
From this disc, these filaments extend in both directions to
Z disc.
Figure 6–1
cross-bridges.
These are
filaments in Figure 6–1
polarized light. Note also the small projections from the sides of the myosin
overlap the myosin, and are called
contain myosin filaments, as well as the ends of the actin filaments where they
to polarized light. The dark bands
illustrated in Figure 6–2. The light bands contain only actin filaments and are
tate and thus cause the myofibrils to have alternate light and dark bands, as
Note in Figure 6–1
The thick filaments in the diagrams are
6–1, parts
tron micrograph of Figure 6–2 and are represented diagrammatically in Figure
the actual muscle contraction. These can be seen in longitudinal view in the elec-
aments,
Each myofibril (Figure 6–1
dots in the cross-sectional view of Figure 6–1
myofibrils,
into bundles to form the muscle tendons that then insert into the bones.
of the sarcolemma fuses with a tendon fiber, and the tendon fibers in turn collect
numerous thin collagen fibrils. At each end of the muscle fiber, this surface layer
plasma membrane,
colemma consists of a true cell membrane, called the
The sarcolemma is the cell membrane of the muscle fiber. The sar-
one nerve ending, located near the middle of the fiber.
Except for about 2 per cent of the fibers, each fiber is usually innervated by only
In most skeletal muscles, each fiber extends the entire length of the muscle.
subunits, also shown in Figure 6–1 and described in subsequent paragraphs.
micrometers in diameter. Each of these fibers is made up of successively smaller
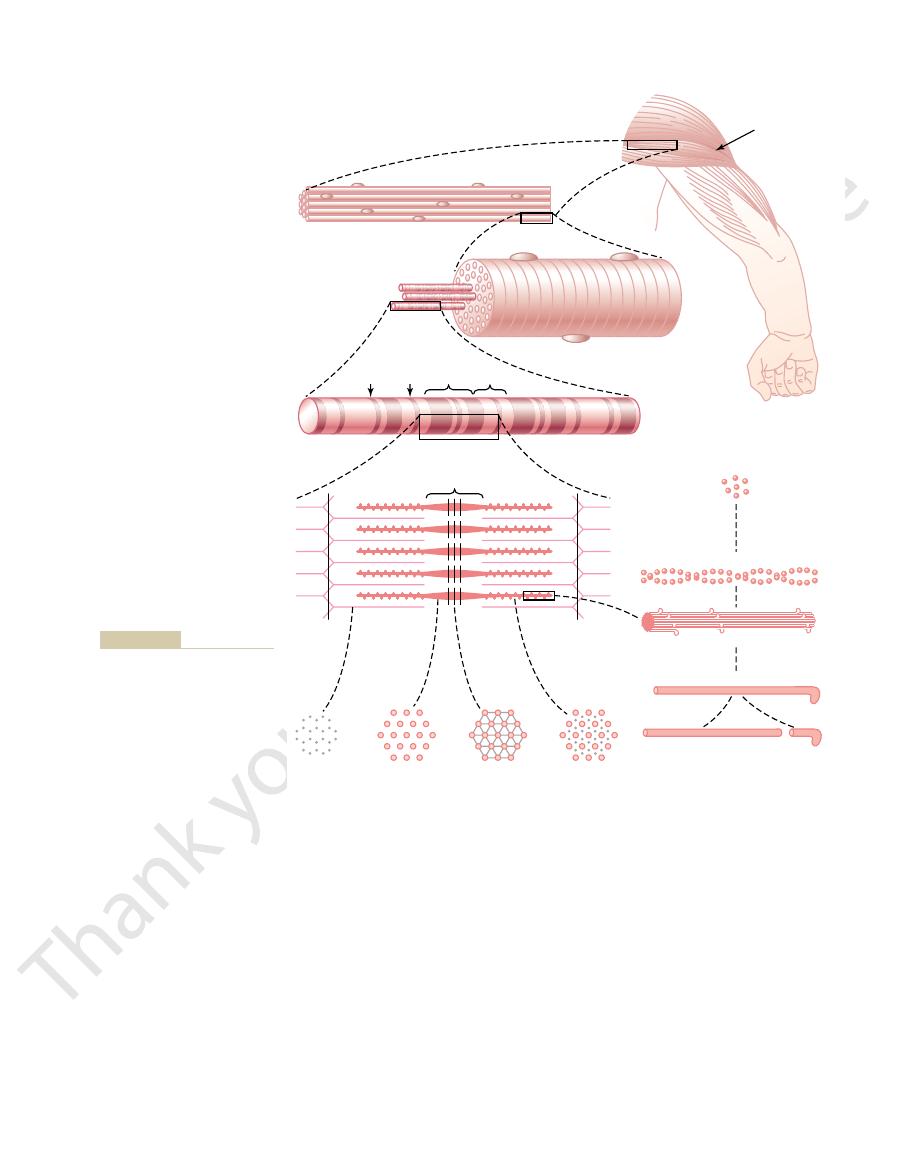
Figure 6–1 shows the organization of skeletal muscle, demonstrating that all
smooth muscle are discussed in Chapter 8, and
is considered mainly; the specialized functions of
muscle. In this chapter, function of skeletal muscle
cardiac muscle. Some of the same basic principles of
About 40 per cent of the body is skeletal muscle,
C
H
A
P
T
E
R
6
72
Contraction of
Skeletal Muscle
and perhaps another 10 per cent is smooth and
contraction apply to all these different types of
cardiac muscle is discussed in Chapter 9.
Physiologic Anatomy of Skeletal Muscle
Skeletal Muscle Fiber
skeletal muscles are composed of numerous fibers ranging from 10 to 80
Sarcolemma.
and an
outer coat made up of a thin layer of polysaccharide material that contains
Myofibrils; Actin and Myosin Filaments.
Each muscle fiber contains several hundred
to several thousand
which are demonstrated by the many small open
C.
D
and E) is composed of about 1500 adjacent myosin filaments and 3000 actin fil-
which are large polymerized protein molecules that are responsible for
E through L.
myosin, and the
thin filaments are actin.
E that the myosin and actin filaments partially interdigi-
called I bands because they are isotropic
A bands because they are anisotropic to
E and L.
It is the interaction
between these cross-bridges and the actin filaments that causes contraction.
E also shows that the ends of the actin filaments are attached to a
so-called
comere will work. There is reason to believe that the
These springy titin molecules act as a
very springy.
ecules in the body. Also, because it is filamentous, it is
million, which makes it one of the largest protein mol-
ficult to maintain. This is achieved by a large number
The side-by-side rela-
Titin Filamentous Molecules.
What Keeps the Myosin and Actin Filaments in Place?
that, at this length, the muscle is capable of generating
beginning to overlap one another. We will see later
filaments, and the tips of the actin filaments are just
the sarcomere is about 2 micrometers. At this length,
shown at the bottom of Figure 6–4, the length of
When the muscle fiber is contracted, as
sarcomere.
The portion of the myofibril (or of the whole muscle
cardiac muscle their striated appearance.
the individual myofibrils.These bands give skeletal and
the entire muscle fiber has light and dark bands, as do
another all the way across the muscle fiber. Therefore,
myofibril to myofibril, attaching the myofibrils to one
ferent from the actin and myosin filaments, passes
interdigitate with the myosin filaments. The Z disc,
Chapter 6
Contraction of Skeletal Muscle
73
which itself is composed of filamentous proteins dif-
crosswise across the myofibril and also crosswise from
fiber) that lies between two successive Z discs is called
a
the actin filaments completely overlap the myosin
its greatest force of contraction.
tionship between the myosin and actin filaments is dif-
of filamentous molecules of a protein called titin. Each
titin molecule has a molecular weight of about 3
framework that holds the myosin and actin filaments
in place so that the contractile machinery of the sar-
F
G
H
I
Sarcomere Z
SKELETAL MUSCLE
G-Actin molecules
F-Actin filament
Myofilaments
Myosin filament
Myosin molecule
Light
meromyosin
Heavy
meromyosin
Muscle fasciculus
Muscle
Muscle fiber
Myofibril
H
band
Z
disc
A
band
H
D
E
N
M
L
K
J
B
C
Z
I
band
A
book of Histology. Philadelphia:
DW: Bloom and Fawcett: A Text-
Keene. Modified from Fawcett
(Drawing by Sylvia Colard
are cross
F, G, H,
from the gross to the molecular
Figure 6–1
Organization of skeletal muscle,
level.
and I
sections at the levels indicated.
WB Saunders, 1986.)

