2
Lecture 1+2 - Proteins & amino
acids metabolism
Introduction
To understand the fate of proteins and amino acids in the
human body
To discuss the amino acid pool
To study the proteolytic pathways and systems
To deal with the formation of ammonia and urea in urea cycle
To take an example of protein synthesis and degradation
To see an amino acid metabolic pathway with possible genetic
abnormality.
Biomedical importance of proteins
1) Structural function: cell membrane, cytoplasm, receptors,
collagen, elastin.
2) Metabolic regulation: enzymes, hormones.
3) Transport functions: albumin, hb, transferrin and
lipoproteins.
4) Blood clotting: fibrinogen, prothrombin.
5) Protection: immunoglobulins.
6) Contraction: actin and myosin.
7) Neurotransmission: adrenaline, acetylcholine, dopamine.
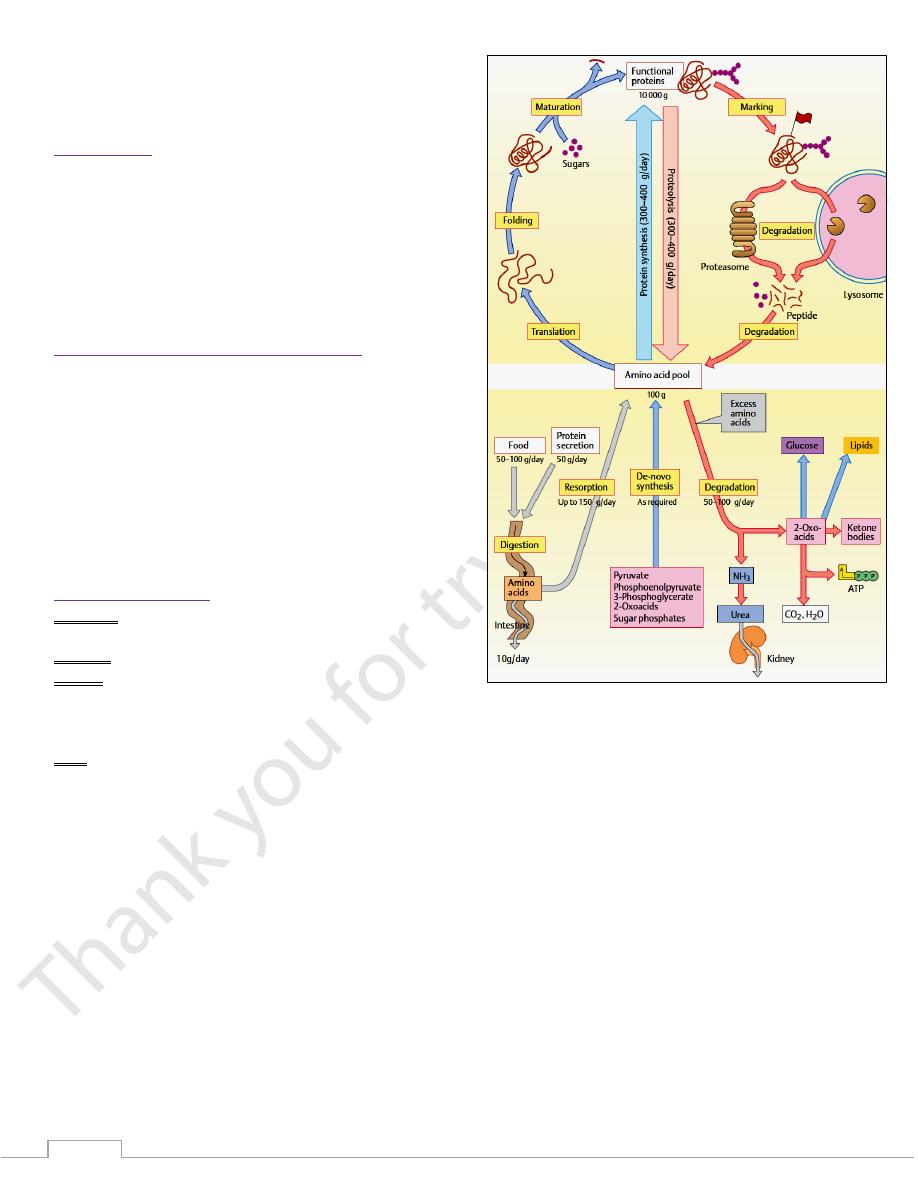
The amino acid pool
Definition: The space that contains all the free amino acids in
cells and extra-cellular fluids.
