
means random molecular movement of substances molecule by molecule, either
Although there are many variations of these basic mechanisms, diffusion
directly through the lipid bilayer or through the proteins, occurs by one of two
Transport through the cell membrane, either
“Diffusion” Versus “Active Transport.”
of molecules or ions that are allowed to cross the membrane.
the interstices of the protein to the other side of the membrane. Both the
bind with molecules or ions that are to be transported; confor-
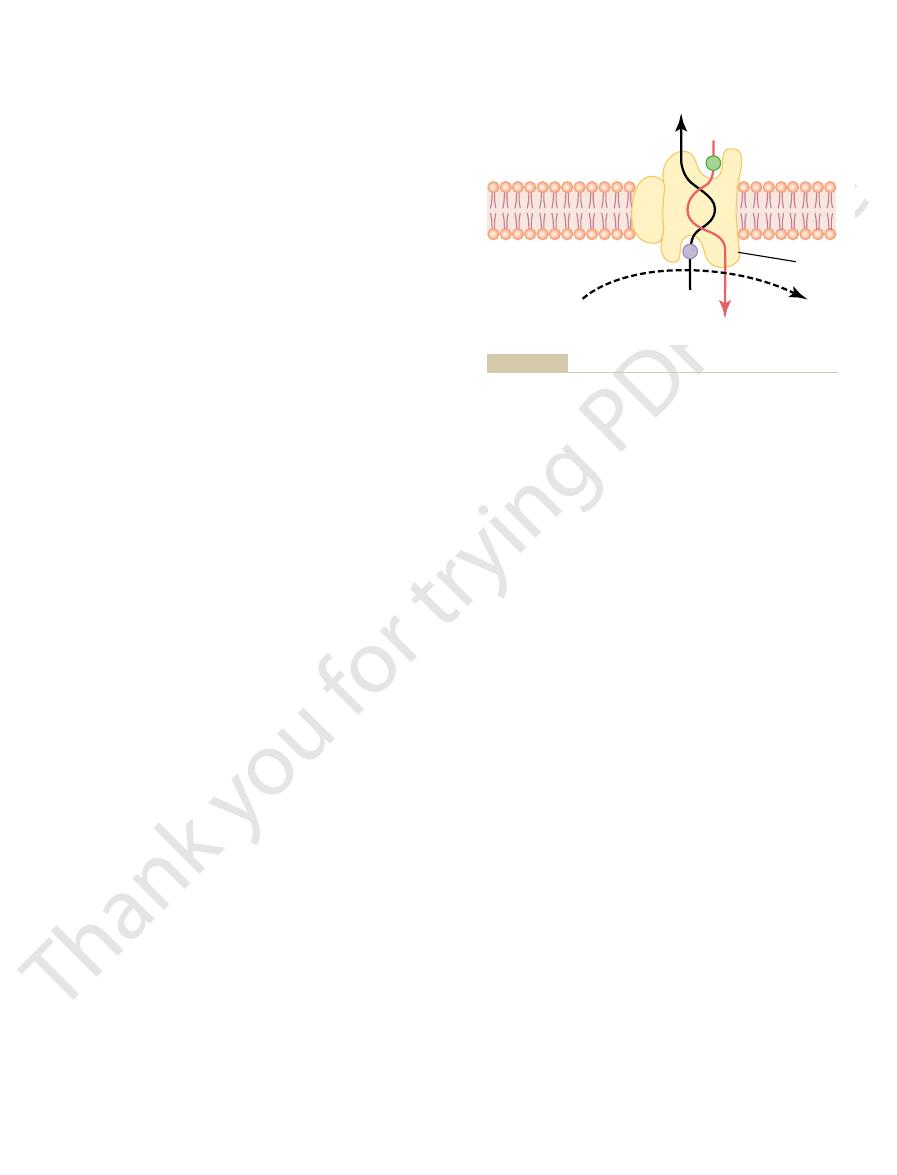
carrier proteins,
Others, called
channel proteins.
selected ions or molecules; these are called
Different proteins function differently. Some have watery spaces all
proteins.
brane. Most of these penetrating proteins, therefore, can function as
the lipid bilayer, constituting an alternative pathway through the cell mem-
transporting substances. Their molecular structures interrupt the continuity of
The protein molecules in the membrane have entirely different properties for
as described later.
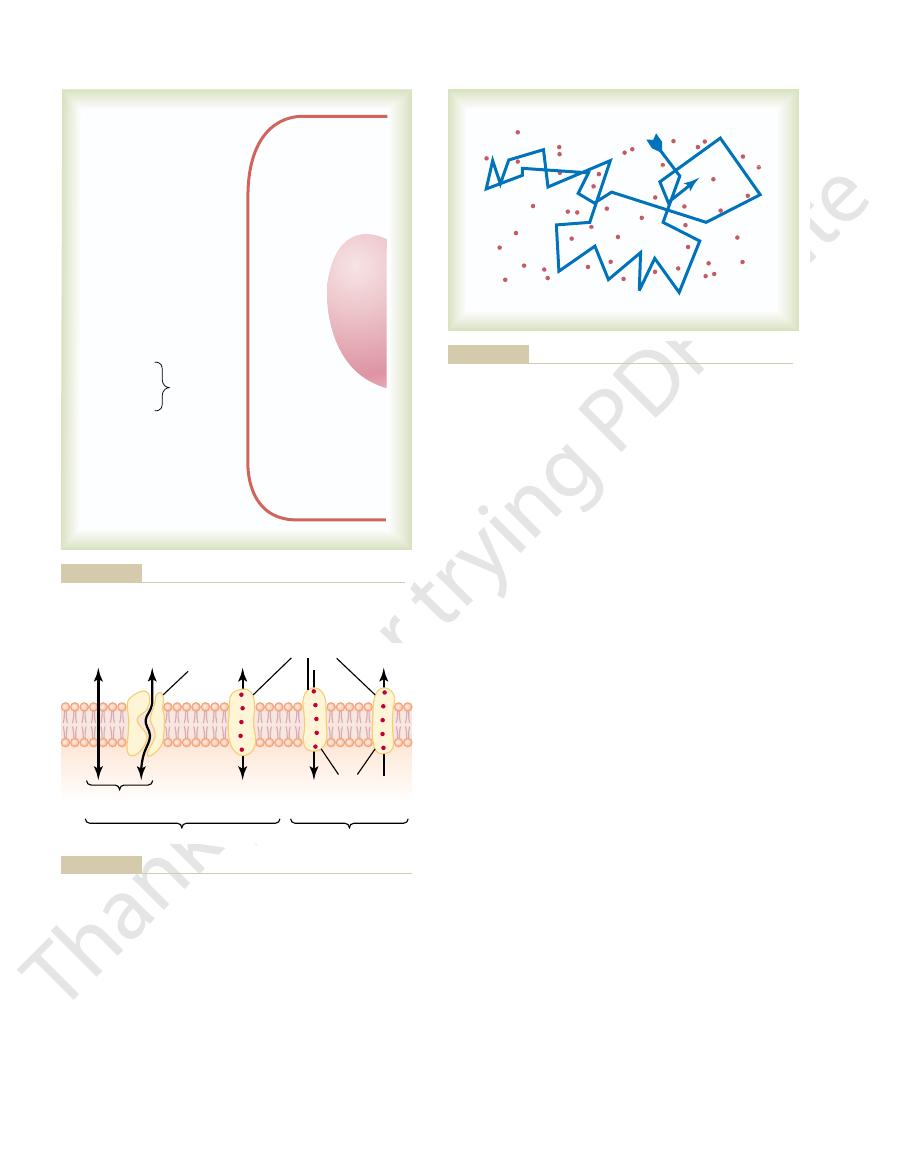
through the lipid substance itself; this is true mainly of lipid-soluble substances,
most arrow, a few substances can penetrate this lipid bilayer, diffusing directly
lular fluid compartments. However, as demonstrated in Figure 4–2 by the left-
molecules and water-soluble substances between the extracellular and intracel-
cellular fluid. Therefore, it constitutes a barrier against movement of water
The lipid bilayer is not miscible with either the extracellular fluid or the intra-
membrane, as shown in Figure 4–2.
protein molecules in the lipid, many of which penetrate all the way through the
lipid bilayer,
is discussed in Chapter 2 and illustrated in Figures 2–3 and 4–2. This membrane
The structure of the membrane covering the outside of every cell of the body
and Cell Membrane Transport Proteins
mechanisms of the cell membranes.
differences are extremely important to the life of the cell. The purpose of this
cellular fluid are considerably greater than those in the extracellular fluid. These
tains very little. But the concentrations of phosphates and proteins in the intra-
of chloride ions, whereas the intracellular fluid con-
Also, the extracellular fluid contains a large amount
Figure 4–1 gives the approximate concentrations of
Transport of Substances Through
C
H
A
P
T
E
R
4
45
the Cell Membrane
important electrolytes and other substances in the
extracellular fluid and intracellular fluid. Note that
the extracellular fluid contains a large amount of
sodium but only a small amount of potassium.
