pyruvic
process. For instance, alanine can be converted directly
cult or impossible. Each amino acid is
teins can be converted easily into carbohydrates; the
other precursors.
During prolonged fasting, the kidneys also synthesize
duction during fasting is from gluconeogenesis, helping
) and by synthesizing glucose,
in the blood for several hours between meals. The liver
cells, and adequate amounts of glucose must be present
tration during fasting. Glucose is the primary substrate
gluconeogenesis.
This process is called
below normal, moderate quantities of glucose can be
When the body
from Proteins and Fats—
Formation of Carbohydrates
cells and is stored as fat in the fat cells. Other steps
muscle cells) approach saturation with glycogen, the
When the glycogen-storing cells (primarily liver and
to 24 hours.
either stored as glycogen or converted into fat. Glucose
When glucose is not immediately required for energy,
for the formation and storage of fat in the body.
instance,
used other than for the formation of ATP
derived from glucose, into long fatty acid chains. This is
becomes abundant to help convert acetyl-CoA, also
tinues to be transported into the cells, and NADPH
becomes slowed because of cellular inactivity, the
When the glycolytic pathway for using glucose
Metabolism and Temperature Regulation
838
Unit XIII
pentose phosphate pathway remains operative (mainly
in the liver) to break down any excess glucose that con-
another way in which energy in the glucose molecule is
—in this
Glucose Conversion to Glycogen
or Fat
the extra glucose that continually enters the cells is
is preferentially stored as glycogen until the cells have
stored as much glycogen as they can—an amount suffi-
cient to supply the energy needs of the body for only 12
additional glucose is converted into fat in liver and fat
in the chemistry of this conversion are discussed in
Chapter 68.
“Gluconeogenesis”
’s stores of carbohydrates decrease
formed from amino acids and the glycerol portion of fat.
Gluconeogenesis is especially important in prevent-
ing an excessive reduction in the blood glucose concen-
for energy in tissues such as the brain and the red blood
plays a key role in maintaining blood glucose levels
during fasting by converting its stored glycogen to
glucose (glycogenolysis
mainly from lactate and amino acids (gluconeogenesis).
Approximately 25 per cent of the liver’s glucose pro-
to provide a steady supply of glucose to the brain.
considerable amounts of glucose from amino acids and
About 60 per cent of the amino acids in the body pro-
remaining 40 per cent have chemical configurations that
make this diffi
converted into glucose by a slightly different chemical
acid is then converted into glucose or stored glycogen.
Several of the more complicated amino acids can be
converted into different sugars that contain three-,
fi
the phosphogluconate pathway and eventually form
glycerol into glucose or glycogen.
Regulation of Gluconeogenesis.
Diminished carbohydrates
in the cells and decreased blood sugar are the basic
ished carbohydrates can directly reverse many of the
ing the conversion of deaminated amino acids and
cortisol
neogenesis.
secrete increased quantities of the hormone corti-
cotropin.
large quantities of
especially
cortisol.
in the form of amino acids in the body fl
proportion of these immediately become deaminated
in the liver and provide ideal substrates for conversion
by which gluconeogenesis is promoted is through
the release of glucocorticoids from the adrenal
cortex.
Glucose-6-phosphate
6-Phosphoglucono-
d
-lactone
6-Phosphogluconic acid
3-Keto-6-phosphogluconic acid
D-Ribulose-5-phosphate
D-Xylulose-5-phosphate
D-Ribose-5-phosphate
D-Sedoheptulose-7-phosphate
+
+
+
D-Glyceraldehyde-3-phosphate
Fructose-6-phosphate
Erythrose-4-phosphate
Net reaction:
Glucose
+
12NADP
+
+
6H
2
O
6CO
2
+
12H
+
12NADPH
2H
2H
CO
2
H
2
O
Figure 67–8
Pentose phosphate pathway for glucose metabolism.

Endocr Metab Disord 4:95, 2003.
Wolfsdorf JI, Weinstein DA: Glycogen storage diseases. Rev
exercise. Acta Physiol Scand 178:443, 2003.
Spriet LL, Watt MJ: Regulatory mechanisms in the interac-
cal basis of certain disease states. J Intern Med 254:517,
Ronquist G, Waldenstrom A: Imbalance of plasma mem-
Clin Endocrinol Metab 17:365, 2003.
its role in health and disease. Best Pract Res
Roden M, Bernroider E: Hepatic glucose metabolism in
Physiol 54:885, 1992.
tion of hepatic gluconeogenesis and glycolysis. Annu Rev
Pilkis SJ, Granner DK: Molecular physiology of the regula-
Lett 545:47, 2003.
cytochrome c oxidase: lessons from other proteins. FEBS
Mills DA, Ferguson-Miller S: Understanding the mechanism
production. Am J Physiol Endocrinol Metab 284:E863,
Lam TK, Carpentier A, Lewis GF, et al: Mechanisms of
FEBS Lett 564:239, 2004.
Kunji ER: The role and structure of mitochondrial carriers.
Krebs HA: The tricarboxylic acid cycle. Harvey Lect 44:165,
humans. Physiol Rev 72:419, 1992.
Jungas RL, Halperin ML, Brosnan JT: Quantitative analysis
metabolism. Am J Physiol Endocrinol Metab 284:E671,
Jiang G, Zhang BB: Glucagon and regulation of glucose
FEBS Lett 545:18, 2003.
Jackson JB: Proton translocation by transhydrogenase.
chondria. FEBS Lett 567:96, 2004.
Gunter TE, Yule DI, Gunter KK, et al: Calcium and mito-
Physiol 58:565, 1996.
review of sites, pathways, and regulation. Annu Rev
Gleeson TT: Post-exercise lactate metabolism: a comparative
deposition. FEBS Lett 546:127, 2003.
Ferrer JC, Favre C, Gomis RR, et al: Control of glycogen
Diabetes 53(Suppl 1):S96, 2004.
Duchen MR: Roles of mitochondria in health and disease.
Physiol Rev 84:1, 2004.
phosphatase-1, a cellular economizer and reset button.
Ceulemans H, Bollen M: Functional diversity of protein
Metab 285:E685, 2003.
of hepatic gluconeogenesis. Am J Physiol Endocrinol
Barthel A, Schmoll D: Novel concepts in insulin regulation
indirect? J Clin Invest 111:434, 2003.
s effect on glucose production: direct or
Barrett EJ: Insulin
78 in relation to the functions of these hormones.
glucagon; this subject is discussed in detail in Chapter
The regulation of blood glucose concentration is inti-
dl unless the person has diabetes mellitus, which is dis-
of carbohydrates, this level seldom rises above 140 mg/
is about 90 mg/dl.After a meal containing large amounts
The normal blood glucose concentration in a person
Metabolism of Carbohydrates, and Formation of Adenosine Triphosphate
Chapter 67
839
Blood Glucose
who has not eaten a meal within the past 3 to 4 hours
cussed in Chapter 78.
mately related to the pancreatic hormones insulin and
References
’
2003.
of amino acid oxidation and related gluconeogenesis in
1948-1949.
the free fatty acid-induced increase in hepatic glucose
2003.
of proton movement linked to oxygen reduction in
humans—
brane ion leak and pump relationship as a new aetiologi-
2003.
tion between carbohydrate and lipid oxidation during
