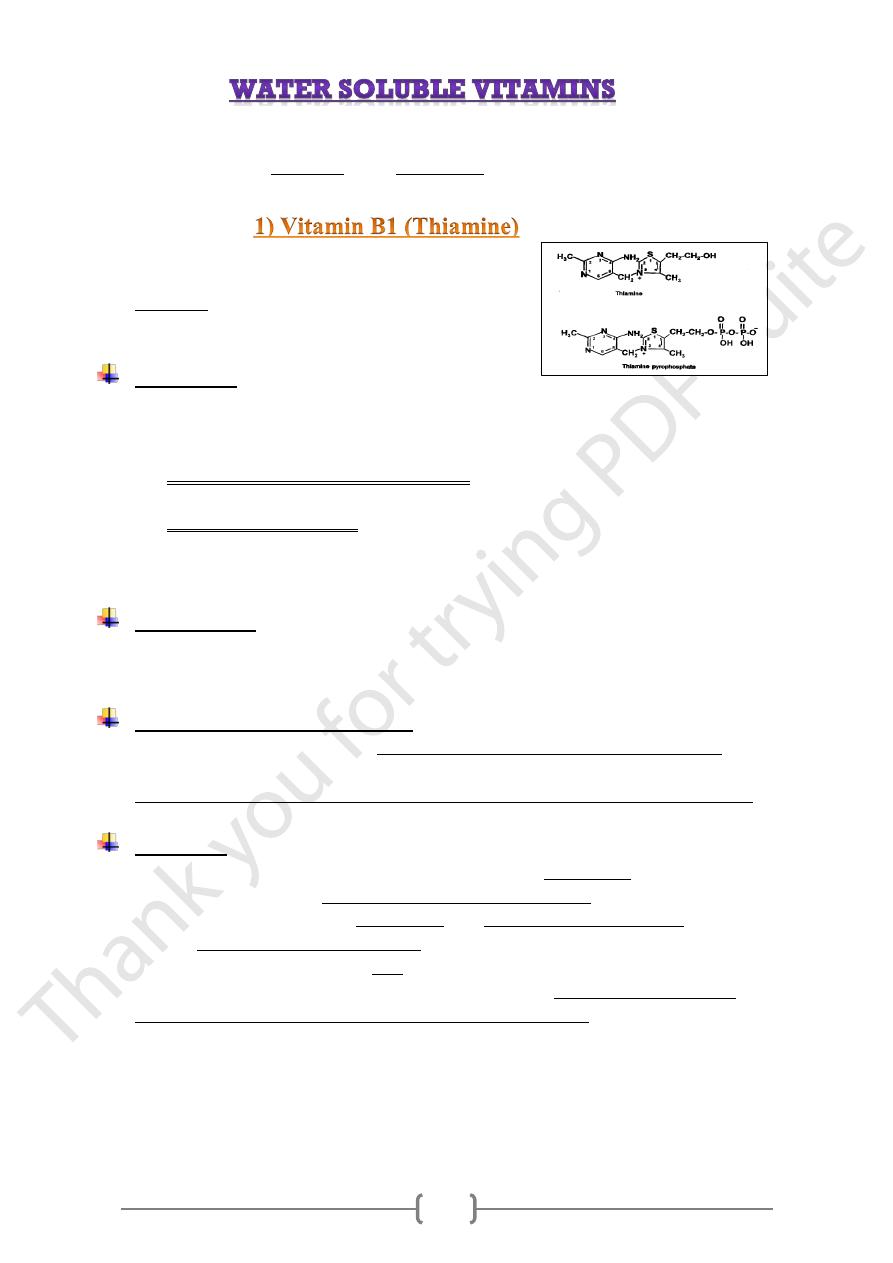
11
Water soluble vitamins function as coenzymes,
is essential for haloenzyme activity.
apoenzymes
for an
coenzyme
affinity of the
The
The free vitamin occurs in the plasma, but the
, predominates in the cellular
, TPP
coenzyme
component
Absorption
The phosphorylated vitamin is dephosphorylated by (membrane-bound alkaline
phosphatase) in the intestinal mucosa. The dephosphorylated vitamers are absorbed in
small intestine by two mechanisms.