Amount: is about 100 g.
Source:
1) Amino acids that are derived from digestion of dietary
proteins & degradation of tissue proteins.
2) Amino acids that are synthesized in the body.
Fate:
A. Anabolic fate
1) Synthesis of tissue proteins.
2) Synthesis of nitrogenous compounds (e.g. Plasma proteins,
hemoglobin, enzymes, protein hormones, purines,
pyrimidines, creatine, neurotransmitters).
3) Conversion to glucose, glycogen, fatty acids, ketone bodies
and steroids.
B. Catabolic fate: oxidation to Co
2
, H
2
O and energy.
3
Protein degradation
There are 2 major enzyme systems responsible for degradation
of damaged or unneeded proteins:
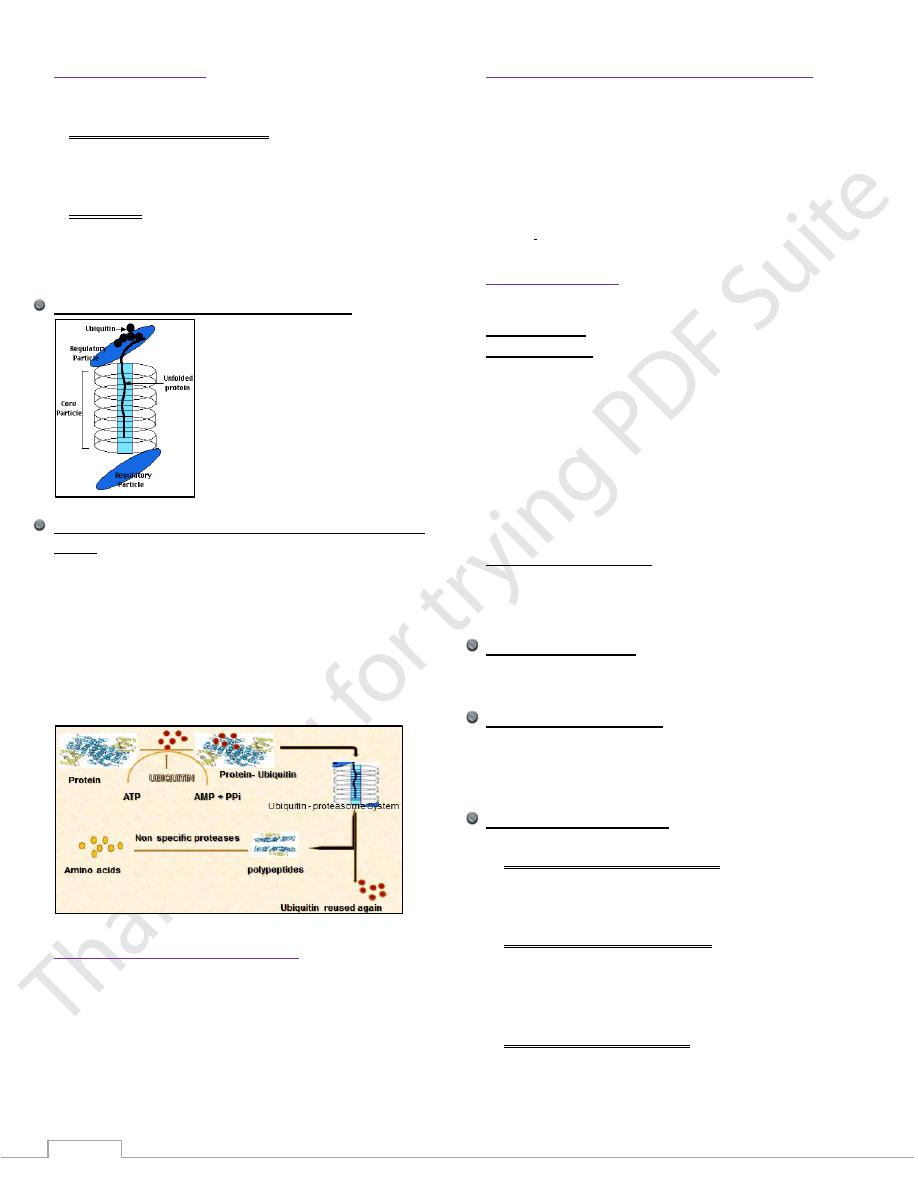
1. Ubiquitin - proteasome system:
Is an energy-dependent system.