another. Conversely, in the contracted state, these actin
state, the ends of the actin filaments extending from
(top) and the contracted state (bottom). In the relaxed
traction. It shows the relaxed state of a sarcomere
Figure
muscle contractile process.
We now describe the molecular machinery of the
myofibrils causes the muscle contraction to cease.
comes along; this removal of calcium ions from the
membrane pump, and they remain stored in
8. After a fraction of a second, the calcium ions are
process.
slide alongside each other, which is the contractile
the actin and myosin filaments, causing them to
7. The calcium ions initiate attractive forces between
fiber. Here it causes the sarcoplasmic reticulum to
membrane, and much of the action potential
6. The action potential depolarizes the muscle
travel along nerve fiber membranes.
5. The action potential travels along the muscle fiber
an action potential at the membrane.
interior of the muscle fiber membrane. This initiates
4. Opening of the acetylcholine-gated channels allows
in the membrane.
gated” channels through protein molecules floating
fiber membrane to open multiple “acetylcholine-
3. The acetylcholine acts on a local area of the muscle
acetylcholine.
2. At each ending, the nerve secretes a small amount
its endings on muscle fibers.
1. An action potential travels along a motor nerve to
occur in the following sequential steps.
The initiation and execution of muscle contraction
contraction, as discussed in Chapter 7.The very rapidly
This reticulum has a special organiza-
extensive reticulum (Figure 6–3), called the
triphosphate (ATP) formed by the mitochondria.
myofibrils. These supply the contracting myofibrils
mitochondria
multiple protein enzymes. Also present are tremen-
ties of potassium, magnesium, and phosphate, plus
lular fluid called sarcoplasm, containing large quanti-
are suspended side by side in the muscle fiber. The
The many myofibrils of each muscle fiber
sarcomere, especially the myosin filaments.
Membrane Physiology, Nerve, and Muscle
74
Unit II
titin molecule itself acts as template for initial forma-
tion of portions of the contractile filaments of the
Sarcoplasm.
spaces between the myofibrils are filled with intracel-
dous numbers of
that lie parallel to the
with large amounts of energy in the form of adenosine
Sarcoplasmic Reticulum.
Also in the sarcoplasm sur-
rounding the myofibrils of each muscle fiber is an
sarcoplas-
mic reticulum.
tion that is extremely important in controlling muscle
contracting types of muscle fibers have especially
extensive sarcoplasmic reticula.
General Mechanism of Muscle
Contraction
of the neurotransmitter substance
large quantities of sodium ions to diffuse to the
membrane in the same way that action potentials
electricity flows through the center of the muscle
release large quantities of calcium ions that have
been stored within this reticulum.
pumped back into the sarcoplasmic reticulum by a
Ca
++
the reticulum until a new muscle action potential
Molecular Mechanism
of Muscle Contraction
Sliding Filament Mechanism of Muscle Contraction.
6–4 demonstrates the basic mechanism of muscle con-
two successive Z discs barely begin to overlap one
filaments have been pulled inward among the myosin
lying between the myofibrils. (From Fawcett DW: The Cell.
Electron micrograph of muscle myofibrils showing the detailed
Figure 6–2
organization of actin and myosin filaments. Note the mitochondria
Philadelphia: WB Saunders, 1981.)
(From Fawcett DW: The Cell. Philadelphia: WB Saunders, 1981.)
ducting the electrical signal into the center of the muscle fiber.
to the exterior of the fiber membrane and are important for con-
(arrows)
brils. Also shown in cross section are T tubules
Sarcoplasmic reticulum in the extracellular spaces between the
Figure 6–3
myofibrils, showing a longitudinal system paralleling the myofi-
that lead

from the center.
almost exactly 1.6 micrometers. Note, however, that
The total length of each myosin filament is uniform,
ing sections.
actual contraction process, as discussed in the follow-
the body. The hinged heads in turn participate in the
the head attaches to the arm. The hinged arms allow
the body of the myosin filament, and the other where
cross-bridges.
the figure. The protruding arms and heads together are
extends the head outward from the body, as shown in
side along with the head, thus providing an
molecules hang outward to the sides of the body. Also,
of the filament, while many heads of the
these filaments is shown in Figure 6–5
vidual myosin molecules. The central portion of one of
The
to each head. These light chains help control the func-
four light chains are also part of the myosin head, two
at one end of the double-helix myosin molecule. The
Thus, there are two free heads
myosin molecule. One end of each of these chains is
to form a double helix, which is called the
The two heavy chains wrap spirally around each other
chains
a molecular weight of about 200,000, and four
heavy chains,
(see Figure 6–5
The
of two actin filaments.
of many molecules to form a myosin filament, as well
vidual molecule; Figure 6–5
weight of about 480,000. Figure 6–5
multiple myosin molecules, each having a molecular
The myosin filament is composed of
Contractile Filaments
Molecular Characteristics of the
energy. In the next few sections, we describe what is
energy bonds in the ATP molecule, which is degraded
process to proceed. This energy comes from high-
tion begins. But energy is needed for the contractile
between the myosin and actin filaments, and contrac-
myofibrils. The calcium ions in turn activate the forces
an action potential travels along the muscle fiber,
resting conditions, these forces are inactive, but when
the myosin filaments with the actin filaments. Under
among the myosin filaments? This is caused by forces
sliding filament mechanism.
myosin filaments. Thus, muscle contraction occurs by a
their maximum extent. Also, the Z discs have been
filaments, so that their ends overlap one another to
Chapter 6
Contraction of Skeletal Muscle
75
pulled by the actin filaments up to the ends of the
But what causes the actin filaments to slide inward
generated by interaction of the cross-bridges from
this causes the sarcoplasmic reticulum to release large
quantities of calcium ions that rapidly surround the
to adenosine diphosphate (ADP) to liberate the
known about the details of these molecular processes
of contraction.
Myosin Filament.
A shows an indi-
B shows the organization
as interaction of this filament on one side with the ends
myosin molecule
A) is composed
of six polypeptide chains—two
each with
light
with molecular weights of about 20,000 each.
tail of the
folded bilaterally into a globular polypeptide structure
called a myosin head.
tion of the head during muscle contraction.
myosin filament is made up of 200 or more indi-
B, displaying the
tails of the myosin molecules bundled together to form
the body
part of the body of each myosin molecule hangs to the
arm that
called
Each cross-bridge is flexible at
two points called hinges—one where the arm leaves
the heads either to be extended far outward from the
body of the myosin filament or to be brought close to
there are no cross-bridge heads in the very center of
the myosin filament for a distance of about 0.2
micrometer because the hinged arms extend away
I
A
I
Z
Z
I
A
Relaxed
Contracted
I
Z
Z
each other.
pulling of the Z membranes toward
(red),
Figure 6–4
Relaxed and contracted states of a myofibril showing (top) sliding
of the actin filaments (pink) into the spaces between the myosin
filaments
and (bottom)
Two heavy chains
Tail
Head
A
B
Light chains
Actin filaments
Hinges
Myosin filament
Body
Cross-bridges
f the cross-
cross-bridges
to form a myosin filament. Also shown are thousands of myosin
Figure 6–5
A, Myosin molecule. B, Combination of many myosin molecules
and interaction between the heads o
bridges with adjacent actin filaments.

causes contraction to occur. Although the precise
active sites of the actin filament, and this, in some way,
by the calcium ions, the heads of the cross-bridges
Myosin Cross-Bridges—The “Walk-Along” Theory of Contrac-
is altered by calcium ions, producing a new condition
mechanism, it does emphasize that the normal relation
traction to proceed. Although this is a hypothetical
ers” the active sites of the actin, thus allowing these to
the groove between the two actin strands. This “uncov-
calcium ions, the troponin complex supposedly under-
ing: When calcium ions combine with troponin C, each
of this is not known, but one suggestion is the follow-
the actin filaments is itself inhibited. The mechanism
the presence of large amounts of calcium ions, the
This brings us to the role of the calcium ions. In
contraction. Before contraction can take place, the
tropomyosin complex. Consequently, the sites cannot
place. Therefore, it is believed that the active sites on
of the myosin molecules. Then, if the troponin-
ATP) binds instantly and strongly with the heads
Inhibition of the Actin Filament by the Troponin-Tropomyosin
Two Actin Filaments, and Calcium Ions
contraction process, as explained in the next section.
the tropomyosin to the actin. The strong affinity of the
for calcium ions. This complex is believed to attach
(troponin T) for tropomyosin, and a third (troponin C)
(troponin I) has a strong affinity for actin, another
controlling muscle contraction. One of the subunits
protein subunits, each of which plays a specific role in
These are actually complexes of three loosely bound
Troponin and Its Role in Muscle Contraction.
the active sites of the actin strands, so that attraction
resting state, the tropomyosin molecules lie on top of
spirally around the sides of the F-actin helix. In the
length of 40 nanometers. These molecules are wrapped
The actin filament also con-
Tropomyosin Molecules.
molecules, as shown in Figure 6–4.
the Z discs; the ends of the filaments protrude in both
Each actin filament is about 1 micrometer long. The
nanometers.
strands of the double helix are staggered, giving one
muscle contraction. The active sites on the two F-actin
ADP. It is believed that these ADP molecules are the
molecular weight of about 42,000. Attached to each
, each having a
G-actin molecules
myosin molecule.
two lighter-colored strands in Figure 6–6. The two
, represented by the
The backbone of the actin filament is a double-
The actin filament is also complex.
tion process.