1) Active transport (thiamin transporter):
Carrier mediated as long as intake is less than 5mg/day.
2) Simple passive diffusion:
When the intakes higher than 5mg/day.
Percentage absorption diminished with increased dose
Food Sources
• Excellent sources are liver, heart, kidney, egg yolk and unrefined grains.
• Milk, sea foods, fruits, and vegetables are not good sources of thiamine.
Tissue distribution and storage
• High concentrations are found in skeletal muscles, heart, liver, kidneys, and brain.
• About 50% of the total thiamine is distributed in the muscles.
• In spinal cord & brain, the thiamine level is about double that of the peripheral nerves.
Transport
• Thiamine is carried to the liver by portal circulation; the free vitamin found in the
plasma but the coenzyme (TPP) is primary cellular component.
• The transport of thiamine into erythrocytes by a facilitated diffusion process, where as
it enters other cells by an active process, (leukocytes have a 10fold higher thiamin
concentration than erythrocytes) why.
• Thiamine transport across the blood brain barrier involves the saturable mechanism
which depends on membrane-bound phosphatase enzyme. why
11
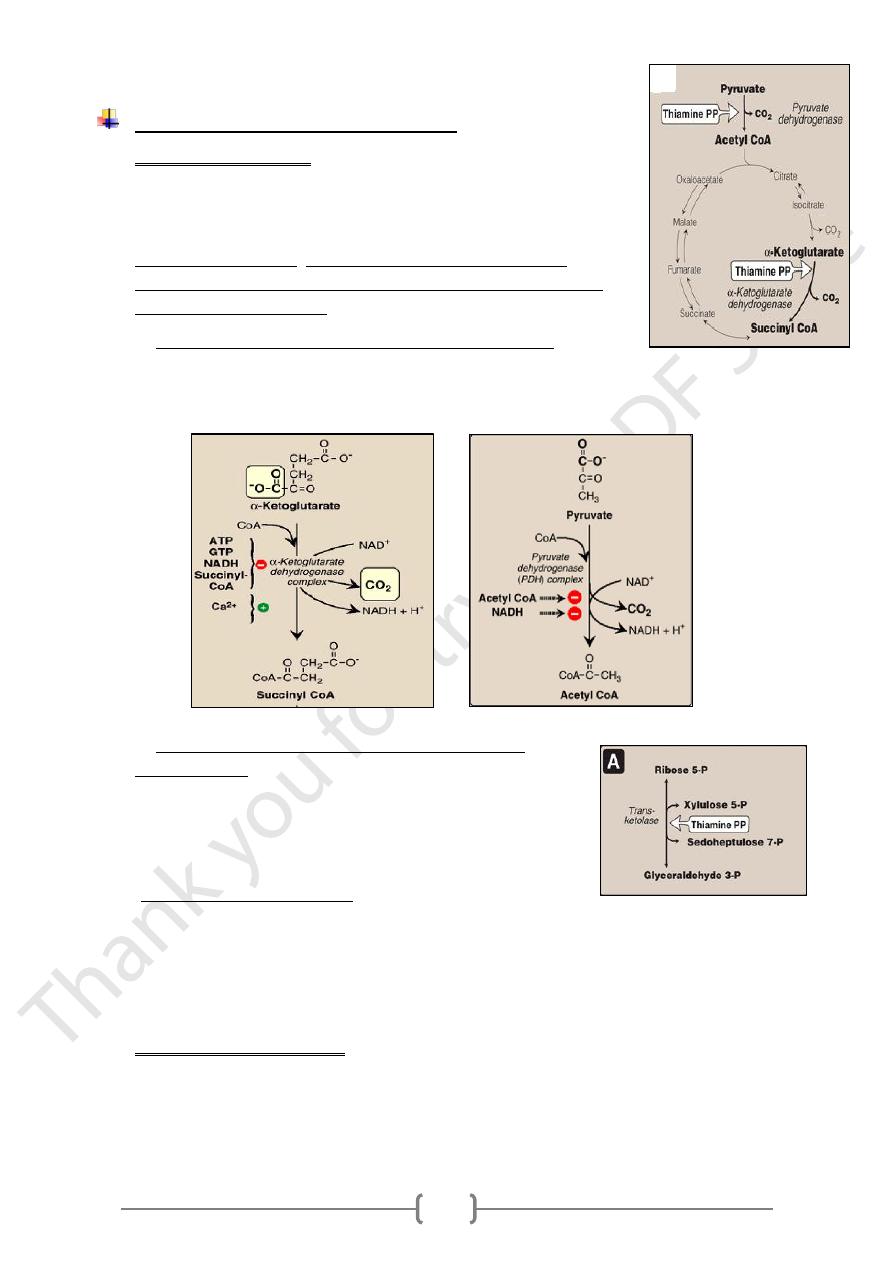
Role thiamin pyrophosphate (TPP);
1) Biochemical functions:
The oxidative decarboxylation of pyruvate and α-ketoglutarate, plays
a key role in energy metabolism of most cells, particularly in
nervous system.