Exactly the opposite is true of the intracellular fluid.
chapter is to explain how the differences are brought about by the transport
The Lipid Barrier of the Cell Membrane,
consists almost entirely of a
but it also contains large numbers of
transport
the way through the molecule and allow free movement of water as well as
mational changes in the protein molecules then move the substances through
channel proteins and the carrier proteins are usually highly selective in the types
basic processes: diffusion or active transport.
through intermolecular spaces in the membrane or in combination with a carrier
The rate of diffusion is determined by the amount of
interaction with carrier proteins in the membrane.
large size.
similar manner, except that the colloids diffuse far
Ions diffuse in the same manner as whole molecules,
times each second. This continual movement of mole-
another, and so forth, randomly bouncing thousands of
molecules first in one direction, then another, then
kinetic energy. Thus, as shown in Figure 4–3, a single
while molecule A slows down, losing some of its
sequently, molecule B gains kinetic energy of motion,
energy of motion of molecule A to molecule B. Con-
molecule A repel molecule B, transferring some of the
cule, B, the electrostatic and other nuclear forces of
a moving molecule, A, approaches a stationary mole-
condition except at absolute zero temperature. When
“heat”—the greater the motion, the higher the tem-
way. Motion of these particles is what physicists call
stant motion, each particle moving its own separate
water molecules and dissolved substances, are in con-
All molecules and ions in the body fluids, including
Diffusion
processes.
energy. Following is a more detailed explanation of
state to a high-concentration state. This movement
an energy gradient, such as from a low-concentration
By contrast, active transport means movement of
of the normal kinetic motion of matter.
protein. The energy that causes diffusion is the energy
Membrane Physiology, Nerve, and Muscle
46
Unit II
ions or other substances across the membrane in com-
bination with a carrier protein in such a way that the
carrier protein causes the substance to move against
requires an additional source of energy besides kinetic
the basic physics and physical chemistry of these two
perature—and the motion never ceases under any
molecule in a solution bounces among the other
cules among one another in liquids or in gases is called
diffusion.
and even suspended colloid particles diffuse in a
less rapidly than molecular substances because of their
Diffusion Through the Cell Membrane
Diffusion through the cell membrane is divided into
two subtypes called simple diffusion and facilitated
diffusion. Simple diffusion means that kinetic move-
ment of molecules or ions occurs through a membrane
opening or through intermolecular spaces without any
(40 mEq/ L)
(5 mEq/ L)
0.5 g/dl-------------- 2 to 95 g/dl
2 mEq/L
75 mEq/ L
10 mEq/ L
4 mEq/ L
58 mEq/ L
0.0001 mEq/ L
140 mEq/ L
10 mEq/ L
1 mEq/L -------------
4 mEq/ L -------------
28 mEq/ L -----------
103 mEq/ L ---------
1.2 mEq/ L ----------
2.4 mEq/ L ----------
4 mEq/ L ------------
142 mEq/ L ---------
Na
+
---------------
K
+
-----------------
Ca
++
--------------
Mg
++
--------------
Cl
–
----------------
HCO
3
–
-----------
Phosphates-----
SO
4
– -------------
Glucose ---------
Amino acids ----
90 mg/dl ------------
30 mg/dl ------------
0 to 20 mg/dl
200 mg/dl ?
Cholesterol
Phospholipids
Neutral fat
PO
2
---------------
PCO
2
-------------
pH -----------------
Proteins ----------
35 mm Hg ---------
46 mm Hg ---------
7.4 -------------------
2 g/dl ----------------
20 mm Hg ?
50 mm Hg ?
7.0
16 g/dl
EXTRACELLULAR
FLUID
INTRACELLULAR
FLUID
Figure 4–1
Chemical compositions of extracellular and intracellular fluids.
Diffusion
diffusion
diffusion
Channel
protein
Simple
Facilitated
Energy
Active transport
Carrier proteins
mechanisms of transport.
Transport pathways through the cell membrane, and the basic
Figure 4–2
Diffusion of a fluid molecule during a thousandth of a second.
Figure 4–3
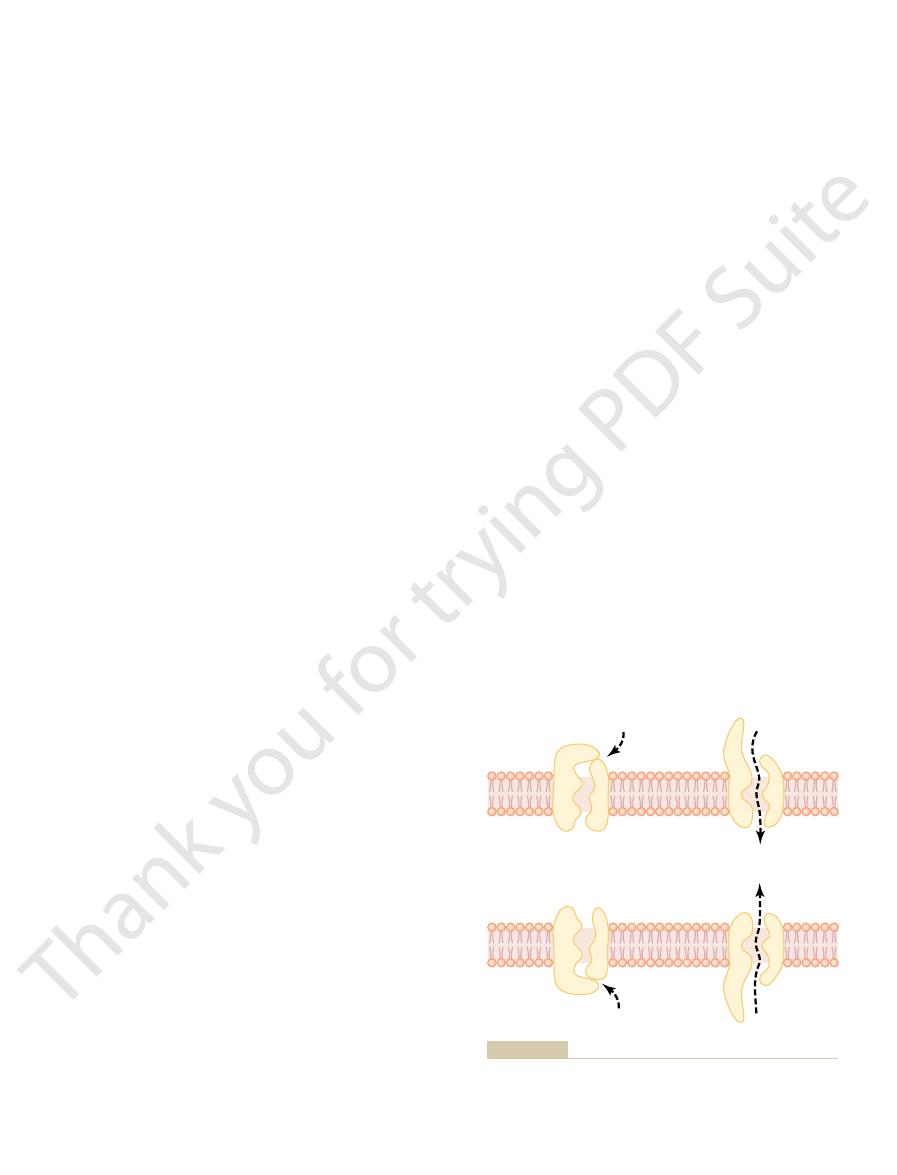
tive force is pulling ions into the channels, and the
bonds are different. Therefore, no strong attrac-
they are not negatively charged,
the sodium channels, only 0.3 by 0.3 nanometer, but
of Figure 4–4. These channels are slightly smaller than
tive for potassium transport, shown in the lower panel
Conversely, another set of protein channels is selec-
selective for passage of sodium ions.
of diffusion. Thus, the sodium channel is specifically
molecules. Once in the channel, the sodium ions
sodium ions into these channels, actually pulling
These strong negative charges can pull small
the channel proteins in the top panel of Figure 4–4.
tively charged,
0.3 by 0.5 nanometer in diameter, but more important,
sodium channel,
protein channels, the so-called
To give an example, one of the most important of the
charges and chemical bonds along its inside surfaces.
its diameter, its shape, and the nature of the electrical
from the characteristics of the channel itself, such as
one or more specific ions or molecules. This results
gates.
stances, and (2) many of the channels can be opened
are distinguished by two important characteristics: (1)
of the membrane to the other. The protein channels
lar fluid. Therefore, substances can move by simple
through the membrane within minutes.
the astonishing rate of water penetration, this amount
about 1000 times less than that of water. Even so, given
molecule is only 20 per cent greater than that of water,
off rapidly. For instance, the diameter of the urea
However, as they become larger, their penetration falls
great as the volume of the red cell itself.
astounding. As an example, the total amount of water
through the membrane. The rapidity with which water
in the membrane lipids, it readily passes through chan-
Diffusion of Water and Other Lipid-Insoluble Molecules Through
therefore, oxygen can be delivered to the interior of
proportional to its lipid solubility. Especially large
For obvious reasons, the rate of diffusion of each of
carbon dioxide, and alcohols are high, so that all these
instance, the lipid solubilities of oxygen, nitrogen,
of the substance. For
Diffusion of Lipid-Soluble Substances Through the Lipid Bilayer.
proteins, as shown to the left in Figure 4–2.
soluble, and (2) through watery channels that pene-
brane by two pathways: (1) through the interstices of
protein. The carrier protein aids passage of the mole-
Facilitated diffusion requires interaction of a carrier
through which the molecules or ions can move.
substance available, the velocity of kinetic motion, and
Transport of Substances Through the Cell Membrane
Chapter 4
47
the number and sizes of openings in the membrane
cules or ions through the membrane by binding
chemically with them and shuttling them through the
membrane in this form.