ATP and to use the energy derived from the ATP’s
explained later, this property allows the head to cleave
ATPase enzyme.
ATPase Activity of the Myosin Head.
120 degrees. This ensures that the cross-bridges extend
Now, to complete the picture, the myosin filament
Membrane Physiology, Nerve, and Muscle
76
Unit II
itself is twisted so that each successive pair of cross-
bridges is axially displaced from the previous pair by
in all directions around the filament.
Another feature
of the myosin head that is essential for muscle con-
traction is that it functions as an
As
high-energy phosphate bond to energize the contrac-
Actin Filament.
It is composed of three protein components: actin,
tropomyosin, and troponin.
stranded F-actin protein molecule
strands are wound in a helix in the same manner as the
Each strand of the double F-actin helix is composed
of polymerized
one of the G-actin molecules is one molecule of
active sites on the actin filaments with which the cross-
bridges of the myosin filaments interact to cause
active site on the overall actin filament about every 2.7
bases of the actin filaments are inserted strongly into
directions to lie in the spaces between the myosin
tains another protein, tropomyosin. Each molecule of
tropomyosin has a molecular weight of 70,000 and a
cannot occur between the actin and myosin filaments
to cause contraction.
Attached
intermittently along the sides of the tropomyosin mol-
ecules are still other protein molecules called troponin.
troponin for calcium ions is believed to initiate the
Interaction of One Myosin Filament,
to Cause Contraction
Complex; Activation by Calcium Ions.
A pure actin filament
without the presence of the troponin-tropomyosin
complex (but in the presence of magnesium ions and
tropomyosin complex is added to the actin filament,
the binding between myosin and actin does not take
the normal actin filament of the relaxed muscle are
inhibited or physically covered by the troponin-
attach to the heads of the myosin filaments to cause
inhibitory effect of the troponin-tropomyosin complex
must itself be inhibited.
inhibitory effect of the troponin-tropomyosin on
molecule of which can bind strongly with up to four
goes a conformational change that in some way tugs
on the tropomyosin molecule and moves it deeper into
attract the myosin cross-bridge heads and cause con-
between the troponin-tropomyosin complex and actin
that leads to contraction.
Interaction Between the “Activated” Actin Filament and the
tion.
As soon as the actin filament becomes activated
from the myosin filaments become attracted to the
Troponin complex
F-actin
Tropomyosin
Active sites
troponin
tropomyosin molecule is a
grooves between the actin strands. Attached to one end of each
molecules that fit in the
tropomyosin
Figure 6–6
Actin filament, composed of two helical strands of F-actin mole-
cules and two strands of
complex that initiates
contraction.

yet reached the center of the myosin filament. With
point, the actin filament has already overlapped all
length decreases to about 2.2 micrometers. At this
filament begins to overlap the myosin filament, the
is zero. Then, as the sarcomere shortens and the actin
point, the tension developed by the activated muscle
myosin filament, with no actin-myosin overlap. At this
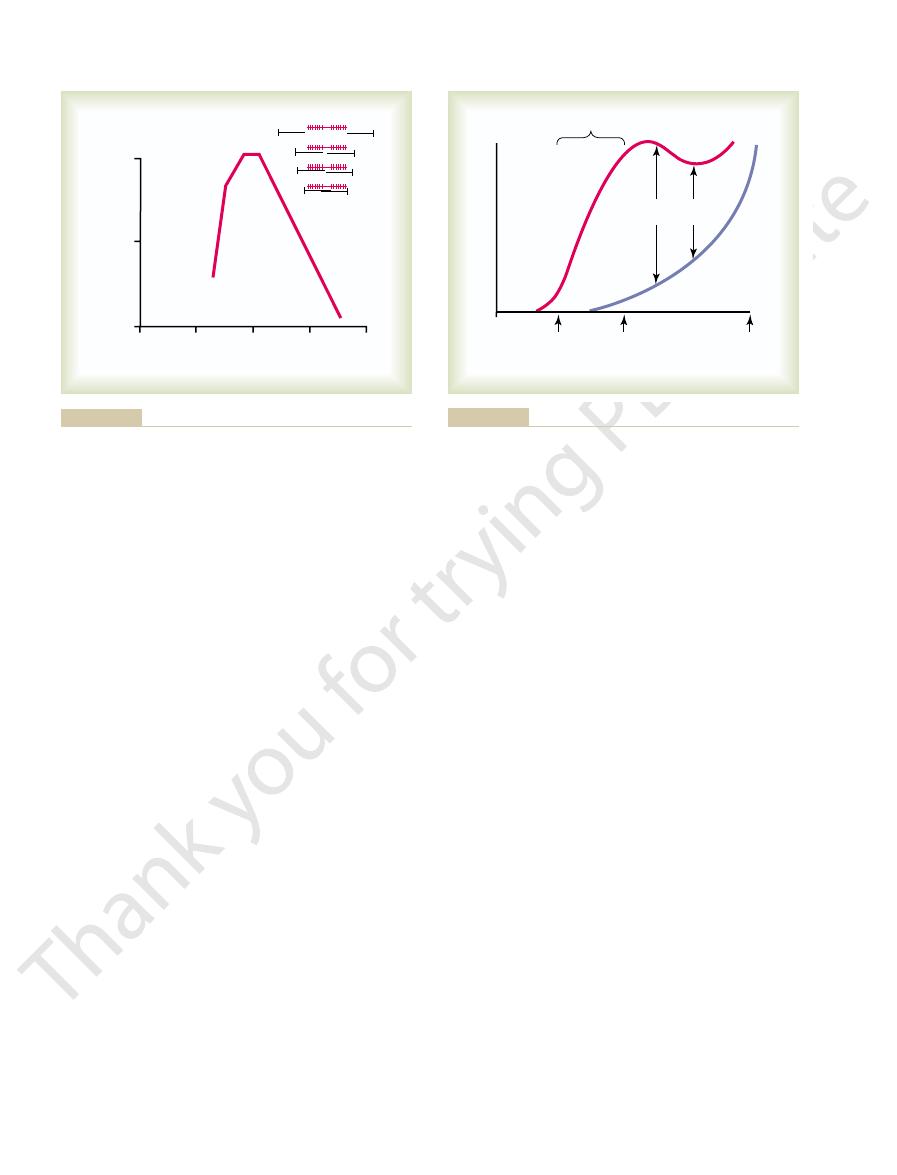
mere lengths. At point D on the diagram, the actin
right, shown in black, are different degrees of overlap
tension developed by a contracting muscle fiber. To the
Figure 6–8 shows the effect of sarcomere length and
Developed by the Contracting Muscle
Filament Overlap on Tension
Effect of Amount of Actin and Myosin
occur.
Thus, the process proceeds again and again until the
stroke.
active site on the actin filament, it becomes
derived from the cleaved ATP) binds with a new
6. When the cocked head (with its stored energy
power stroke cycle.
perpendicular condition, ready to begin the new
the energy again “cocks” the head back to its
cycle, leading to a new power stroke. That is,
new molecule of ATP is cleaved to begin the next
5. After the head has detached from the actin, the
This binding of new ATP causes detachment of
release of the ADP, a new molecule of ATP binds.
previously attached to the head. At the site of
release of the ADP and phosphate ion that were
4. Once the head of the cross-bridge tilts, this allows
was cleaved earlier.
that occurred in the head when the ATP molecule
“cocked” spring, by the conformational change
power stroke is the energy already stored, like a
actin filament. The energy that activates the
power stroke
This provides the
head to tilt toward the arm of the cross-bridge.
conformational change in the head, prompting the
3. The bond between the head of the cross-bridge
with these, as shown in Figure 6–7.
are uncovered, and the myosin heads then bind
with calcium ions, active sites on the actin filament
2. When the troponin-tropomyosin complex binds
ion, bound to the head. In this state, the
leaves the cleavage products, ADP plus phosphate
myosin head immediately cleaves the ATP but
bridges bind with ATP. The ATPase activity of the
1. Before contraction begins, the heads of the cross-
The
Fenn effect.