activity of these two enzymes is
the
,
In thiamine deficiency
of ATP and, thus,
decreased production
decreased, resulting in a
impaired cellular function.
1. Oxidative decarboxylation by dehydrogenase complex.
A. The conversion of α-ketoglutarate to succinyl CoA release CO
2
and produce
the NADH in the TCA cycle.
B. The conversion of Pyruvate (end product of aerobic glycolysis) to acetyl coA.
2. Formation of α –ketoses catalyzed by transketolase
unit of a ketose
carbon
-
transfers the two
Transketolase
onto the aldehyde carbon of an aldose sugar (the conversion
of a ketose sugar into an aldose with 2 carbons less)
[Note: Thiamine deficiency is diagnosed by an increase in
erythrocyte transketolase activity.]
n pentose phosphate pathway
I
The metabolic significance of the pentose phosphate pathway is not to obtain
energy from the oxidation of glucose.
Its primary purpose is to generate NADPH, which serves as a hydrogen and
electron donor in reductive biosynthetic reactions, including the biosynthesis of
fatty acids
2) Neurophysiologic functions
Involve in metabolism of four types of neurotransmitters (acetylcholine,
catecholamine, serotonin, and amino acids)
Abnormal metabolism of 4 types of neurotransmitters has been reported in
thiamine deficiency.

11
Deficiency (clinical sings)
Caused by inadequate intake, decreased absorption, and defective transport of
thiamine, impaired biosynthesis of TPP, increased requirement, and increased loss of
thiamine.
result in three syndromes:
1) Chronic peripheral neuritis (beriberi): which may or may not be associated with
heart failure and edema;
2) Acute pernicious (shoshin beriberi): heart failure without peripheral neuritis;
3) Wernicke’s- Korsakoff’s syndrome :
• As consequences of failure of energy metabolism, affects neurons functions &
CNS as a result of thiamin deficiency on neurotransmitter function & nerve
conduction.
• This is because brain cells are unable to produce sufficient ATP (via the TCA
cycle) for proper function if pyruvate dehydrogenase is inactive.
Toxicity
Excess thiamine is easily cleared by the kidneys.
Although there is some evidence of toxicity from large doses given parenterally,
there is no evidence of thiamine toxicity by oral administration. why
Vitamin B6 includes six vitamer: Pyridoxine (alcohol) (principal form), pyridoxal
(aldehyde), pyridoxamine (amine) & their 5- phosphates;
They have equal biological activity.
Absorption & transport
• Intestinal mucosal cells have (pyridoxine kinase)
so that there is net accumulation of pyridoxal phosphate by metabolic trapping.
• Tissue concentrations of pyridoxal phosphate are controlled by the balance between
phosphorylation and dephosphorylation.