Is mainly degrades endogenous proteins: (proteins
synthesized within the cell).
2. Lysosomes.
Are non - energy-dependent enzymes.
Primarily degrade extracellular proteins: (plasma
proteins and membrane proteins).
Structure of the ubiquitin - proteasome system
Steps of protein degradation by the proteasome- ubiquitin
system
1) Protein intended for degradation is tagged with molecules of
ubiquitin.
2) Ubiquinated proteins are recognized by the proteasome which
transports them to the proteolytic core.
3) Ubiquitin is released and reused again.
4) Peptide fragments produced by the proteasome are degraded
into amino acids.
5) Released amino acids are reused in new protein synthesis.
The result of protein catabolism
Proteins are degraded into amino acids.
First step in amino acid degradation is the removal of the
amino group and formation of ammonia.
Ammonium ion is converted into urea in the liver and
execrated by the kidneys.
Carbon atoms are converted to other major metabolic
intermediates.
Protein Requirement in Health and Disease
The normal daily requirement of protein for adults is 0.8 - 1 g
/ Kg body mass.
Protein requirement is increased in:
Healthy conditions: growth, pregnancy, lactation &
adolescence.
Disease states: illness, major trauma and surgery.
Recommended daily allowance (RDA) for protein should be
reduced in: hepatic and renal failure
Nitrogen balance
It is the relationship between nitrogen intake & output.
Nitrogen intake is in the form of dietary proteins.
Nitrogen Output (Nitrogen excretion or loss)
Occurs through several routes:
1) Stools: in the form of undigested proteins.
2) Urine: as non-protein nitrogenous compounds (NPN) e.g.
ammonia, urea, creatinine, uric acid…etc.
3) Hair and nails.
4) Desquamated epithelial cells from skin, gastro- intestinal,
genitourinary, and respiratory tracts.
5) Sweat as urea.
6) Menstrual blood.
States of nitrogen balance
A- Nitrogen equilibrium.
B. Positive nitrogen balance.
C. Negative nitrogen balance.
Nitrogen equilibrium
Nitrogen intake is equal to nitrogen output.
It occurs in healthy adults on balanced diets
Positive nitrogen balance
Nitrogen intake > nitrogen output.
Causes of positive nitrogen balance
(1) Growing children. (2) Pregnancy. (3) Covalence.
Negative nitrogen balance
Causes of Negative Nitrogen Balance
A. Inadequate protein intake due to:
1. Starvation.
2. Malnutrition.
3. Malabsorption.
B. Excessive loss of proteins due to:
1. Chronic hemorrhage.
2. Extensive burns.
2. Albuminuria due to kidney disease.
3. During pregnancy and lactation on an inadequate diet.
C. Increased protein catabolism
1. Chronic metabolic diseases: Diabetes mellitus.
2. Hormonal abnormalities: Cushing's syndrome (increased
glucocorticoids), Hyperthyroidism.
4
3. Chronic Infectious diseases: Tuberculosis, AIDS.
4- after surgical operations.
5. Cancer.
Metabolism of amino nitrogen:
The nitrogen of AAs is metabolized through the following
processes:
1) Transamination of amino acids.
2) Oxidative deamination of glutamate.
3) Transport of Ammonia.
4) Reactions in the urea cycle.