ATP that is cleaved, which is called the
performed by the muscle, the greater the amount of
contraction process; the greater the amount of work
amounts of ATP are cleaved to form ADP during the
tracts, work is performed and energy is required. Large
When a muscle con-
ATP as the Source of Energy for Contraction—Chemical Events
filament at any given time, the greater, theoretically,
in a continuous repeated cycle. Therefore, the greater
independently of all others, each attaching and pulling
along the actin filament, pulling the ends of two suc-
moves another step. Thus, the heads of the cross-
to cause a new power stroke, and the actin filament
down along the actin filament; then the head tilts again
position, it combines with a new active site farther
the head returns to its extended direction. In this
automatically breaks away from the active site. Next,
Then, immediately after tilting, the head
power stroke.
ment along with it. This tilt of the head is called the
cross-bridge. The new alignment of forces causes the
when a head attaches to an active site, this attach-
active sites of an actin filament. It is postulated that
mechanism for contraction. The figure shows the heads
Figure 6–7 demonstrates this postulated walk-along
“ratchet”
dence exists is the “walk-along” theory (or
theoretical, one hypothesis for which considerable evi-
Chapter 6
Contraction of Skeletal Muscle
77
manner by which this interaction between the cross-
bridges and the actin causes contraction is still partly
theory) of contraction.
of two cross-bridges attaching to and disengaging from
ment simultaneously causes profound changes in the
intramolecular forces between the head and arm of its
head to tilt toward the arm and to drag the actin fila-
bridges bend back and forth and step by step walk
cessive actin filaments toward the center of the myosin
filament.
Each one of the cross-bridges is believed to operate
the number of cross-bridges in contact with the actin
the force of contraction.
in the Motion of the Myosin Heads.
following sequence of events is believed to be the
means by which this occurs:
conformation of the head is such that it extends
perpendicularly toward the actin filament but is
not yet attached to the actin.
and the active site of the actin filament causes a
for pulling the
the head from the actin.
uncocked and once again provides a new power
actin filaments pull the Z membrane up against
the ends of the myosin filaments or until the load on
the muscle becomes too great for further pulling to
amount of myosin-actin filament overlap on the active
of the myosin and actin filaments at different sarco-
filament has pulled all the way out to the end of the
tension increases progressively until the sarcomere
the cross-bridges of the myosin filament but has not
Actin filament
Active sites
Myosin filament
Hinges
Power
stroke
Movement
“Walk-along” mechanism for contraction of the muscle.
Figure 6–7
In mathematical terms, work is defined by the
This means that
When a muscle contracts against a load, it performs
Work Output During Muscle
Energetics of Muscle
caused by muscle contraction. Therefore, the net force
This decreasing velocity of contraction with load is
results, despite activation of the muscle fiber.
the maximum force that the muscle can exert, the veloc-
6–10. That is, when the load has been increased to equal
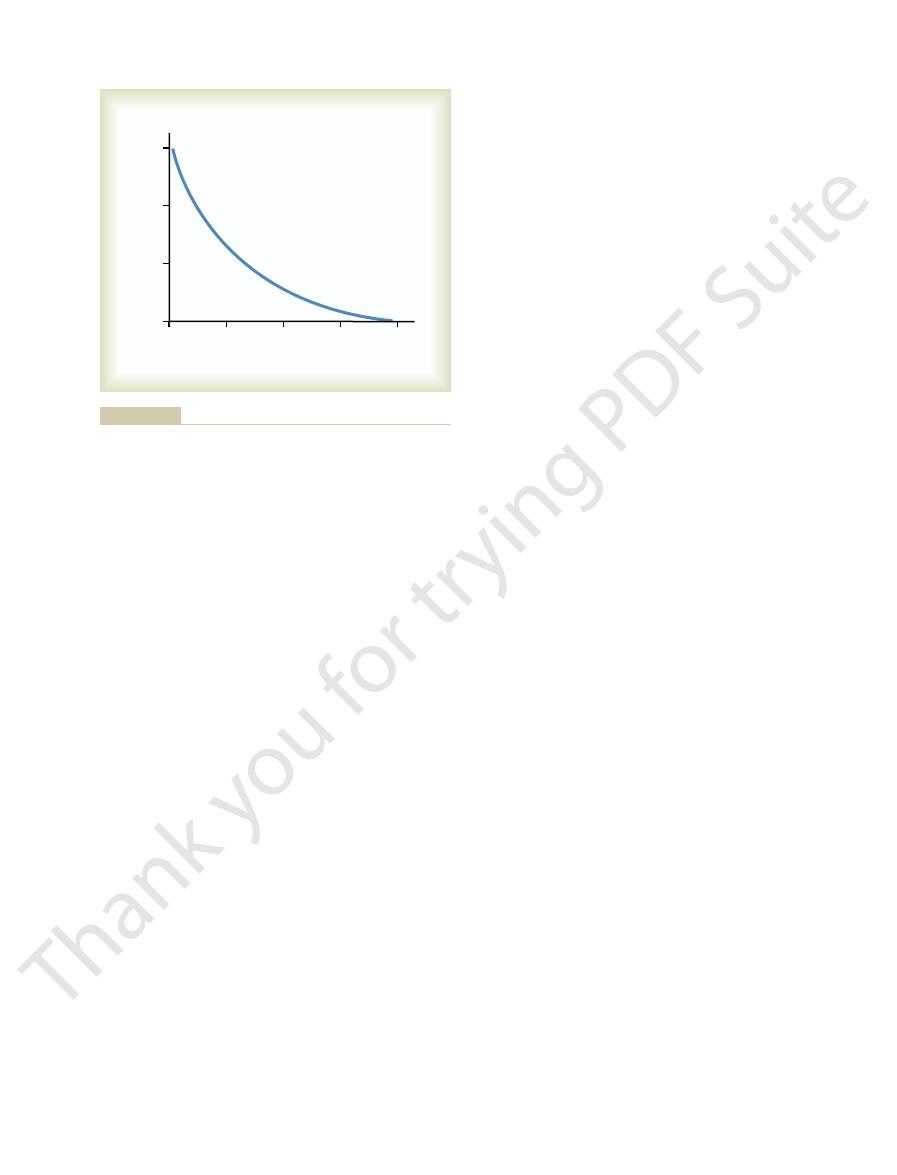
gressively less as the load increases, as shown in Figure
are applied, the velocity of contraction becomes pro-
in about 0.1 second for the average muscle. When loads
Relation of Velocity of Contraction
eters. This is demonstrated by the decreased length of
muscle is stretched beyond its normal length—that is,
contraction, called
However, the
of about 2 micrometers, it contracts upon activation
length, which is at a sarcomere length
Note in Figure 6–9 that when the muscle is at its
as noted in Figure 6–9.
individual muscle fiber, but it exhibits the same general
the same amount. Therefore, the curve has somewhat
amount of connective tissue in it; also, the sarcomeres
single muscle fiber. The whole muscle has a large
tension of the intact, whole muscle rather than of a
that in Figure 6–8, but the curve in Figure 6–9 depicts
The top curve of Figure 6–9 is similar to
of contraction approaches zero, but the entire muscle
are crumpled and, as shown in the figure, the strength
sarcomere lengths, the ends of the myosin filaments
aments. Then, as contraction proceeds to still shorter
contraction decreases rapidly. At this point, the two Z
about 1.65 micrometers, at point A, the strength of
addition to overlapping the myosin filaments. As the
of about 2 micrometers. At this point, the ends of the
tension until point B is reached, at a sarcomere length
further shortening, the sarcomere maintains full
Membrane Physiology, Nerve, and Muscle
78
Unit II
two actin filaments begin to overlap each other in
sarcomere length falls from 2 micrometers down to
discs of the sarcomere abut the ends of the myosin fil-
has now contracted to its shortest length.