Reaction type
1) Transamination
The presence of the α-amino group keeps amino acids away
from oxidative breakdown, therefore removing the α-amino
group is essential for producing energy from any amino acid,
.
and is an obligatory step in the catabolism of all amino acids
2) Deamination
glutamate → α-ketoglutarate + NH
3
3) Decarboxylation
Histidine → histamine + CO
2
4) Condensation
succinyl CoA → aminolevulinic acid
Glycine +
11
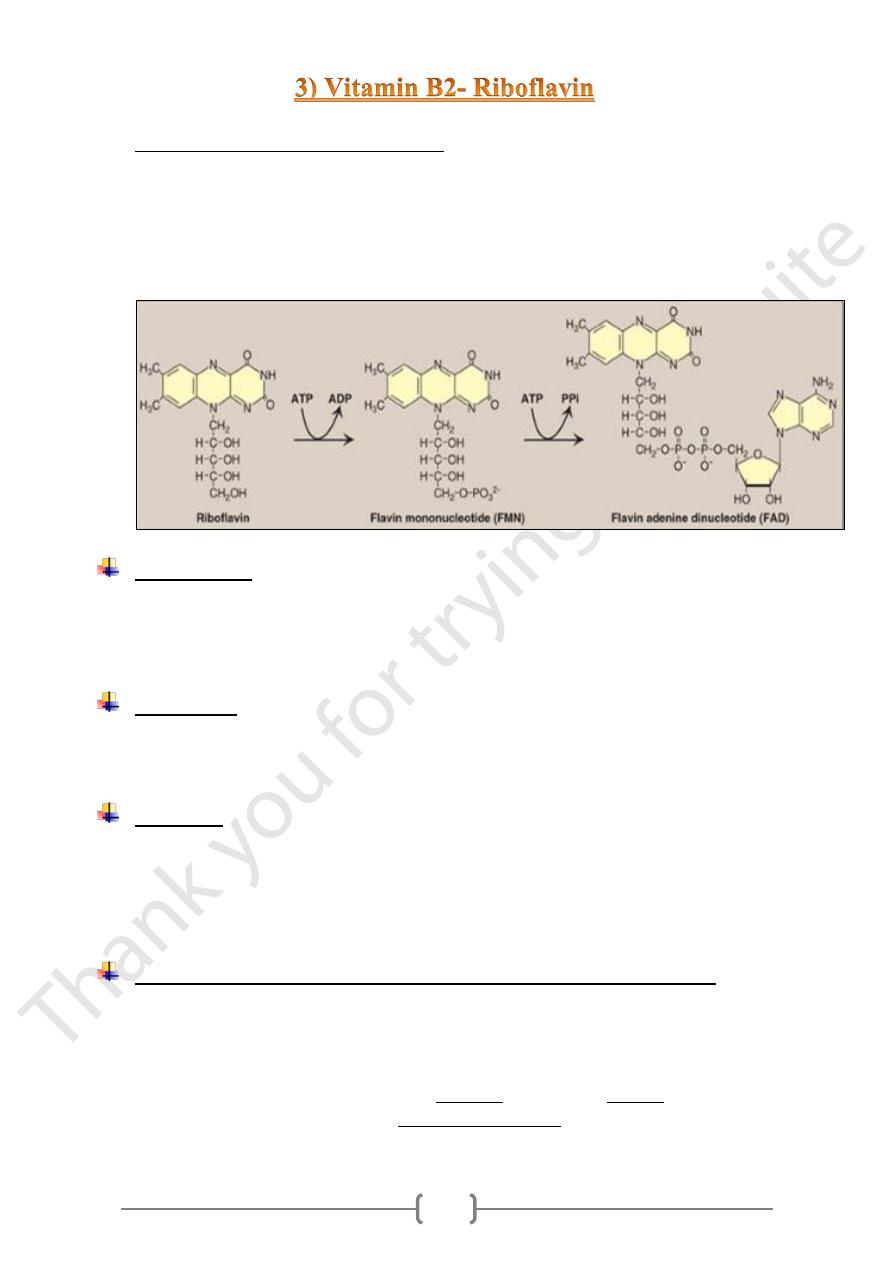
• The metabolically active coenzymes are:
a) Flavin mononucleotide (FMN). (By flavokinase )
b) Flavin adenine dinucleotide (FAD). (By FAD synthetase.)