Simple diffusion can occur through the cell mem-
the lipid bilayer if the diffusing substance is lipid
trate all the way through some of the large transport
One of the most important factors that determines
how rapidly a substance diffuses through the lipid
bilayer is the lipid solubility
can dissolve directly in the lipid bilayer and diffuse
through the cell membrane in the same manner that
diffusion of water solutes occurs in a watery solution.
these substances through the membrane is directly
amounts of oxygen can be transported in this way;
the cell almost as though the cell membrane did not
exist.
Protein Channels.
Even though water is highly insoluble
nels in protein molecules that penetrate all the way
molecules can move through most cell membranes is
that diffuses in each direction through the red cell
membrane during each second is about 100 times as
Other lipid-insoluble molecules can pass through
the protein pore channels in the same way as water
molecules if they are water soluble and small enough.
yet its penetration through the cell membrane pores is
of urea penetration still allows rapid transport of urea
Diffusion Through Protein Channels,
and “Gating” of These Channels
Computerized three-dimensional reconstructions of
protein channels have demonstrated tubular pathways
all the way from the extracellular to the intracellu-
diffusion directly along these channels from one side
they are often selectively permeable to certain sub-
or closed by
Selective Permeability of Protein Channels.
Many of the
protein channels are highly selective for transport of
is only
the inner surfaces of this channel are strongly nega-
as shown by the negative signs inside
dehy-
drated
the sodium ions away from their hydrating water
diffuse in either direction according to the usual laws
and their chemical
potassium ions are not pulled away from the water
Gate
closed
Gate open
Outside
Inside
Na
+
Na
+
Gate
closed
Gate open
Outside
Inside
K
+
K
+
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
to open or close “gates” guarding the channels.
Also shown are conformational changes in the protein molecules
Transport of sodium and potassium ions through protein channels.
Figure 4–4
milliseconds. This demonstrates the rapidity with
snaps open and then snaps closed, each open state
either “all or none.” That is, the gate of the channel
membrane. Note that the channel conducts current
teristic of most voltage-gated channels. This figure
Figure 4–5
Open-State Versus Closed-State of Gated Channels.
than this diameter to pass through. This gate is
of this channel, providing a negatively charged pore
acetylcholine channel.
ligand gating.
chemical gating
protein molecule that opens or closes the gate. This
substance (a ligand) with the protein; this causes a
Chemical (ligand) gating.
action potential, as is discussed more fully in
becomes positively charged. The opening of these
intracellular ends of the potassium channels, and
of Figure 4–4, the potassium gates are on the
responsible for nerve signals. In the bottom panel
sodium pores. This is the basic mechanism for
membrane loses its negative charge, these gates
tightly closed; conversely, when the inside of the
on the inside of the cell membrane, this presumably
4–4, when there is a strong negative charge
membrane. For instance, in the top panel of Figure
In this instance, the molecular
Voltage gating.
The opening and closing of gates are controlled in
the shape of the protein molecule itself.
extensions of the transport protein molecule, which
for selective gating of sodium and potassium ions. It is
channels. This is shown in both panels of Figure 4–4
larger hydrated sodium ions are rejected, thus provid-
can pass easily through this small channel, whereas the
sium. Therefore, the smaller hydrated potassium ions
molecules that hydrate them. The hydrated form of
Membrane Physiology, Nerve, and Muscle
48
Unit II
the potassium ion is considerably smaller than the
hydrated form of sodium because the sodium ion
attracts far more water molecules than does potas-
ing selective permeability for a specific ion.
Gating of Protein Channels.
Gating of protein channels
provides a means of controlling ion permeability of the
believed that some of the gates are actual gatelike
can close the opening of the channel or can be lifted
away from the opening by a conformational change in
two principal ways:
1.
conformation of the gate or of its chemical bonds
responds to the electrical potential across the cell
could cause the outside sodium gates to remain
would open suddenly and allow tremendous
quantities of sodium to pass inward through the
eliciting action potentials in nerves that are
they open when the inside of the cell membrane
gates is partly responsible for terminating the
Chapter 5.
2.
Some protein channel
gates are opened by the binding of a chemical
conformational or chemical bonding change in the
is called
or
One of
the most important instances of chemical gating
is the effect of acetylcholine on the so-called
Acetylcholine opens the gate
about 0.65 nanometer in diameter that allows
uncharged molecules or positive ions smaller
exceedingly important for the transmission of nerve
signals from one nerve cell to another (see Chapter
45) and from nerve cells to muscle cells to cause
muscle contraction (see Chapter 7).
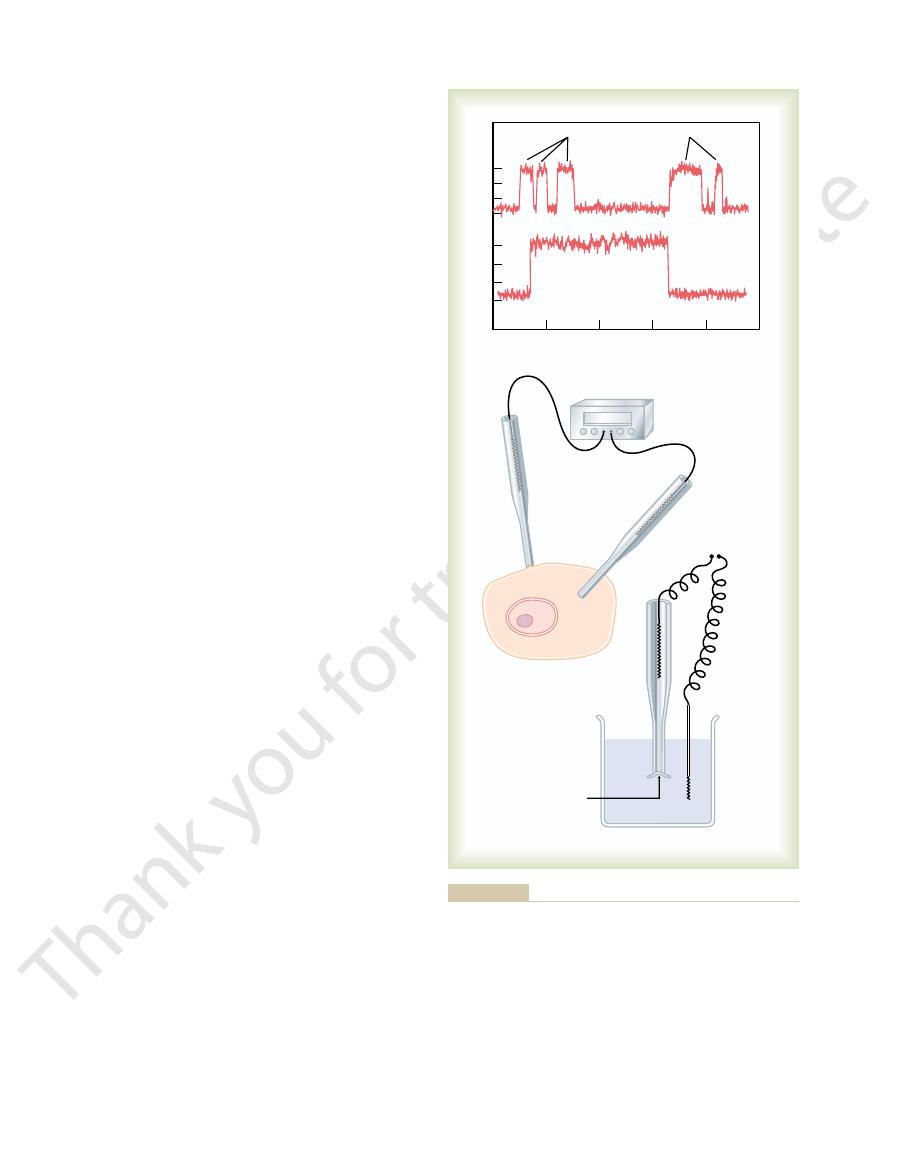
A shows an especially interesting charac-
shows two recordings of electrical current flowing
through a single sodium channel when there was an
approximate 25-millivolt potential gradient across the
lasting for only a fraction of a millisecond up to several
To recorder
6
8
10
2
4
Milliseconds
Open sodium channel
Picoamperes
Recorder
0
A
B
3
3
0
0
Membrane
“patch”
torn away from the cell.