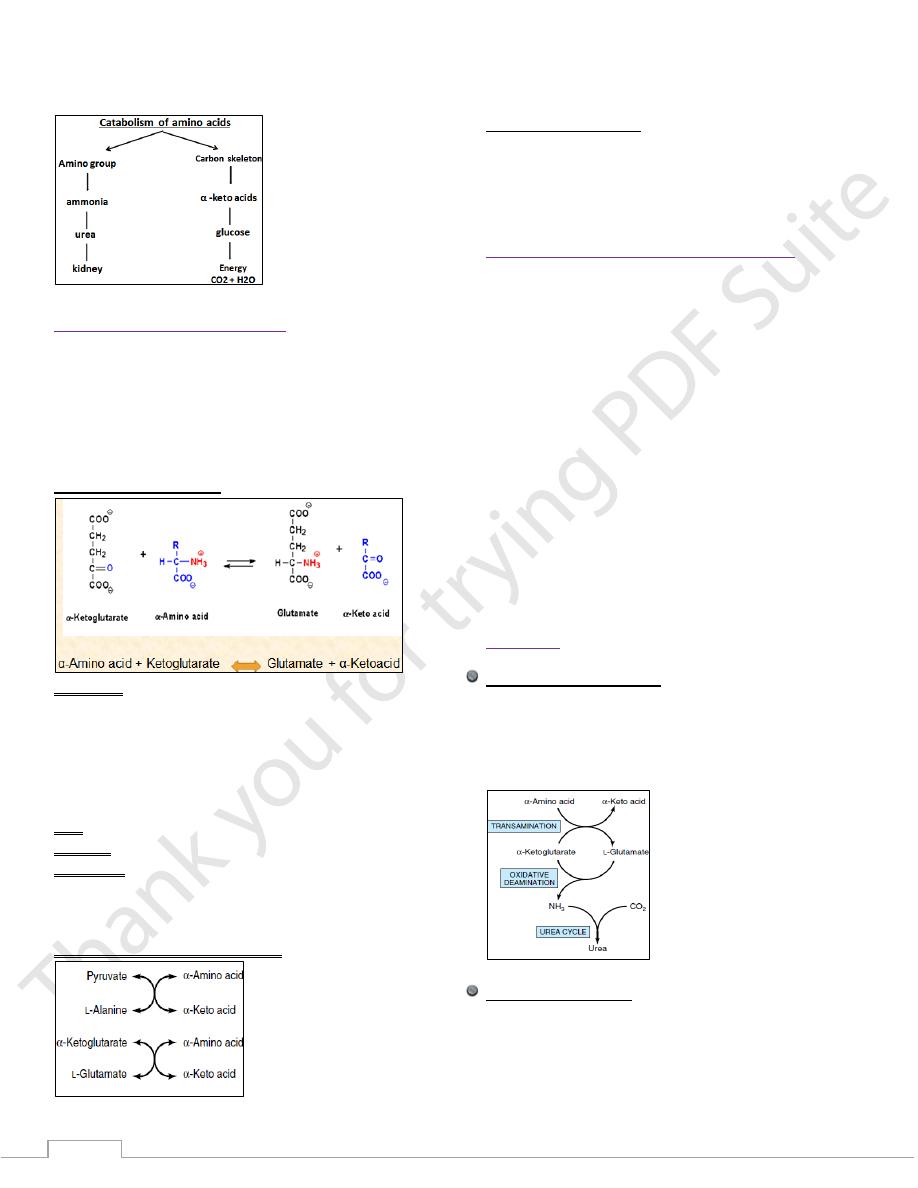
First - Transamination:
Definition:
Is the first step in catabolism of AAs.
It is the transfer of the amino group from one AA to an α-
ketoglutarate (α-kg).
The products are α-keto acid + glutamate.
The reaction is reversible - function in both catabolisms of
AAs + biosynthesis of non-essential AAs.
Site: all tissues
Enzyme: transaminase (aminotransferase)
Coenzyme: pyridoxal phosphate.
The two most important aminotransferase reactions are
catalyzed by:
Alanine aminotransferase aspartate aminotransferase
General aminotransferase activities
Alanine aminotransferase (top) and
glutamate aminotransferase (bottom).