• They are reversibly accepting two hydrogen atoms, forming FMNH2 or FADH2 &
have a central role as a coenzyme in oxidation- reduction reaction.
• The conversion of riboflavin to coenzymes occurs within the cellular cytoplasm of
most tissue.
Food sources
• Milk, dairy products, liver and green leafy vegetables.
• Intestinal bacteria synthesize riboflavin, and fecal losses of the vitamin may be 5-6
folds higher than intake.
• The vitamin is readily destroyed by UV components of sunlight (photodegradation).
Absorption
• free riboflavin , absorbed in the small intestines by a saturable transport mechanism;
• Riboflavin is phosphorylated in the intestinal mucosa by flavokinase ; this metabolic
trapping is essential for concentrative uptake of riboflavin into enterocytes .
Excretion
• Once metabolic requirements have been achieved, riboflavin are excreted by kidney,
so that any excess absorbed is excreted in urine & riboflavin does not accumulate in
the body even when oral intake is very high.
• When dietary deficiency is present, any riboflavin present in the body can be
efficiently conserved & reutilized.
With the onset of riboflavin deficiency, an adaptation occurs through:
1) Fall in the hepatic free riboflavin metabolism to undetectable levels, with a sparing of
FMN and FAD for critical metabolic functions.
2) In early stages of deficiency, there is an increase in the synthesis of reduced
glutathione,
In response to the decrease conversion of oxidized glutathione to reduced glutathione,
as a results of decreasing activity of glutathione reductase (require FAD as coenzyme)
11
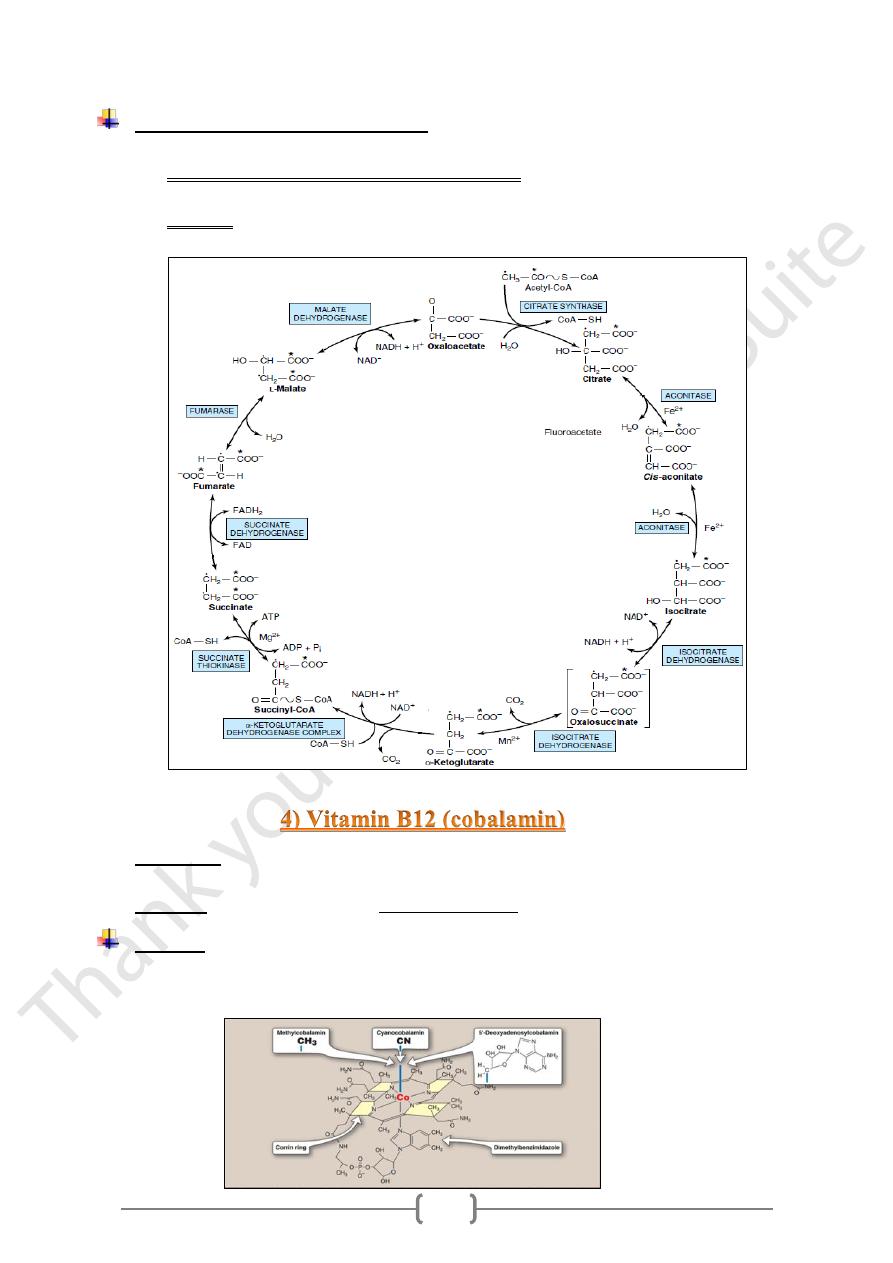
Metabolic function of Riboflavin
An electron carrier in oxidation - reduction rx.
1) In the mitochondrial electron transport chain.
FAD reversibly accepting 2H atoms, forming FADH2
2) In TCA: Succinate dehydrogenated to fumarate by succinate dehydrogenase,
producing the reduced coenzyme FADH2.
• In plasma, the major vitamen is methylcobalamin accounting for 80% of plasma
vitamin B12.
• In tissues, the major vitamen is adenosylcobalamin (70% in liver).
Sources
1) Cobalamin is synthesized by certain microorganisms in SI.
2) Diets: essentially of animal origin & not found in plant.
11
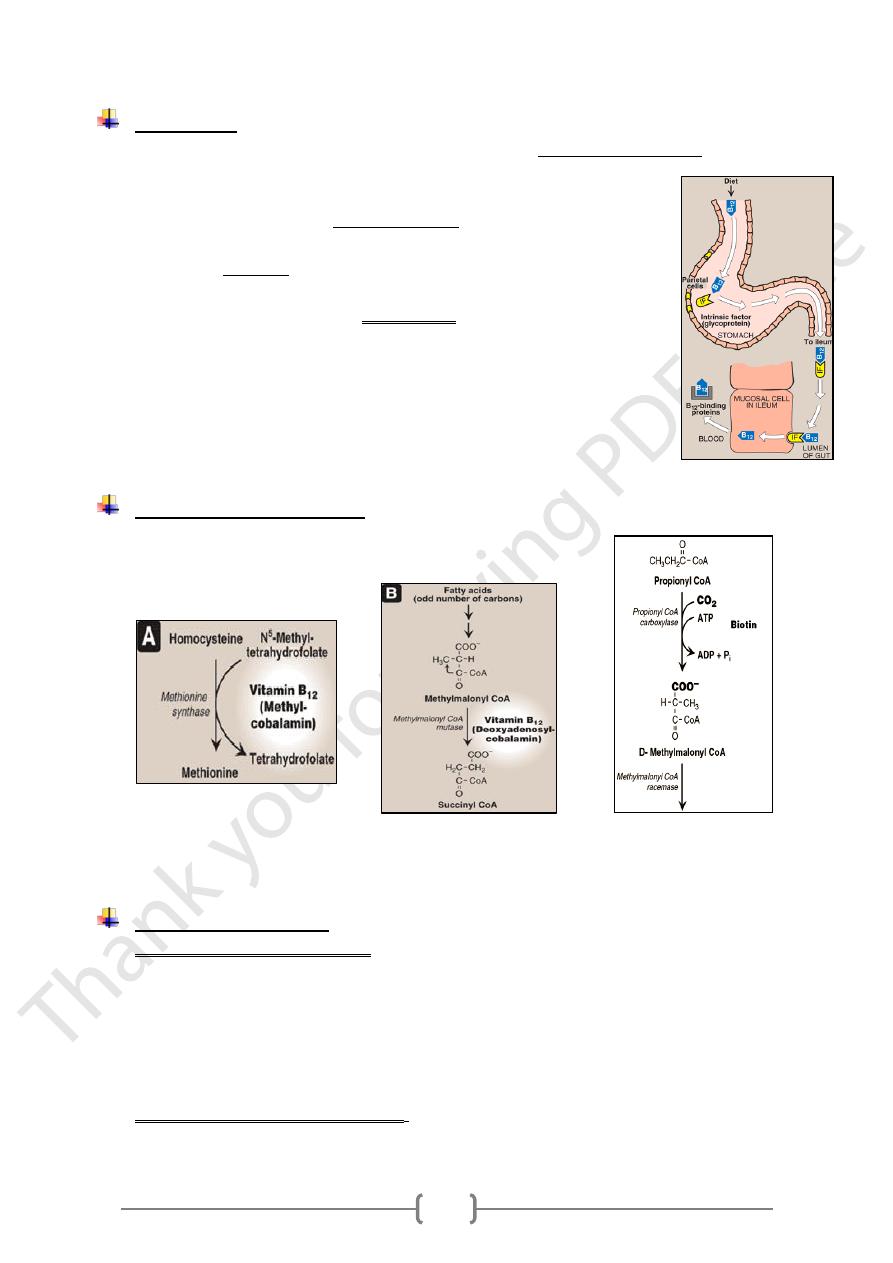
Absorption
• Vit.B12 released from food in the stomach by action of gastric acid and pepsin which
release the vitamin from protein binding and to make it available for
absorption.
• Liberated vit. B12 binds to intrinsic factor IF (is a glycoprotein produced
from gastric mucosa by parietal cells).