Effect of Muscle Length on Force of Contraction in the Whole
Intact Muscle.
in different parts of the muscle do not always contract
different dimensions from those shown for the
form for the slope in the normal range of contraction,
normal resting
with the approximate maximum force of contraction.
increase in tension that occurs during
active tension, decreases as the
to a sarcomere length greater than about 2.2 microm-
the arrow in the figure at greater than normal muscle
length.
to Load
A skeletal muscle contracts extremely rapidly when it
contracts against no load—to a state of full contraction
ity of contraction becomes zero and no contraction
caused by the fact that a load on a contracting muscle
is a reverse force that opposes the contractile force
that is available to cause velocity of shortening is
correspondingly reduced.
Contraction
Contraction
work.
energy is transferred from the
muscle to the external load to lift an object to a greater
height or to overcome resistance to movement.
following equation:
3
4
2
1
0
100
50
0
Length of sarcomere (micrometers)
A
B C
D
C
B
A
D
Tension developed
(per cent)
vertebrate striated muscle fibers. J Physiol 171:28P, 1964.)
AM, Huxley AF, Julian FJ: The length-tension diagram of single
comere lengths from point A to point D. (Modified from Gordon
tive positions of the actin and myosin filaments at different sar-
2.0 to 2.2 micrometers in length. At the upper right are the rela-
showing maximum strength of contraction when the sarcomere is
Length-tension diagram for a single fully contracted sarcomere,
Figure 6–8
0
Length
Tension of muscle
1/2
normal
2 x
normal
Normal
Increase in tension
during contraction
Tension during
contraction
Normal range of contraction
Tension
before contraction
D
Relation of muscle length to tension in the muscle both before and
Figure 6–9
during muscle contraction.
ATP itself can later be converted into work.
even then, only 40 to 45 per cent of the energy in the
in foodstuffs is lost during the formation of ATP, and
this low efficiency is that about one half of the energy
cent, with the remainder becoming heat. The reason for
work, even under the best conditions, is less than 25 per
The percentage of the input energy to muscle (the
The efficiency of an
physiology.
In addition, the importance of the different mecha-
The detailed mechanisms of these energetic
energy can come from stored carbohydrates.
periods of 2 to 4 hours, as much as one half of the
greatest proportion of energy comes from fats, but for
and protein. For extremely long-term maximal muscle
foodstuffs that are consumed are carbohydrates, fats,
term contraction is derived from this source. The
of all energy used by the muscles for sustained, long-
lular foodstuffs to liberate ATP. More than 95 per cent
This means combining oxygen with the
oxidative
The third and final source of energy is
minute.
lar foodstuffs reacting with oxygen. However, so
times as rapid as ATP formation in response to cellu-
mation of ATP by the glycolytic process is about 2.5
from the blood is not available. Second, the rate of for-
to more than a minute, even when oxygen delivery
in the absence of oxygen, so that muscle contraction
twofold. First, the glycolytic reactions can occur even
The importance of this glycolysis mechanism is
phosphocreatine.
that is used to convert ADP to ATP; the ATP can
muscle cells. Rapid enzymatic breakdown of the glyco-
is “glycolysis” of
used to reconstitute both ATP and phosphocreatine,
The second important source of energy, which is
seconds.
the combined energy of both the stored ATP and
only about five times as great as the ATP. Therefore,
reconstitute the ATP. However, the total amount of
causes bonding of a new phosphate ion to ADP to
creatine is instantly cleaved, and its released energy
more fully in Chapters 67 and 72. Therefore, phospho-
energy than that of each ATP bond, as is discussed
bonds of ATP. The high-energy phosphate bond of
phosphocreatine,
tute the ATP is the substance
The first source of energy that is used to reconsti-
traction. There are several sources of the energy for
second, which allows the muscle to continue its con-
lated to form new ATP within another fraction of a
as described in Chapter 2, the ADP is rephosphory-
to the contracting machinery of the muscle fiber. Then,
ADP, which transfers energy from the ATP molecule
for only 1 to 2 seconds at most.The ATP is split to form
4 millimolar, is sufficient to maintain full contraction
The concentration of ATP in the muscle fiber, about
for propagation of muscle fiber action potentials.
ulum after the contraction is over, and (2) pumping
which the cross-bridges pull the actin filaments, but
on energy supplied by ATP. Most of this energy is
We have already seen that muscle contraction depends
tion, as described in the following sections.
the distance of movement against the load. The energy
in which W is the work output, L is the load, and D is
Chapter 6
Contraction of Skeletal Muscle
79
W
= L ¥ D
required to perform the work is derived from the
chemical reactions in the muscle cells during contrac-
Sources of Energy for Muscle
Contraction
required to actuate the walk-along mechanism by
small amounts are required for (1) pumping calcium
ions from the sarcoplasm into the sarcoplasmic retic-
sodium and potassium ions through the muscle fiber
membrane to maintain appropriate ionic environment
this rephosphorylation.
which
carries a high-energy phosphate bond similar to the
phosphocreatine has a slightly higher amount of free
phosphocreatine in the muscle fiber is also very little—
the phosphocreatine in the muscle is capable of
causing maximal muscle contraction for only 5 to 8
glycogen previously stored in the
gen to pyruvic acid and lactic acid liberates energy
then be used directly to energize additional muscle
contraction and also to re-form the stores of
can be sustained for many seconds and sometimes up
many end products of glycolysis accumulate in the
muscle cells that glycolysis also loses its capability to
sustain maximum muscle contraction after about 1
metabolism.
end products of glycolysis and with various other cel-
activity—over a period of many hours—by far the
processes are discussed in Chapters 67 through 72.
nisms of energy release during performance of
different sports is discussed in Chapter 84 on sports
Efficiency of Muscle Contraction.
engine or a motor is calculated as the percentage of
energy input that is converted into work instead of heat.
chemical energy in nutrients) that can be converted into
Velocity of contraction (cm
/sec)
2
3
4
0
1
30
20
10
0
Load-opposing contraction (kg)
with a cross section of 1 square centimeter and a length of 8
Figure 6–10
Relation of load to velocity of contraction in a skeletal muscle
centimeters.
Membrane Physiology, Nerve, and Muscle
80
Unit II
0
40
80
120
160
200
Milliseconds
Force of contraction
Duration of
depolarization
Ocular
muscle
Gastrocnemius
Soleus
energy by the glycolytic process. (4) Less extensive
release of calcium ions to initiate contraction. (3) Large
traction. (2) Extensive sarcoplasmic reticulum for rapid
Fast Fibers.
between these two types of fibers are as follows.
composed mainly of “slow” fibers. The differences
numbers of the slow variety. Conversely, the muscles
composed mainly of “fast” fibers with only small
these two extremes. The muscles that react rapidly are
muscle fibers, with still other fibers gradated between
slow
Chapter 84 on sports physiology, every muscle of the
Fast Versus Slow Muscle Fibers.
long-term support of the body against gravity.