Second - Deamination
There are three main enzymes
1. L-glutamate dehydrogenase
2. L-amino acid oxidases
3. D-amino acid oxidases
Biosynthesis of ammonia and urea cycle
The continuous degradation and synthesis of cellular
proteins each day is about 1–2% of the total body protein,
principally muscle protein.
High rates of protein degradation occur in tissues
undergoing structural rearrangement:- uterine tissue
during pregnancy, skeletal muscle in starvation, bone
remodulation.
approximately 75% of the liberated amino acids are
reutilized.
The amino acids in excess of those needed for the synthesis
for protein or other biomolecules cannot be stored in the
body.
They will be used as a metabolic fuel.
First the a amino group is removed and converted to
ammonia then to urea.
Then the carbon skeleton is converted into a major
metabolic intermediates for energy utilization.
Ammonia
Biosynthesis of ammonia
Biosynthesis occurs in three stages:
(1) Transamination of amino acids.
(2) Oxidative deamination of glutamate.
(3) Transport of ammonia.
Steps in biosynthesis of ammonia
Ammonia Transport
1) Ammonia is transported in the blood from the sites of
production to the sites of elimination.
2) Ammonia is present in low level in the blood due to:
A. Many tissues release amino acid nitrogen in the form of
glutamine and alanine rather than free NH3.
5
B. The rapid removal of ammonia by the liver.
3) Glutamine is removed by intracellular renal amino acid
deamination by glutaminase to glutamate.
4) Urea formed in the liver from NH3 and excreted by the kidney
is the most important route for NH3 disposal.
5) Formation & Excretion of ammonia by renal tubular cells
maintains acid-base balance.
Ammonia production from intracellular renal amino acids,
especially glutamine is:
Increases in metabolic acidosis (high H ion conc.)
Decreases in metabolic alkalosis (low H ion conc.)
6) NH
3
is produced by the intestinal bacteria as well.
Fates of ammonia
1) NH3 is captured by liver cells to form urea.
2) Directly excreted in urine (by the action of glutaminase in the
kidney → liberation and reabsorption of glutamate while
ammonia is excreted in urine as NH4 ions).
3) Synthesis of nucleotides.