To the right, recording is from a membrane patch that has been
recording is performed from a “patch” of a living cell membrane.
ing current flow through a single protein channel. To the left,
The “patch-clamp” method for record-
Record of current flow through a single voltage-gated sodium
Figure 4–5
A,
channel, demonstrating the “all or none” principle for opening and
closing of the channel. B,
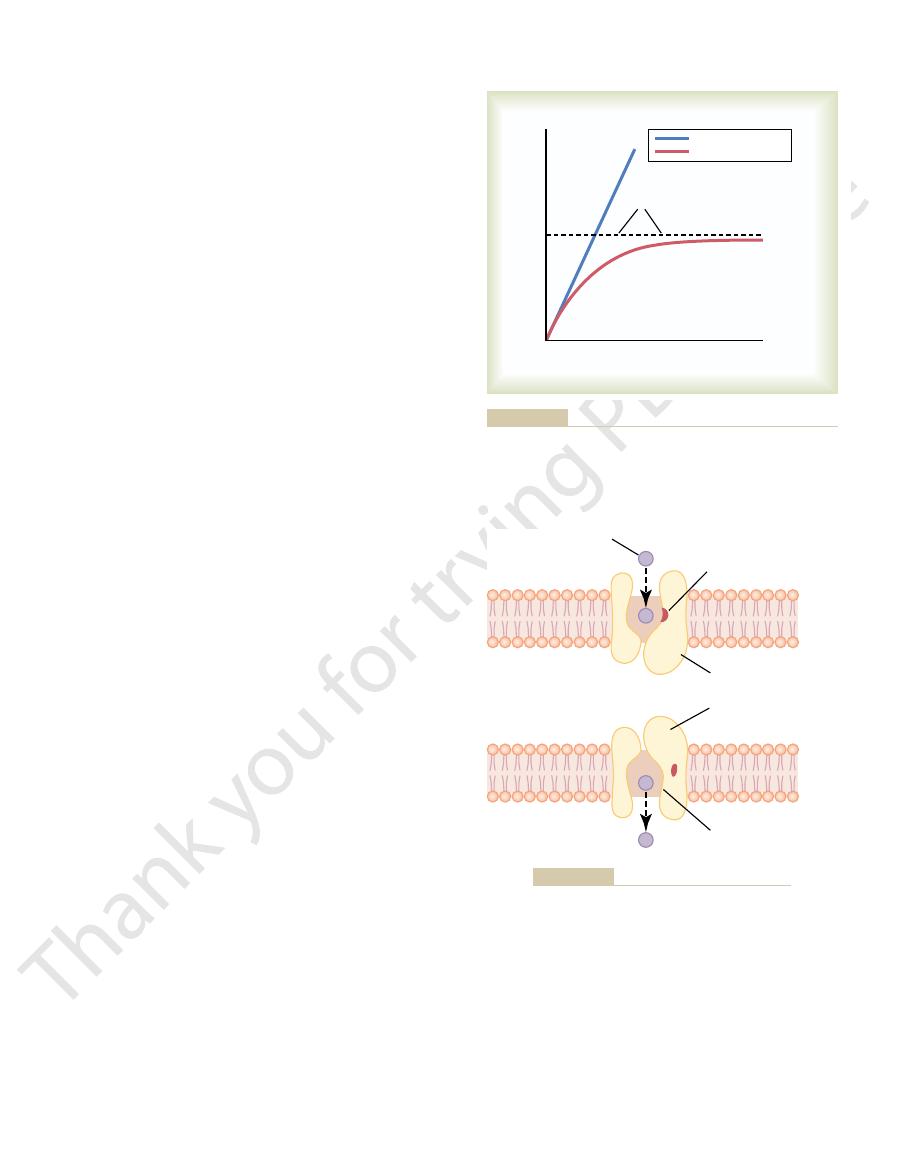
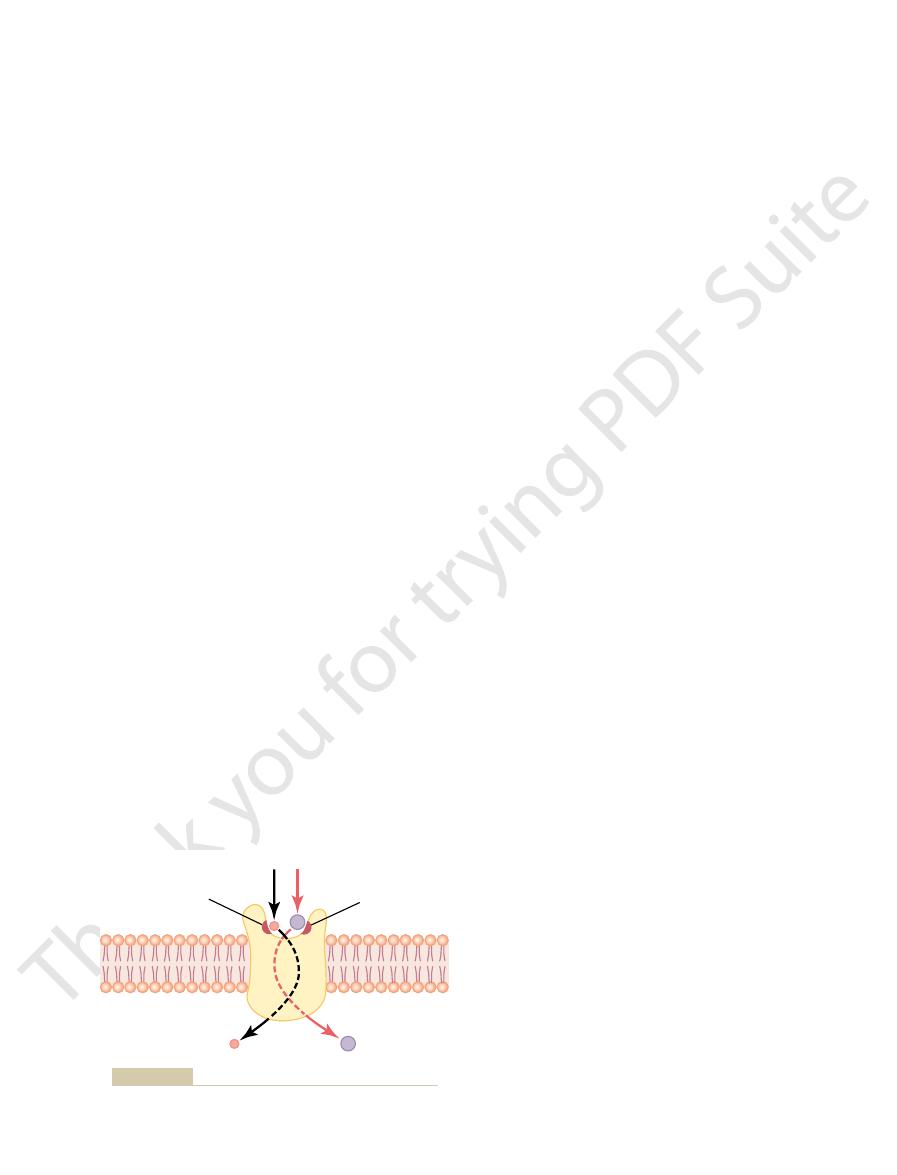
Figure 4–7. This figure shows a carrier protein with a
What is it that limits the rate of facilitated diffusion?
diffusion, the rate of diffusion cannot rise greater than
increase proportionately, but in the case of facilitated
increases, the rate of simple diffusion continues to
fusion is demonstrated in Figure 4–6. The figure shows
concentration of the diffusing substance increases. This
, as the
diffusion approaches a maximum, called V
ing substance, in facilitated diffusion the rate of
the following important way: Although the rate of
Facilitated diffusion differs from simple diffusion in
of the substance to the other side.
protein to help. That is, the carrier
carrier-mediated dif-
Facilitated diffusion is also called
properties.
across the membrane, one can determine the transport
concentrations of different ions, as well as the voltage
in the membrane patch being studied. By varying the
a given voltage.