running and jumping, and the soleus muscle is con-
specific objects to provide accuracy of vision. The gas-
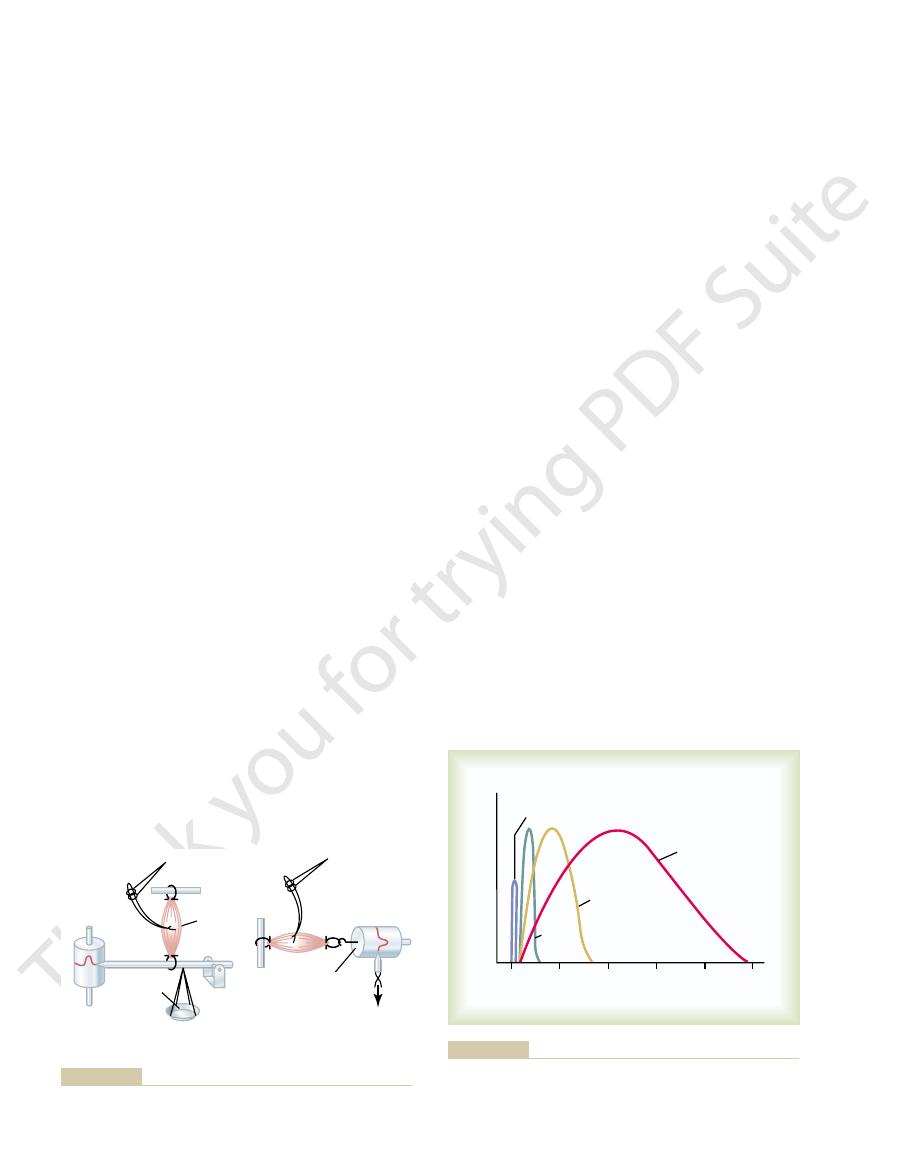
respective muscles. Ocular movements must be
tion of about 1/3 second. It is interesting that these dura-
soleus muscle, which has a duration of contrac-
a duration of contraction of about 1/15 second; and the
than 1/40 second; the gastrocnemius muscle, which has
of three types of skeletal muscle: an ocular muscle,
Figure 6–12 shows records of isometric contractions
contraction differ among muscles.
siderably from one muscle to another. Therefore, it is no
Finally, the energetics of muscle contraction vary con-
micrometers in diameter or as large as 80 micrometers.
stapedius. Further, the fibers may be as small as 10
quadriceps muscle, a half million times as large as the
a millimeter or so in diameter, up to the very large
middle ear, measuring only a few millimeters long and
The human body has many sizes of skeletal
Characteristics of Isometric Twitches Recorded from Different
functional characteristics of different muscle types.
in force of muscle contraction itself. Therefore, the iso-
However, the isometric system records strictly changes
the muscle contracts, as well as the inertia of the load.
muscle lifting a pan of weights. The characteristics of
this is illustrated on the left in the figure, showing a
tonic system, the muscle shortens against a fixed load;
length, as shown on the right in Figure 6–11. In the iso-
In the isometric system, the muscle contracts against
muscle contraction are shown in Figure 6–11.
the contraction. Systems for recording the two types of
Isometric Versus Isotonic Contraction.
stimulus through the muscle itself, giving rise to a single,
This can be
muscle twitches.
ciency is developed when the velocity of contraction is
efficiency of contraction. Ordinarily, maximum effi-
friction within the muscle itself, and this, too, reduces the
as zero. Conversely, if contraction is too rapid, large pro-
thereby decreasing the conversion efficiency to as little
traction, even though little or no work is performed,
contracts slowly or without any movement, small
muscle contracts at a moderate velocity. If the muscle
Maximum efficiency can be realized only when the
Duration of isometric contractions for different types of mammalian
Figure 6–12
skeletal muscles, showing a latent period between the action
potential (depolarization) and muscle contraction.
amounts of maintenance heat are released during con-
portions of the energy are used to overcome viscous
about 30 per cent of maximum.
Characteristics of Whole
Muscle Contraction
Many features of muscle contraction can be demon-
strated by eliciting single
accomplished by instantaneous electrical excitation of
the nerve to a muscle or by passing a short electrical
sudden contraction lasting for a fraction of a second.
Muscle contraction is
said to be isometric when the muscle does not shorten
during contraction and isotonic when it does shorten but
the tension on the muscle remains constant throughout
a force transducer without decreasing the muscle
isotonic contraction depend on the load against which
metric system is most often used when comparing the
Muscles.
muscles—from the very small stapedius muscle in the
wonder that the mechanical characteristics of muscle
which has a duration of isometric contraction of less
tions of contraction are adapted to the functions of the
extremely rapid to maintain fixation of the eyes on
trocnemius muscle must contract moderately rapidly
to provide sufficient velocity of limb movement for
cerned principally with slow contraction for continual,
As we discuss more fully in
body is composed of a mixture of so-called fast and
that respond slowly but with prolonged contraction are
(1) Large fibers for great strength of con-
amounts of glycolytic enzymes for rapid release of
blood supply because oxidative metabolism is of
To electronic
ISOTONIC SYSTEM
ISOMETRIC SYSTEM
Weights
Kymograph
Muscle
Stimulating
electrodes
Stimulating
electrodes
Electronic force
transducer
recorder
Isotonic and isometric systems for recording muscle contractions.
Figure 6–11
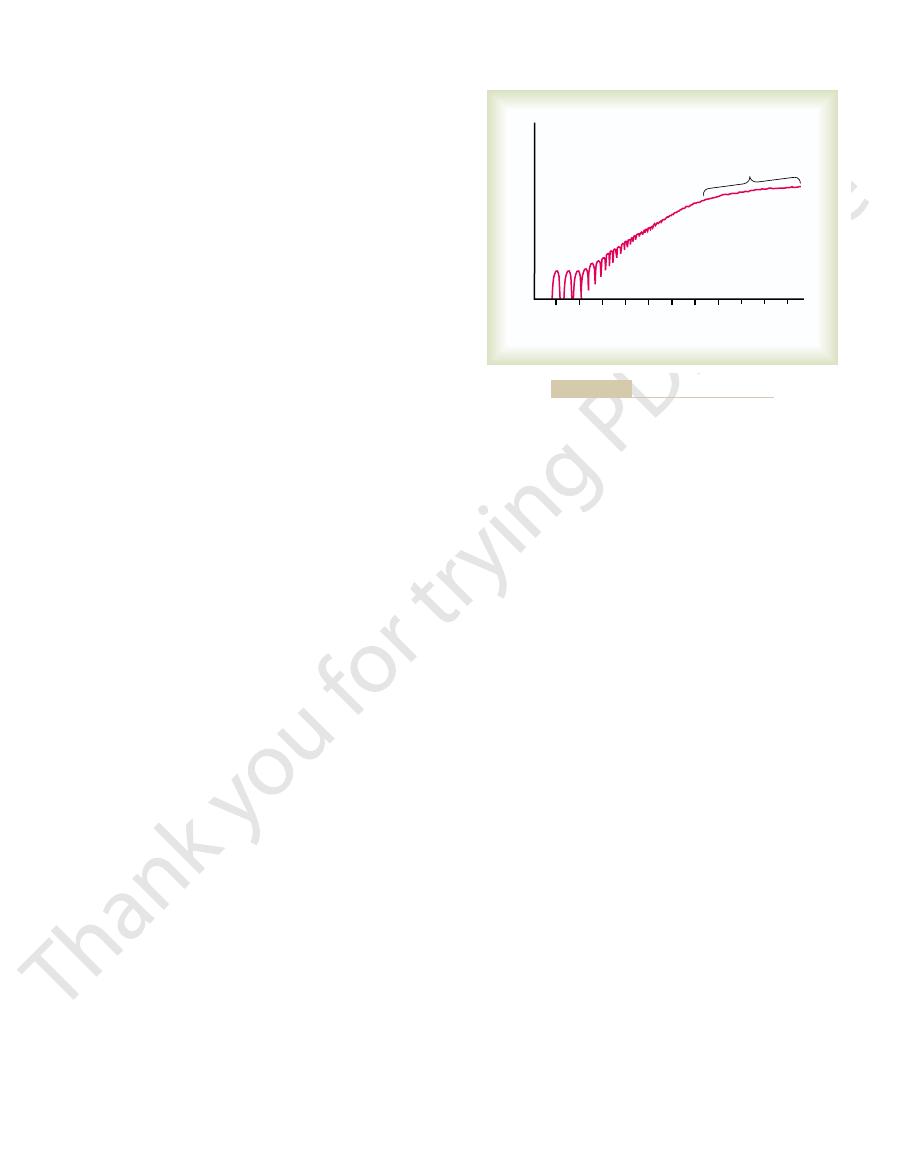
tract after a long period of rest, its initial strength of
When a muscle begins to con-
Staircase Effect (Treppe).