4) Synthesis of heme.
5) Synthesis of non-essential AAs by the reverse of L-glutamate
dehydrogenase.
Types of hyperammonemia
1) Hereditary hyperammonemia :
It is due to deficiency of
-any enzyme of urea cycle,
2) Acquired hyperammonemia
1) Liver cirrhosis
2) Biliary obstruction
Urea
Structure of urea
NH2 -CO- NH2
What is urea
1. It is the end-product of deamination of AAs.
2. is formed in the liver from ammonia .
3. It forms about 80 -90 % of total urine nitrogen.
4. Its excess in blood is called ureamia.
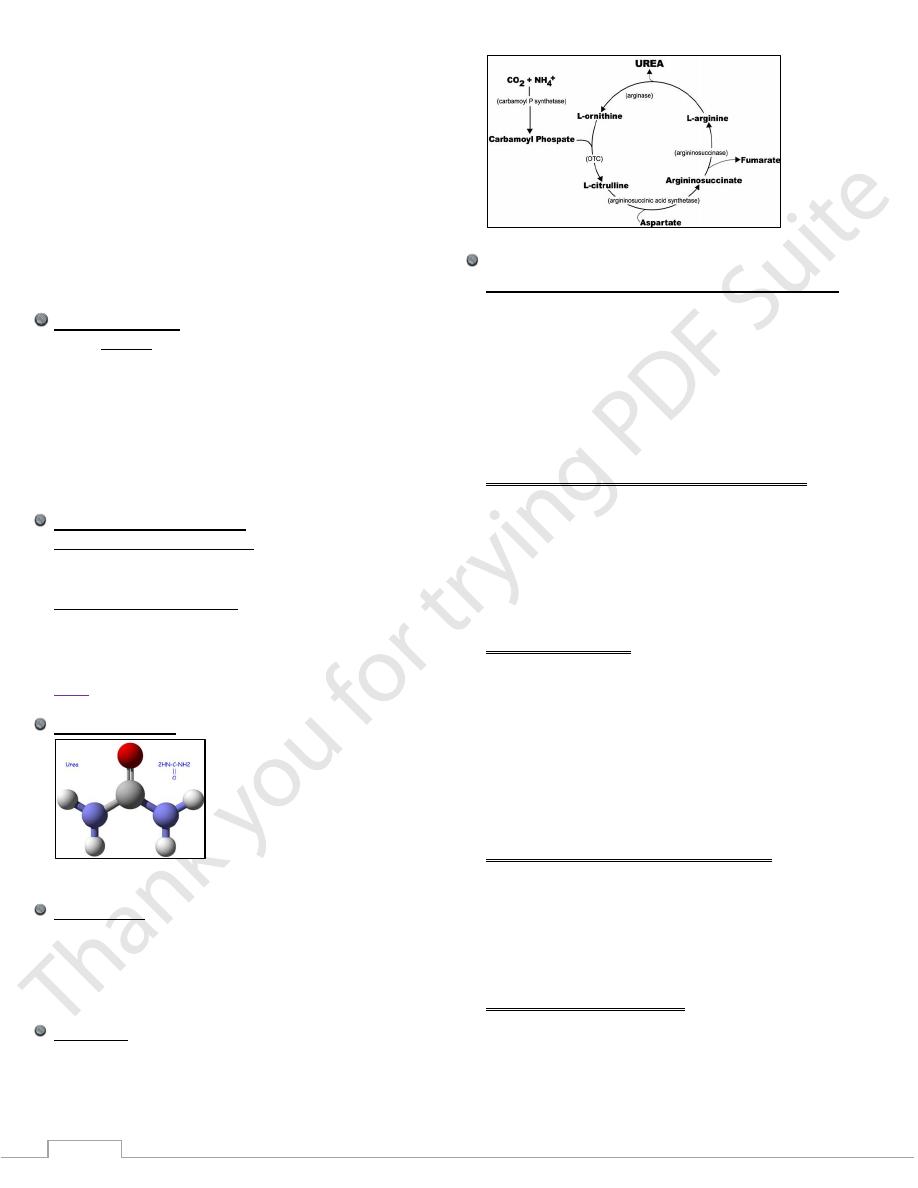
Urea cycle
Synthesis of urea occurs in the liver in 5 reactions.
The first 2 reactions proceed in mitochondria of liver cells.
While the remaining 3 proceed in cytosol of liver cells.
Urea and Uremia
(Definition, causes, clinical picture and treatment)
Urea is the main product of protein metabolism. It is the
chemical form in which unrequired nitrogen is excreted
mainly by kidneys, small amounts are lost via skin & GIT.
Urea is formed in the liver from ammonia and CO2 in a series
of enzyme mediated reaction (the urea cycle).
Normal blood urea: 20 – 40 mg/dl.
Uremia: is the accumulation of urea with other nitrogenous
compounds mainly due to renal failure.
Clinical picture of hyperammonemia and ureamia
1) Vomiting
2) Intermittent ataxia
3) Irritability
4) Lethargy
5) Coma
6) Mental retardation (hereditary type)
7) Death
Main causes of uremia
1) Pre-renal causes:
a) Increased protein catabolism: High protein diet, GIT
hemorrhages trauma, surgery and starvation.
b) Impaired renal perfusion: Loss of extracellular fluid,
hypoproteinaemia and heart failure.
2) Renal causes: (reduced glomerular filtration rate):
Acute or chronic renal disease.
3) Post renal causes (any obstruction to urine flow): Stones,
ureter and urethral strictures and prostatic hypertrophy.
Treatment of Hyperammonemia and uremia
Restriction of protein intake.
haemo and peritoneal dialysis.
Liver transplantation.
Renal transplantation.
Gene Therapy Offers Promise for Correcting Defects in
Urea Biosynthesis.
Causes of reduced plasma urea
1- Low protein diet.
2- Sever starvation.
3- Malabsorption syndrome.
4- Sever liver diseases.
5- Water retention.