the membrane can be set at will—that is, “clamped” to
as desired. Also, the voltage between the two sides of
This allows the concentrations of ions both inside the
can be torn away from the cell. The pipette with
Alternatively, as shown to the right in Figure 4–5
membrane “patch” at the tip of the pipette through
pipette touch the cell membrane. The result is a minute
of the pipette. This creates a seal where the edges of the
the outside of a cell membrane. Then suction is applied
diameter of only 1 or 2 micrometers, is abutted against
Very simply, a micropipette, having a tip
in Figure 4–5
achieved by using the “patch-clamp” method illustrated
This has been
protein channels as shown in Figure 4–5
gates tend to snap open and closed intermittently,
At in-between voltages, as shown in the figure, the
level, it may remain open either all or most of the time.
or almost all the time, whereas at another voltage
potential, the channel may remain closed all the time
closing of the protein molecular gates. At one voltage
Because the binding force of the receptor is weak, the
pore now opens to the opposite side of the membrane.
cal change occurs in the carrier protein, so that the
in a fraction of a second, a conformational or chemi-
transported enters the pore and becomes bound. Then,
the inside of the protein carrier. The molecule to be
partway through. It also shows a binding “receptor” on
Transport of Substances Through the Cell Membrane
Chapter 4
49
pore large enough to transport a specific molecule
thermal motion of the attached molecule causes it to
break away and to be released on the opposite side of
which changes can occur during the opening and
giving an average current flow somewhere between
the minimum and the maximum.
Patch-Clamp Method for Recording Ion Current Flow Through
Single Channels.
One might wonder how it is technically
possible to record ion current flow through single
A.
B.
inside the pipette to pull the membrane against the tip
which electrical current flow can be recorded.
B,
the small cell membrane patch at the end of the pipette
its sealed patch is then inserted into a free solution.
micropipette and in the outside solution to be altered
It has been possible to make such patches small
enough so that only a single channel protein is found
characteristics of the single channel and also its gating
Facilitated Diffusion
fusion because a substance transported in this manner
diffuses through the membrane using a specific carrier
facilitates diffusion
simple diffusion through an open channel increases
proportionately with the concentration of the diffus-
max
difference between simple diffusion and facilitated dif-
that as the concentration of the diffusing substance
the V
max
level.
A probable answer is the mechanism illustrated in
Facilitated diffusion
Simple diffusion
Concentration of substance
Rate of diffusion
V
max
shows that facilitated diffusion approaches a maximum rate called
a membrane by simple diffusion and facilitated diffusion. This
Effect of concentration of a substance on rate of diffusion through
Figure 4–6
the V
max
.
Transported
molecule
Binding point
Carrier protein
and
conformational
change
Release
of binding
Postulated mechanism for facilitated diffusion.
Figure 4–7

times, considerable pressure difference develops
side 2. This equation is extremely important in under-
concentration on side 1, and C
between side 1 and side 2 of the membrane, C
determined from the following formula, called the
body temperature (37°C), the electrical difference
enough, the two effects balance each other. At normal
right. When the concentration difference rises high
ference now tends to move the ions to the left, while
electrical potential difference. The concentration dif-
of Figure 4–8
right, creating the condition shown in the right panel
net diffusion occurs from left to right.After much time,
whereas the negative charge repels them. Therefore,
brane. The positive charge attracts the negative ions,
the left, creating an electrical gradient across the mem-
brane, but a positive charge has been applied to the
the left panel of Figure 4–8
tration difference exists to cause movement. Thus, in
applied across the membrane, as shown in Figure
tration inside.
the concentration on the inside, or:
Therefore, the rate of net diffusion into the cell is pro-
the membrane.
outward
the outside of the membrane each second. Conversely,
outside,
inward
concentration on the inside. The rate at which the sub-
Figure 4–8
factors.
direction. This net rate is determined by several
through the cell membrane. What is usually important
Factors That Affect Net Rate
in the body, as discussed in Chapter 78.
glucose as much as 10-fold to 20-fold. This is the prin-
similar to that of glucose, including galactose. Also,
molecular weight of about 45,000; it can also transport
carrier molecule has been discovered, and it has a
In the case of glucose, the
amino acids.
“diffuse”—in either direction through the membrane.
allows the transported molecule to move—that is, to
states. Note specifically, though, that this mechanism
the membrane. The rate at which molecules can be
Membrane Physiology, Nerve, and Muscle
50
Unit II
transported by this mechanism can never be greater
than the rate at which the carrier protein molecule
can undergo change back and forth between its two
Among the most important substances that cross
cell membranes by facilitated diffusion are glucose and
most of the
several other monosaccharides that have structures
insulin can increase the rate of facilitated diffusion of
cipal mechanism by which insulin controls glucose use
of Diffusion
By now it is evident that many substances can diffuse
is the net rate of diffusion of a substance in the desired
Effect of Concentration Difference on Net Diffusion Through a
Membrane.
A shows a cell membrane with a
substance in high concentration on the outside and low
stance diffuses
is proportional to the con-
centration of molecules on the
because this
concentration determines how many molecules strike
the rate at which molecules diffuse
is propor-
tional to their concentration inside
portional to the concentration on the outside minus
Net diffusion
µ (C
o
- C
i
)
in which C
o
is concentration outside and C
i
is concen-
Effect of Membrane Electrical Potential on Diffusion of Ions—
The “Nernst Potential.”
If an electrical potential is
4–8B, the electrical charges of the ions cause them to
move through the membrane even though no concen-
B, the concentration of
negative ions is the same on both sides of the mem-
right side of the membrane and a negative charge to
large quantities of negative ions have moved to the
B, in which a concentration difference of
the ions has developed in the direction opposite to the
the electrical difference tends to move them to the
that will balance a given concentration difference of
univalent ions—such as sodium (Na
+
) ions—can be
Nernst equation:
in which EMF is the electromotive force (voltage)
1
is the
2
is the concentration on
standing the transmission of nerve impulses and is dis-
cussed in much greater detail in Chapter 5.
Effect of a Pressure Difference Across the Membrane.
At
EMF in millivolts
61 log
C
C
1
2
(
)
= ±
-
- -
–
+
+
-
-
-
-
-
-
-
-
-
-
-
- -
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
- - -
-
-
–
-
-
-
-
-
Outside
A
B
C
Inside
Membrane
Piston
P
2
P
1
C
o
C
i
cause diffusion of molecules and ions through a cell membrane.
, and pressure difference
ence affecting negative ions
, electrical potential differ-
Effect of concentration difference
Figure 4–8
(A)
(B)
(C) to

than small particles, move at slower velocities (v). The
That is, large particles, which have greater mass (m)
the same amount of pressure against the membrane.
in a solution, regardless of its mass, exerts, on average,
the particles. The reason for this is that each particle
not by the mass
are molecules or ions, is determined by the
sure exerted by particles in a solution, whether they
The osmotic pres-
nondiffusible solute.
enough to oppose the osmotic effect. The pressure dif-
farther apart, until eventually a pressure difference
water from chamber B into chamber A causes the
that will not penetrate the membrane. Osmosis of
uid, one containing pure water and the
10, which shows
osmosis is demonstrated in Figure 4
The principle of a pressure difference opposing
would be slowed, stopped, or even reversed. The exact
chloride solution, osmosis of water into this solution
If in Figure 4
Osmotic Pressure
that is,
the water concentration has been reduced. Thus, net
where there is pure water, than on the right side, where
water molecules strike the channels on the left side,
9, more
water. As a result, in the example of Figure 4
ions are present and, therefore, has reduced the con-
so to sodium and chloride ions. Yet the presence of the
ant sodium and chloride ions, and the membrane is
culty. Therefore, sodium chloride solution is actually a
pass through the cell membrane with ease, whereas
sodium chloride on the other side. Water molecules
9, with pure water on
conditions shown in Figure 4
To give an example of osmosis, let us assume the
osmosis.
ment. This process of net movement of water caused
shrink, depending on the direction of the water move-
cell membrane, causing the cell either to swell or to
happens, net movement of water does occur across the
differences for other substances can occur. When this
can develop across a membrane, just as concentration
certain conditions, a
volume of the cell remains constant. However, under
movement of water occurs. Therefore, the
normally,
Yet,
volume of the cell itself.
through the cell membrane is water. Enough water
of Water
Net Diffusion
Osmosis Across Selectively
to the other side.
the pore on this side and, therefore, more molecules to
pore,
This effect is demonstrated in Figure 4
the high-pressure side toward the low-pressure side.
side. The result is that increased amounts of energy are
greater than on the other side. In most instances, this
on one side of a membrane than on the other, this
a given instant. Therefore, when the pressure is higher
20 mm Hg greater inside the capillary than outside.
brane in all tissues of the body. The pressure is about
This occurs, for instance, at the blood capillary mem-
between the two sides of a diffusible membrane.