• vit.B12 –IF complex in small intestine, bound to receptors on mucosal
cells & enter the cells (enterocytes).
• The absorption of vitamin B12 is limited by the number of vit.B12 –IF
complex binding sites.
• Inside the enterocytes the complex is dissociated,& vit.B12 is then bound
with transcobalamin II (Tc-II) [a vitamin B12 binding protein
synthesized in the enterocytes].
• B12-TcII complex is then transported across the cell membrane & then
released into the plasma of mucosal capillaries & into portal vein.
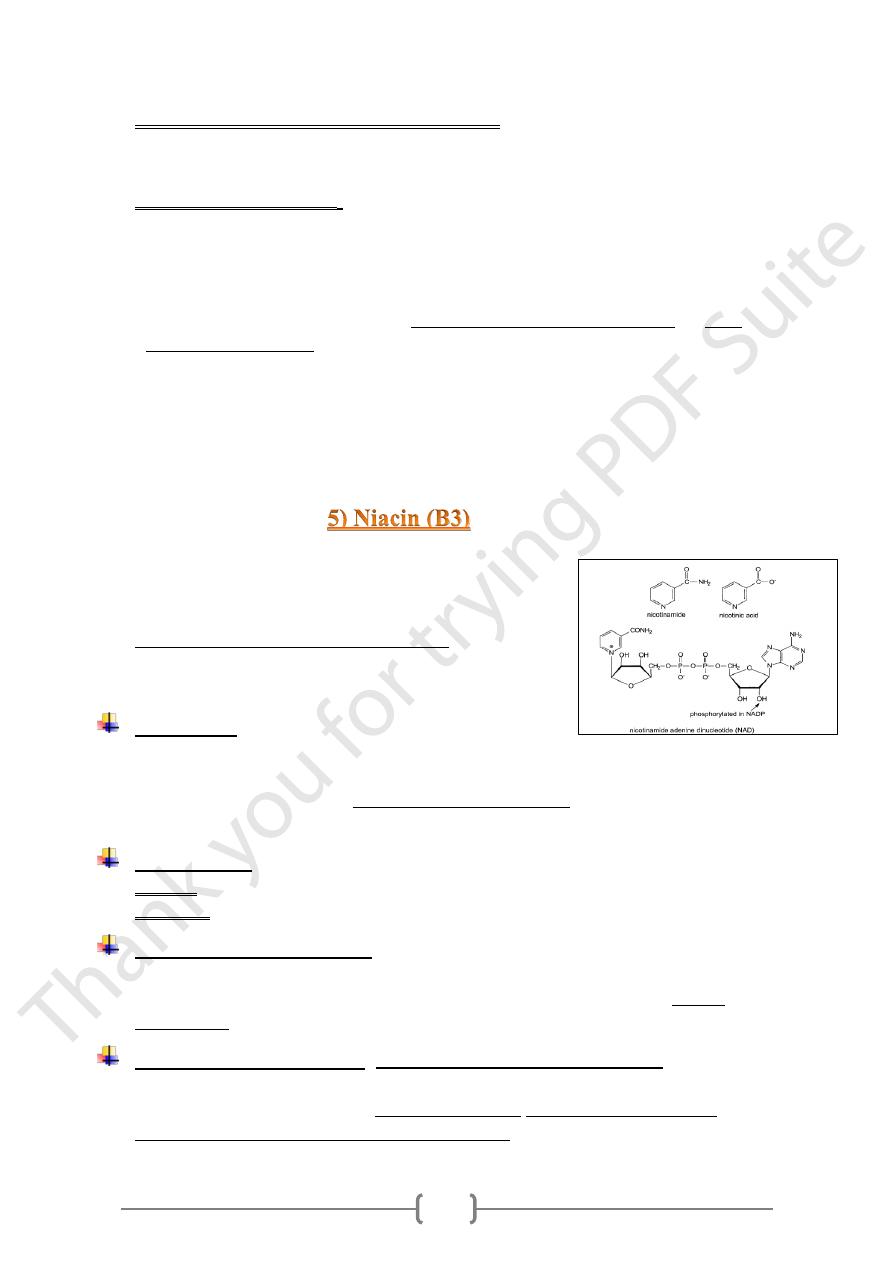
Metabolic roles of vit.B12
1) There are 2 vitamin B12-dependent enzymes:
a. Methionine synthetase, b. Methyl malonyl CoA mutase.
2) Vitamin B12 has a role in the metabolism of cyanide, forming Cyancobalamin, lead to
prevent the binding of cyanide to cytochrome oxidase.
Cobalamin deficiency
• Have many underlying causes:
a) Inadequate dietary intake.
b) Increased metabolic requirements ( pregnancy)
c) Impaired vitamin activation or utilization in tissues.
d) Pernicious anemia.
an autoimmune disease in which chronic atrophic gastritis results from antibodies
to gastric parietal cells & IF, directed against parietal cell .
• The effects of cobalamin deficiency are most pronounced in rapidly dividing cells,