bone.
of tension may be applied to the patellar tendon. Thus,
16 square inches of muscle belly, as much as 800 pounds
inch. Because a quadriceps muscle can have as much as
square centimeter of muscle, or 50 pounds per square
The maximum strength
any relaxation between the action potentials.
muscle sarcoplasm, even between action potentials, so
further effect in increasing contractile force. This occurs
tional increase in frequency beyond that point has no
of contraction reaches its maximum, so that any addi-
At a slightly higher frequency, the strength
and continuous, as shown in the figure. This is called
become so rapid that they fuse together, and the whole
critical level, the successive contractions eventually
increasing frequency. When the frequency reaches a
contraction is added partially to the first, so that the
before the preceding one is over. As a result, the second
quency of stimulation. Then, as the frequency increases,
tetanization. To the left are displayed individual twitch
shows the principles of frequency summation and
Figure 6–13
Frequency Summation and Tetanization.
of nerve signals.
alternates among motor units one after the other, thus
chronously by the spinal cord, so that contraction
the larger ones, so they naturally are excited first.
driven by small motor nerve fibers, and the small
when large amounts of force are required. The cause of
steps, whereas the steps become progressively greater
It is important, because it allows the gradations of
ple.
size princi-
force of the smallest units. This is called the
units begin to be excited as well, with the largest motor
strength of the signal increases, larger and larger motor
preference to the larger motor units. Then, as the
system sends a weak signal to contract a muscle, the
When the central nervous
and (2) by increasing the frequency of contraction,
simultaneously, which is called
muscle contraction. Summation occurs in two ways: (1)
ual segments.
units in microbundles of 3 to 15 fibers. This interdigita-
The muscle fibers in each motor unit are not all
figure for all the muscles of the body is questionable, but
hundred muscle fibers in a motor unit. An average
control, such as the soleus muscle, may have several
Conversely, large muscles that do not require fine
muscle fibers (for instance, as few as two or three muscle
general, small muscles that react rapidly and whose
ing on the type of muscle. All the muscle fibers inner-
innervates multiple muscle fibers, the number depend-
white muscle.
red muscle,
to the mitochondria. The myoglobin gives the slow
until needed; this also greatly speeds oxygen transport
cells. Myoglobin combines with oxygen and stores it
Fibers contain large amounts of myoglobin, an iron-
also to support high levels of oxidative metabolism. (5)
oxygen. (4) Greatly increased numbers of mitochondria,
smaller nerve fibers. (3) More extensive blood vessel
(1) Smaller fibers. (2) Also innervated by
because oxidative metabolism is secondary.
secondary importance. (5) Fewer mitochondria, also
Chapter 6
Contraction of Skeletal Muscle
81
Slow Fibers.
system and capillaries to supply extra amounts of
containing protein similar to hemoglobin in red blood
muscle a reddish appearance and the name
whereas a deficit of red myoglobin in fast muscle gives
it the name
Mechanics of Skeletal Muscle
Contraction
Motor Unit.
Each motoneuron that leaves the spinal cord
vated by a single nerve fiber are called a motor unit. In
control must be exact have more nerve fibers for fewer
fibers per motor unit in some of the laryngeal muscles).
a good guess would be about 80 to 100 muscle fibers to
a motor unit.
bunched together in the muscle but overlap other motor
tion allows the separate motor units to contract in
support of one another rather than entirely as individ-
Muscle Contractions of Different Force—Force Summation.
Summation means the adding together of individual
twitch contractions to increase the intensity of overall
by increasing the number of motor units contracting
multiple fiber summa-
tion,
which is called frequency summation and can lead to
tetanization.
Multiple Fiber Summation.
smaller motor units of the muscle may be stimulated in
units often having as much as 50 times the contractile
muscle force during weak contraction to occur in small
this size principle is that the smaller motor units are
motoneurons in the spinal cord are more excitable than
Another important feature of multiple fiber summa-
tion is that the different motor units are driven asyn-
providing smooth contraction even at low frequencies
contractions occurring one after another at low fre-
there comes a point where each new contraction occurs
total strength of contraction rises progressively with
muscle contraction appears to be completely smooth
tetanization.
because enough calcium ions are maintained in the
that full contractile state is sustained without allowing
Maximum Strength of Contraction.
of tetanic contraction of a muscle operating at a normal
muscle length averages between 3 and 4 kilograms per
one can readily understand how it is possible for
muscles to pull their tendons out of their insertions in
Changes in Muscle Strength at the Onset of Contraction—The
5
10 15 20 25 30 35 40 45 50 55
Rate of stimulation (times per second)
Strength of muscle contraction
Tetanization
Frequency summation and tetanization.
Figure 6–13

Remodeling of Muscle
positioning process.
We learn in Chapter 54 that the motor nervous
system directs the positioning of the arm or leg.
of the agonist and antagonist muscles, the nervous
Thus, by varying the ratios of the degree of activation
other. At this point, movement of the arm or leg stops.
muscle decreases, whereas the strength of the shorter
toward its midposition, the strength of the longer
muscle on the opposite side. As an arm or leg moves
Therefore, the elongated muscle on one side of a joint
demonstrated in Figure 6–9, showing maximum strength
with more force than a shortened muscle, which was
equally. Remember that an elongated muscle contracts
this, agonist and antagonist muscles are excited about
is to be placed in a midrange position. To achieve
muscles. For instance, let us assume that an arm or a leg
as an arm or a leg, is determined by the relative degrees
The position of each separate part of the body, such
muscles, and it is controlled by the motor control centers
antagonist muscles on opposite sides of joints. This
Virtually all body movements are
physioanatomy.
systems, and their movements is called
tances. The study of different types of muscles, lever
are long and contract a long distance, and some are
reason, there are many different types of muscle; some
of which need large distances of movement. For this
body, some of which need great strength and others
lever. Many types of movement are required in the
the length of the lever arm, and (4) the position of the
tion, (2) its distance from the fulcrum of the lever, (3)
In short, an analysis of the lever systems of the body
also much less than 43 pounds.
much less than 2 inches anterior to the fulcrum, and the
arm is fully extended, the attachment of the biceps is
pounds of muscle force, or about 43 pounds. When the
inches. Therefore, the amount of lifting power of the
is about 2 inches anterior to the fulcrum at the elbow,
with the upper arm, the tendon attachment of the biceps
about 300 pounds. When the forearm is at right angles
inches, the maximum force of contraction would be
muscle to lift the forearm. If we assume that a large
bones in turn form various types of lever systems. Figure
tension to their points of insertion into bones, and the
Lever Systems of the Body.
supply, especially loss of oxygen.
contraction. Interruption of blood flow through a con-
longed muscle activity, thus further diminishing muscle
7, can diminish at least a small amount after intense pro-
neuromuscular junction, which is discussed in Chapter
same work output. However, experiments have also
depletion of muscle glycogen. Therefore, fatigue results
muscle leads to the well-known state of muscle fatigue.
itself. Both of these are discussed in relation to muscle
spinal cord. These, in turn, are controlled partly by
stimulate the fibers, skeletal muscle tone results entirely
muscle tone.
certain amount of tautness usually remains. This is
Even when muscles are at rest, a
Skeletal Muscle Tone.
immediately.
increasing calcium ions in the cytosol because of the
are not known, it is believed to be caused primarily by
treppe.
traction increases to a plateau, a phenomenon called the
to 50 muscle twitches later. That is, the strength of con-
Membrane Physiology, Nerve, and Muscle
82
Unit II
contraction may be as little as one half its strength 10
staircase effect, or
Although all the possible causes of the staircase effect
release of more and more ions from the sarcoplasmic
reticulum with each successive muscle action potential
and failure of the sarcoplasm to recapture the ions
called
Because normal skeletal muscle
fibers do not contract without an action potential to
from a low rate of nerve impulses coming from the
signals transmitted from the brain to the appropriate
spinal cord anterior motoneurons and partly by signals
that originate in muscle spindles located in the muscle
spindle and spinal cord function in Chapter 54.
Muscle Fatigue.