Transport of Substances Through the Cell Membrane
Chapter 4
51
Pressure actually means the sum of all the forces of
the different molecules striking a unit surface area at
means that the sum of all the forces of the molecules
striking the channels on that side of the membrane is
is caused by greater numbers of molecules striking the
membrane per second on one side than on the other
available to cause net movement of molecules from
–8C, which
shows a piston developing high pressure on one side
of a “
” thereby causing more molecules to strike
“diffuse”
Permeable Membranes—
“
”
By far the most abundant substance that diffuses
ordinarily diffuses in each direction through the red
cell membrane per second to equal about 100 times the
the amount that
diffuses in the two directions is balanced so precisely
that zero net
concentration difference for water
by a concentration difference of water is called
–
one side of the cell membrane and a solution of
sodium and chloride ions pass through only with diffi-
mixture of permeant water molecules and nonperme-
said to be selectively permeable to water but much less
sodium and chloride has displaced some of the water
molecules on the side of the membrane where these
centration of water molecules to less than that of pure
–
movement of water occurs from left to right—
osmosis occurs from the pure water into the sodium
chloride solution.
–9 pressure were applied to the sodium
amount of pressure required to stop osmosis is called
the osmotic pressure of the sodium chloride solution.
–
a selectively permeable membrane separating two
columns of fl
other containing a solution of water and any solute
levels of the fluid columns to become farther and
develops between the two sides of the membrane great
ference across the membrane at this point is equal to
the osmotic pressure of the solution that contains the
Importance of Number of Osmotic Particles (Molar Concentra-
tion) in Determining Osmotic Pressure.
number of
particles per unit volume of fluid,
of
small particles move at higher velocities in such a way
Water
NaCl solution
Osmosis
Figure 4–9
Osmosis at a cell membrane when a sodium chloride solution is
placed on one side of the membrane and water is placed on the
other side.

lality of 1 milliosmole per kilogram. The normal
in each kilogram of water
Thus, a solution that has
58.5 grams, is equal to 2 osmoles.
ciated, 1 gram molecular weight of sodium chloride,
the nondissociated solute. Therefore, when fully disso-
ions, 1 gram molecular weight of the solute will
into ions. Conversely, if a solute dissociates into two
is 1 gram molecular weight of glucose, is equal to 1
cally active solute. Thus, 180 grams of glucose, which
is used in place of grams.
of a solution in terms of numbers of particles, the unit
To express the concentration
ciated molecule), not in terms of mass of the solute.
particle. Consequently, the factor that determines the
that their average kinetic energies (k), determined by
Membrane Physiology, Nerve, and Muscle
52
Unit II
the equation
are the same for each small particle as for each large
osmotic pressure of a solution is the concentration of
the solution in terms of number of particles (which is
the same as its molar concentration if it is a nondisso-
“Osmolality”—The Osmole.
called the osmole
One osmole is 1 gram molecular weight of osmoti-
osmole of glucose because glucose does not dissociate
become 2 osmoles because the number of osmotically
active particles is now twice as great as is the case for
1 osmole of solute dissolved
is said to have an osmolality
of 1 osmole per kilogram, and a solution that has
1/1000 osmole dissolved per kilogram has an osmo-
k
mv
2
2
=
ferent sugars, and most of the amino acids.
ions, chloride ions, iodide ions, urate ions, several dif-
ions, potassium ions, calcium ions, iron ions, hydrogen
sure gradient), the process is called
ions to the outside of cells. When a cell membrane
two sides of the membrane. Instead, some energy
effects could occur by simple diffusion, because simple
is especially true for sodium ions. Neither of these two
uid are great. This
versely, it is important to keep the concentrations of
This is true, for instance, for potassium ions. Con-
At times, a large concentration of a substance is
Substances Through
“Active Transport” of
studies.
lality, this is the usual practice in almost all physiologic
larity and osmolality are less than 1 per cent. Because it
the body, the quantitative differences between osmo-
osmotic pressure, for dilute solutions such as those in
used instead. Although, strictly speaking, it is osmoles
rather than osmoles per kilogram of water, is
osmolarity,
to determine osmolality,
uring kilograms of water in a solution, which is required
The Term “Osmolarity.”
culated value.
potential. Therefore, on average, the actual osmotic
consequently, they cannot move entirely unrestrained
and chloride ions, are highly attracted to one another;
uids, such as sodium
about 5500 mm Hg. The reason for this difference is
The measured value for this, however, averages only
uids of 5790 mm Hg.
osmotic pressure.
solution. Likewise,
C, a concentration of 1 osmole per
temperature, 37
300 milliosmoles per kilogram of water.
osmolality of the extracellular and intracellular fluids
is about
Relation of Osmolality to Osmotic Pressure.
At normal body
°
liter will cause 19,300 mm Hg osmotic pressure in the
1 milliosmole per liter concentra-
tion is equivalent to 19.3 mm Hg
Multiplying this value by the 300 milliosmolar con-
centration of the body fluids gives a total calculated
osmotic pressure of the body fl
that many of the ions in the body fl
in the fluids and create their full osmotic pressure
pressure of the body fluids is about 0.93 times the cal-
Because of the difficulty of meas-
which is the
osmolar concentration expressed as osmoles per liter of
solution
per kilogram of water (osmolality) that determines
is far more practical to measure osmolarity than osmo-
Membranes
required in the intracellular fluid even though the
extracellular fluid contains only a small concentration.
other ions very low inside the cell even though their
concentrations in the extracellular fl
diffusion eventually equilibrates concentrations on the
source must cause excess movement of potassium ions
to the inside of cells and excess movement of sodium
moves molecules or ions “uphill” against a concentra-
tion gradient (or “uphill” against an electrical or pres-
active transport.
Different substances that are actively transported
through at least some cell membranes include sodium
diffusible
Water
Semipermeable
membrane
B
A
Non-
solute
permeable membrane.
Demonstration of osmotic pressure caused by osmosis at a semi-
Figure 4–10
to the interior of the cell. Unless this is checked, the
potassium, sodium, and other positive ions as well. All
cannot escape from the cell. Most of these are nega-
the volume is as follows: Inside the cell are large
swell until they burst. The mechanism for controlling
function of this pump, most cells of the body would
pump is to control the volume of each cell. Without
Pump for Controlling Cell Volume.
out of
energy
nerve cells, 60 to 70 per cent of the cells
reaction. For some cells, such as electrically active
, determine the direction of the enzyme
phosphate, as well as the electrochemical gradients for
cell. The relative concentrations of ATP, ADP, and
produce ATP or use the energy to change its confor-
therefore, can either donate its phosphate to ADP to
pump,
phate. The phosphorylated form of the Na
pump will synthesize ATP from ADP and phos-
the chemical energy of ATP hydrolysis, these ions will
can run in reverse. If the electrochemical gradients for
ATPase pump
As with other enzymes, the Na
sium ions to the inside.
change in the protein carrier molecule, extruding the
phosphate bond of energy. This liberated energy is
cleaves one molecule of ATP, splitting it to adenosine
function of the protein becomes activated. This then
and three sodium ions bind on the inside, the ATPase
To put the pump into perspective: When two potas-
binding sites has ATPase activity.
3. The inside portion of this protein near the sodium
outside.
2. It has two
1. It has three
ular weight of about 55,000. Although the function of
subunit, with a molec-
subunit, with a molecular weight of about 100,000,
two separate globular proteins: a larger one called the
pump. The
Figure 4
the basis of nerve function, transmitting nerve signals
cells. Indeed, Chapter 5 shows that this pump is also
differences across the cell membrane, as well as for
the outside to the inside. This pump is responsible for
pump, a transport process that pumps
The active transport mechanism that has been
hydrogen, chloride, and a few other ions.
active transport are sodium, potassium, calcium,
Primary Active Transport
transport, with more detailed explanations of their
chemical gradient. Following are some examples
However, in active transport, the carrier protein func-
cell membrane, as is true for facilitated diffusion.
primary active transport. In both instances, transport
two sides of a cell membrane, created originally by
pound. In secondary active transport, the energy is
(ATP) or of some other high-energy phosphate com-
In primary active transport, the energy is derived
Primary Active Transport and Secondary Active Transport.
Transport of Substances Through the Cell Membrane
Chapter 4
53
Active transport is divided into two types according to
the source of the energy used to cause the transport:
primary active transport and secondary active trans-
port.
directly from breakdown of adenosine triphosphate
derived secondarily from energy that has been stored
in the form of ionic concentration differences of
secondary molecular or ionic substances between the
depends on carrier proteins that penetrate through the
tions differently from the carrier in facilitated diffu-
sion because it is capable of imparting energy to the
transported substance to move it against the electro-
of primary active transport and secondary active
principles of function.
Sodium-Potassium Pump
Among the substances that are transported by primary
studied in greatest detail is the sodium-potassium
(Na
+
-K
+
)
sodium ions outward through the cell membrane of all
cells and at the same time pumps potassium ions from
maintaining the sodium and potassium concentration
establishing a negative electrical voltage inside the
throughout the nervous system.
–11 shows the basic physical components of
the Na
+
-K
+
carrier protein is a complex of
a
and a smaller one called the
b
the smaller protein is not known (except that it might
anchor the protein complex in the lipid membrane),
the larger protein has three specific features that are
important for the functioning of the pump:
receptor sites for binding sodium ions
on the portion of the protein that protrudes to the
inside of the cell.
receptor sites for potassium ions on the
sium ions bind on the outside of the carrier protein
diphosphate (ADP) and liberating a high-energy
then believed to cause a chemical and conformational
three sodium ions to the outside and the two potas-
+
-K
+
Na
+
and K
+
are experimentally increased enough so
that the energy stored in their gradients is greater than
move down their concentration gradients and the Na
+
-
K
+
+
-K
+
mation and pump Na
+
out of the cell and K
+
into the
Na
+
and K
+
’
requirement may be devoted to pumping Na
+
the cell and K
+
into the cell.
Importance of the Na
+
-K
+
One of the most important functions of the Na
+
-K
+
numbers of proteins and other organic molecules that
tively charged and therefore attract large numbers of
these molecules and ions then cause osmosis of water
ATPase
ATP
Inside
Outside
ADP
+
Pi
3Na
+
3Na
+
2K
+
2K
+
adenosine diphosphate; ATP, adenosine triphosphate; Pi,
Postulated mechanism of the sodium-potassium pump. ADP,
Figure 4–11
phosphate ion.

to be co-transported. Once they both are attached, the
brane. The carrier in this instance serves as an attach-
a coupling mechanism is required. This is achieved by
For sodium to pull another substance along with it,
form of
brane.This phenomenon is called
diffuse to the interior. Under appropriate conditions,
very low concentration inside. This gradient represents
primary active transport, a large concentration gradi-
When sodium ions are transported out of cells by
Co-Transport and Counter-Transport
Secondary Active Transport
alone.
renal tubules and many glandular cells, expend as
can be tremendous. Some cells, such as those lining the
2800 calories. One can see that the energy expenditure
is about 1400 calories; or to concentrate it 100-fold,
Thus, in terms of calories, the amount of energy
is concentrated, as expressed by the following
In other words, the energy required is proportional
it 1000-fold requires three times as much energy.
requires twice as much energy, and to concentrate
trate a substance 10-fold, to concentrate it 100-fold
port. Compared with the energy required to concen-
The amount of energy required to transport a sub-
Energetics of Primary Active Transport
uids. The hydrogen ions can be secreted into the
port. In this case, large amounts of hydrogen ions are
the gastric gland parietal cells, the hydrogen ion con-
stomach digestive secretions. At the secretory ends of
This is the basis for secreting hydrochloric acid in the
transporting hydrogen ions of any part of the body.
In the gastric glands, the deep-lying
and cortical collecting ducts of the kidneys.
glands of the stomach, and (2) in the late distal tubules
hydrogen ions is very important: (1) in the gastric
At two places in the body, primary active transport of
Primary Active Transport of Hydrogen Ions
carrier protein. The difference is that this protein has
capability to cleave ATP as the ATPase of the sodium
and functions as an enzyme ATPase, having the same
instances, the carrier protein penetrates the membrane
and the mitochondria in all cells. In each of these
cell, such as the sarcoplasmic reticulum of muscle cells
the cell. The other pumps calcium ions into one or
primary active transport calcium pumps. One is in the
uid. This is achieved mainly by two
intracellular cytosol of virtually all cells in the body, at
calcium pump.
Primary Active Transport of Calcium Ions
bers for transmitting nerve and muscle signals.
membrane. As discussed in Chapter 5, this electrical
Therefore, the Na
inside the cell; that is, it causes negativity on the inside.
the exterior for each cycle of the pump. This creates pos-
The fact that the
veillance role in maintaining normal cell volume.
Therefore, the Na
pump, moving still more
If a cell begins to swell for any reason, this auto-
net loss of ions out of the cell, which initiates osmosis
strong tendency to stay there. Thus, this represents a
once the sodium ions are on the outside, they have a
able to sodium ions than to potassium ions, so that
to the interior. Also, the membrane is far less perme-
pump.
nitely until it bursts. The normal
Membrane Physiology, Nerve, and Muscle
54
Unit II
cell will swell indefi
mechanism for preventing this is the Na
+
-K
+
Note again that this device pumps three Na
+
ions to
the outside of the cell for every two K
+
ions pumped
of water out of the cell as well.
matically activates the Na
+
-K
+
ions to the exterior and carrying water with them.
+
-K
+
pump performs a continual sur-
Electrogenic Nature of the Na
+
-K
+
Pump.
Na
+
-K
+
pump moves three Na
+
ions to the exterior for
every two K
+
ions to the interior means that a net of one
positive charge is moved from the interior of the cell to
itivity outside the cell but leaves a deficit of positive ions
+
-K
+
pump is said to be electrogenic
because it creates an electrical potential across the cell
potential is a basic requirement in nerve and muscle
fi
Another important primary active transport mecha-
nism is the
Calcium ions are normally
maintained at extremely low concentration in the
a concentration about 10,000 times less than that in the
extracellular fl
cell membrane and pumps calcium to the outside of
more of the intracellular vesicular organelles of the
a highly specific binding site for calcium instead of for
sodium.
parietal cells
have the most potent primary active mechanism for
centration is increased as much as a millionfold and
then released into the stomach along with chloride
ions to form hydrochloric acid.
In the renal tubules are special intercalated cells in
the late distal tubules and cortical collecting ducts that
also transport hydrogen ions by primary active trans-
secreted from the blood into the urine for the purpose
of eliminating excess hydrogen ions from the body
fl
urine against a concentration gradient of about
900-fold.
stance actively through a membrane is determined by
how much the substance is concentrated during trans-
to the logarithm of the degree that the substance
formula:
required to concentrate 1 osmole of substance 10-fold
for concentrating substances in cells or for removing
substances from cells against a concentration gradient
much as 90 per cent of their energy for this purpose
—
ent of sodium ions across the cell membrane usually
develops—high concentration outside the cell and
a storehouse of energy because the excess sodium
outside the cell membrane is always attempting to
this diffusion energy of sodium can pull other sub-
stances along with the sodium through the cell mem-
co-transport; it is one
secondary active transport.
means of still another carrier protein in the cell mem-
ment point for both the sodium ion and the substance
energy gradient of the sodium ion causes both the
sodium ion and the other substance to be transported
together to the interior of the cell.
Energy in calories per osmole
1400 log
C
C
1
2
(
)
=
across these membranes, which in turn causes osmosis
This creates a high sodium ion concentration gradient
the surrounding connective tissue and blood vessels.
and lateral membranes of the cells, sodium ions are
lumen into the interior of the cell. Then, at the basal
Therefore, sodium and water diffuse readily from the
the cells is permeable to both sodium ions and water.
The brush border on the luminal surfaces of
kisses.