Prolonged and strong contraction of a
Studies in athletes have shown that muscle fatigue
increases in almost direct proportion to the rate of
mainly from inability of the contractile and metabolic
processes of the muscle fibers to continue supplying the
shown that transmission of the nerve signal through the
tracting muscle leads to almost complete muscle fatigue
within 1 or 2 minutes because of the loss of nutrient
Muscles operate by applying
6–14 shows the lever system activated by the biceps
biceps muscle has a cross-sectional area of 6 square
and the total length of the forearm lever is about 14
biceps at the hand would be only one seventh of the 300
force with which the hand can be brought forward is
depends on knowledge of (1) the point of muscle inser-
short but have large cross-sectional areas and can
provide extreme strength of contraction over short dis-
kinesiology
and is an important scientific component of human
“Positioning” of a Body Part by Contraction of Agonist and Antag-
onist Muscles on Opposite Sides of a Joint—“Coactivation”
of Antagonist Muscles.
caused by simultaneous contraction of agonist and
is called coactivation of the agonist and antagonist
of the brain and spinal cord.
of contraction of the agonist and antagonist sets of
of contraction at full functional muscle length and
almost no strength of contraction at half normal length.
can contract with far greater force than the shorter
muscle increases until the two strengths equal each
system has additional important mechanisms to com-
pensate for different muscle loads when directing this
to Match Function
All the muscles of the body are continually being
remodeled to match the functions that are required of
Figure 6–14
Lever system activated by the biceps muscle.

traction in striated muscle. Physiol Rev 80:853, 2000.
Gordon AM, Homsher E, Regnier M: Regulation of con-
hypertrophy and atrophy. Nat Cell Biol 5:87, 2003.
Glass DJ: Signalling pathways that mediate skeletal muscle
Trends Mol Med 8:344, 2003.
Glass DJ: Molecular mechanisms modulating muscle mass.
tractility. Physiol Rev 83:1269, 2003.
Clausen T: Na
iology. Adv Physiol Educ 27:171, 2003.
Brooks SV: Current topics for teaching skeletal muscle phys-
plasticity, and disease. Physiol Rev 80:1215, 2000.
in skeletal muscle: its crucial role for muscle function,
Berchtold MW, Brinkmeier H, Muntener M: Calcium ion
rapidly at higher temperatures.
released from lysosomes. All these events occur more
proteins deteriorate about 15 to 25 hours later, which
process. The muscles remain in rigor until the muscle
ATP, which is required to cause separation of the cross-
action potentials. This rigidity results from loss of all the
the muscles contract and become rigid, even without
called “rigor mortis”; that is,
Several hours after death, all the muscles of the body go
for each motoneuron coming from the spinal cord. This
macromotor units,
of the paralyzed muscle fibers. This causes large motor
occurs in poliomyelitis, the remaining nerve fibers
nerve fibers to a muscle are destroyed, as commonly
When some but not all
stretched during the atrophying process.
contractures. This is achieved by daily stretching of the
Therefore, one of the most important problems
tracture.
tinue shortening for many months, which is called
during denervation atrophy also has a tendency to con-
The fibrous tissue that replaces the muscle fibers
regrow.
fatty tissue. The fibers that do remain are composed of
In the final stage of denervation atrophy, most of the
after 1 to 2 years.
becomes less and less, with no further return of function
that time onward, the capability of functional return
of function can occur in as little as 3 months, but from
supply to the muscle grows back rapidly, full return
appear in the muscle fibers themselves. If the nerve
about 2 months, degenerative changes also begin to
Therefore, atrophy begins almost immediately. After
that are required to maintain normal muscle size.
nerve supply, it no longer receives the contractile signals
When a muscle loses its
Effects of Muscle Denervation.
anism is linear splitting of previously enlarged fibers.
When it does occur, the mech-
hypertrophy process. This increase in fiber number is
by a few percentage points), in addition to the fiber
extreme muscle force generation, the actual number of
ends of the muscle fibers can actually disappear. It is by
ened to less than its normal length, sarcomeres at the
Conversely, when a muscle continually remains short-
hypertrophy.
muscle, illustrating the rapidity of this type of
to the tendons. In fact, new sarcomeres can be added
added at the ends of the muscle fibers, where they attach
than normal length. This causes new sarcomeres to be
occurs.
than the rate of replacement. Therefore, muscle atrophy
When a muscle remains unused for many weeks, the
is especially true of the enzymes for glycolysis, allowing
enzyme systems that provide energy also increase. This
Along with the increasing size of myofibrils, the
muscle to form new myofibrils, but how important this
50 per cent. In turn, some of the myofibrils themselves
filaments in the myofibrils, often increasing as much as
greater when hypertrophy is developing, leading also to
hypertrophy is not known. It is known, however, that the
The manner in which forceful contraction leads to
significant hypertrophy within 6 to 10 weeks.
muscle is loaded during the contractile process. Only a
fiber hypertrophy.
ual muscle fibers; this is called simply
each muscle fiber, causing enlargement of the individ-
Virtually all muscle hypertrophy results from an
atrophy.
When it decreases, the process is called
trophy.
mass of a muscle increases, this is called
When the total
Muscle Hypertrophy and Muscle Atrophy.
little as 2 weeks.
some smaller, more active muscles can be replaced in as
quite rapid, within a few weeks. Indeed, experiments in
altered at least slightly. This remodeling process is often
plies are altered, and even the types of muscle fibers are
altered, their strengths are altered, their vascular sup-
them. Their diameters are altered, their lengths are
Chapter 6
Contraction of Skeletal Muscle
83
animals have shown that muscle contractile proteins in
muscle hyper-
muscle
increase in the number of actin and myosin filaments in
Hypertrophy occurs to a much greater extent when the
few strong contractions each day are required to cause
rate of synthesis of muscle contractile proteins is far
progressively greater numbers of both actin and myosin
have been observed to split within hypertrophying
is in usual muscle hypertrophy is still unknown.
rapid supply of energy during short-term forceful
muscle contraction.
rate of decay of the contractile proteins is more rapid
Adjustment of Muscle Length.
Another type of hyper-
trophy occurs when muscles are stretched to greater
as rapidly as several per minute in newly developing
these processes that muscles are continually remodeled
to have the appropriate length for proper muscle
contraction.
Hyperplasia of Muscle Fibers.
Under rare conditions of
muscle fibers has been observed to increase (but only
called fiber hyperplasia.
muscle fibers are destroyed and replaced by fibrous and
a long cell membrane with a lineup of muscle cell nuclei
but with few or no contractile properties and little or no
capability of regenerating myofibrils if a nerve does
con-
in the practice of physical therapy is to keep atrophying
muscles from developing debilitating and disfiguring
muscles or use of appliances that keep the muscles
Recovery of Muscle Contraction in Poliomyelitis: Devel-
opment of Macromotor Units.
branch off to form new axons that then innervate many
units called
which can contain as
many as five times the normal number of muscle fibers
decreases the fineness of control one has over the
muscles but does allow the muscles to regain varying
degrees of strength.
Rigor Mortis
into a state of contracture
bridges from the actin filaments during the relaxation
presumably results from autolysis caused by enzymes
References
+
-K
+
pump regulation and skeletal muscle con-

binding myosins. Biophys Chem 59:357, 1996.
Szent-Gyorgyi AG: Regulation of contraction by calcium
tal muscle. Physiol Rev 81:209, 2001.
Stamler JS, Meissner G: Physiology of nitric oxide in skele-
90:1158, 2001.
contraction in skeletal and cardiac muscle. J Appl Physiol
Sieck GC, Regnier M: Plasticity and energetic demands of
Malden, MA: Blackwell Science, 1998.
Matthews GG: Cellular Physiology of Nerve and Muscle.
skeletal muscle performance. News Physiol Sci 18:222,
MacIntosh BR: Role of calcium sensitivity modulation in
84:649, 2004.
and skeletal muscle to mechanical loading. Physiol Rev
Kjær M: Role of extracellular matrix in adaptation of tendon
channelopathies. J Neurol 249:1493, 2002.
Jurkat-Rott K, Lerche H, Lehmann-Horn F: Skeletal muscle
nisms. Annu Rev Physiol 58:1, 1996.
Huxley HE: A personal view of muscle and motility mecha-
passive shortening of muscle. Nature (Lond) 193:280,
Huxley AF, Gordon AM: Striation patterns in active and
rolls.” News Physiol Sci 16:49, 2001.
muscle contractile activation: tropomyosin “rocks and
Gordon AM, Regnier M, Homsher E: Skeletal and cardiac
Membrane Physiology, Nerve, and Muscle
84
Unit II
1962.
2003.