intestines, gallbladder, and renal tubules. This
Figure 4
in the sheet, and then (2) either
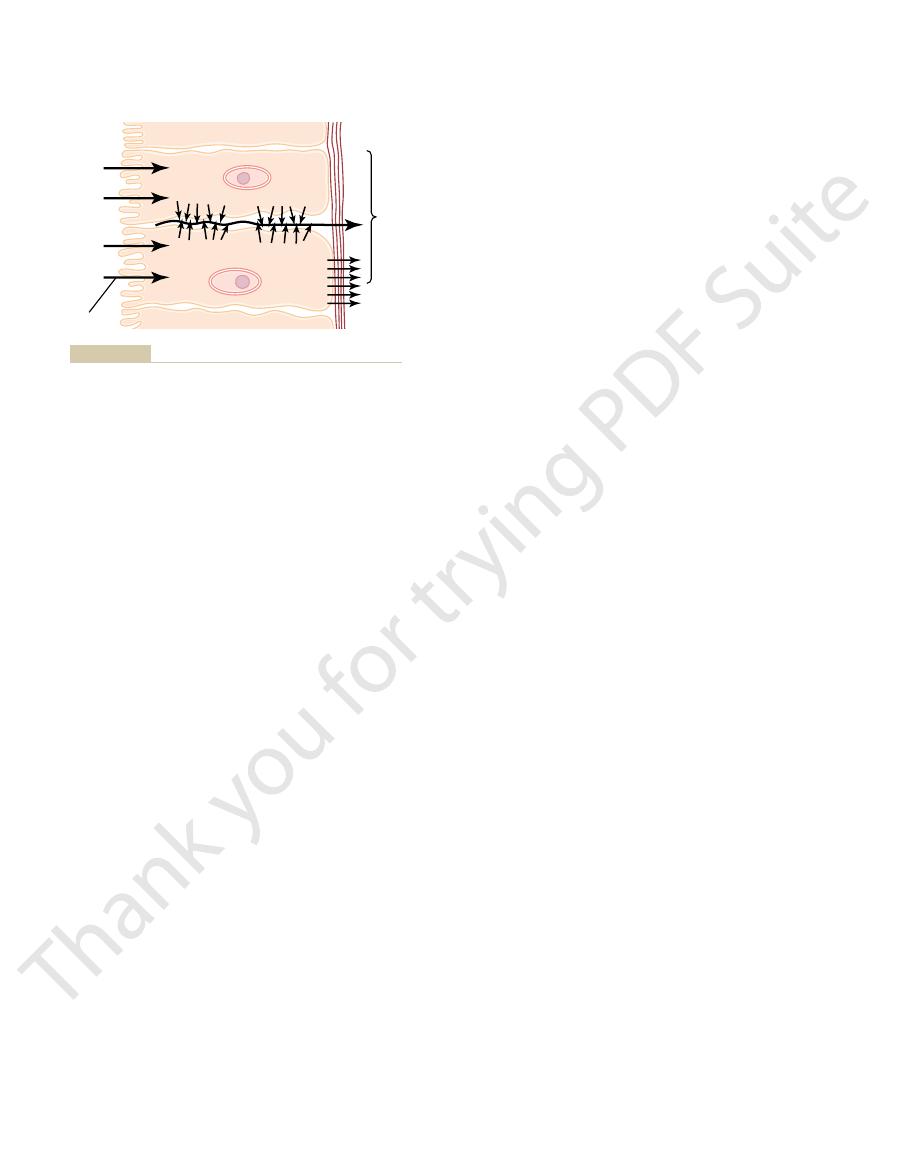
The basic mechanism for transport of a substance
other membranes.
exocrine glands, (4) epithelium of the gallbladder, and
epithelium of the renal tubules, (3) epithelium of all
type occurs through the (1) intestinal epithelium, (2)
simply through the cell membrane. Transport of this
At many places in the body, substances must be trans-
Active Transport Through
Chapter 30.
uids, as discussed in detail in
of hydrogen ions,
tubules, but it can transport extremely
concentrating hydrogen ions, counter-transport is not
transported into the tubule lumen. As a mechanism for
of the tubular cell, while hydrogen ions are counter-
of the kidneys, where sodium ions
proximal tubules
several tissues. An especially important example is in
Sodium-hydrogen counter-transport occurs in
transport of calcium that occurs in some cells.
transport mode. This is in addition to primary active
both bound to the same transport protein in a counter-
moving to the interior and calcium ions to the exterior,
all or almost all cell membranes, with sodium ions
Sodium-calcium counter-transport occurs through
sodium-hydrogen counter-transport.
sodium-calcium counter-transport
Two especially important counter-transport mecha-
Hydrogen Ions
Sodium Counter-Transport of Calcium and
iodine ions, iron ions, and urate ions.
least some cells include co-transport of chloride ions,
blood, as is discussed in later chapters.
c molecular characteristics.
ed, each of which is
ferent set of transport proteins. Five
same manner as for glucose, except that it uses a dif-
cell at the same time. Hence, this is a
formational change takes place automatically, and the
attaches. When they both become attached, the con-
inside, which provides energy for the transport. A
sodium and one for glucose. Also, the concentration of
has two binding sites on its exterior side, one for
12. Note that the transport carrier protein
in Figure 4
mechanism of this is entirely by co-transport, as shown
most cells against large concentration gradients; the
Co-Transport of Glucose and Amino Acids
exterior.
have bound, a conformational change occurs, and
interior projection of the carrier protein. Once both
the substance to be counter-transported binds to the
jects to the exterior surface of the membrane, while
must be transported to the outside. Therefore, the
concentration gradient. However, this time, the sub-
counter-transport,
Transport of Substances Through the Cell Membrane
Chapter 4
55
In
sodium ions again attempt to
diffuse to the interior of the cell because of their large
stance to be transported is on the inside of the cell and
sodium ion binds to the carrier protein where it pro-
energy released by the sodium ion moving to the
interior causes the other substance to move to the
Along with Sodium Ions
Glucose and many amino acids are transported into
–
sodium ions is very high on the outside and very low
special property of the transport protein is that a con-
formational change to allow sodium movement to the
interior will not occur until a glucose molecule also
sodium and glucose are transported to the inside of the
sodium-glucose
co-transport mechanism.
Sodium co-transport of the amino acids occurs in the
amino acid trans-
port proteins have been identifi
responsible for transporting one subset of amino acids
with specifi
Sodium co-transport of glucose and amino acids
occurs especially through the epithelial cells of the
intestinal tract and the renal tubules of the kidneys to
promote absorption of these substances into the
Other important co-transport mechanisms in at
nisms (transport in a direction opposite to the primary
ion) are
and
the
move from the lumen of the tubule to the interior
nearly as powerful as the primary active transport of
hydrogen ions that occurs in the more distal renal
large numbers
thus making it a key to hydrogen
ion control in the body fl
Cellular Sheets
ported all the way through a cellular sheet instead of
(5) membrane of the choroid plexus of the brain and
through a cellular sheet is (1) active transport through
the cell membrane on one side of the transporting cells
simple diffusion or
facilitated diffusion through the membrane on the
opposite side of the cell.
–13 shows a mechanism for transport
of sodium ions through the epithelial sheet of the
figure
shows that the epithelial cells are connected together
tightly at the luminal pole by means of junctions called
“
”
actively transported into the extracellular fluid of
Na-binding
site
Na
+
Na
+
Glucose
Glucose
Glucose-binding
site
Postulated mechanism for sodium co-transport of glucose.
Figure 4–12
Rev 80:211, 2000.
Russell JM: Sodium-potassium-chloride cotransport. Physiol
Rev Physiol 64:877, 2002.
action between genetic and environmental factors. Annu
sodium channel and the control of sodium balance: inter-
Rossier BC, Pradervand S, Schild L, Hummler E: Epithelial
exchange molecule: an overview. Ann N Y Acad Sci 976:1,
Philipson KD, Nicoll DA, Ottolia M, et al: The Na
porters. News Physiol Sci 19:80, 2004.
Peres A, Giovannardi S, Bossi E, Fesce R: Electrophysiolog-
MacKinnon R: Potassium channels. FEBS Lett 555:62, 2003.
a shared structure. Physiol Rev 82:735, 2002.
degenerin family of ion channels: a variety of functions for
Kellenberger S, Schild L: Epithelial sodium channel/
Physiol Rev 82:769, 2002.
Kaupp UB, Seifert R: Cyclic nucleotide-gated ion channels.
Physiol Rev 82:503, 2002.
structure and physiological function of chloride channels.
Jentsch TJ, Stein V, Weinreich F, Zdebik AA: Molecular
channels. Pharmacol Rev 55:607, 2003.
Dolphin AC: G protein modulation of voltage-gated calcium
Annu Rev Physiol 62:919, 2000.
De Weer P: A century of thinking about cell membranes.
proton transfer pathways. Physiol Rev 83:475, 2003.
Decoursey TE: Voltage-gated proton channels and other
cells. Kidney Int 60:427, 2001.
Caplan MJ: Ion pump sorting in polarized renal epithelial
family of ion channels. J Physiol 520:631, 1999.
Benos DJ, Stanton BA: Functional domains within the
mechanisms for human diseases. FEBS Lett 555:72, 2003.
Agre P, Kozono D: Aquaporin water channels: molecular
different types of transport discussed in this chapter.
Throughout this text are numerous examples of the
ltrate by the renal tubules.
the blood from the intestine; they are also the way the
nutrients, ions, and other substances are absorbed into
These are the mechanisms by which almost all the
transport not only of sodium ions but also of water.
of water as well. Thus, active transport of sodium ions
Membrane Physiology, Nerve, and Muscle
56
Unit II
at the basolateral sides of the epithelial cells results in
same substances are reabsorbed from the glomerular
fi
References
degenerin/epithelial sodium channel (Deg/ENaC) super-
ical insights into the mechanism of ion-coupled cotrans-
+
/Ca
2+
2002.
Diffusion
Active
transport
Brush
border
Basement
membrane
Connective tissue
Osmosis
Osmosis
Lumen
Active
transport
Active
transport
Osmosis
Na
+
Na
+
and
H
2
O
Na
+
Na
+
Na
+
Basic mechanism of active transport across a layer of cells.
Figure 4–13