such as the erythropoietic tissue of bone marrow and the mucosal cells of the intestine
11
• Vit.B12 deficiency induces 2 clinical complexes:
1) The Megaloblastic anemia.
2) Characteristic neuropathy
• The Megaloblastic anemia: due to
a) Cobalamin deficiency: respond to cobalamin therapy.
b) Folate deficiency: respond to folic acid therapy.
o Folic acid can partially reverse the hematological abnormalities of B12 deficiency
and, therefore, can mask a cobalamin deficiency.
o Rapidly dividing cells need both the N5-N10-methylene tetrahydrofolate and N10-
formyl tetrahydrofolate for the synthesis of nucleotides required for DNA
replication, resulting in the symptoms of megaloblastic anemia.
o In vitamin B12 deficiency, the utilization of the
o N5-methyl tetrahydrofolate is impaired, because the methylated form cannot be
converted directly to other forms of tetrahydrofolate, so that folate is trapped as N5-
methyl tetrahydrofolate, which accumulates & the levels of the other forms decrease.
• Niacin (B3) describes 2 compounds:
1- Nicotinic acid. 2- Nicotinamide.
They have equal biological activity.
• The biologically active forms (coenzymes):
1) Nicotinamide adenine dinucleotide (NAD).
2) Nicotinamide adenine dinucleotide phosphate (NADP)
Absorption
• Once absorbed (Passive diffusion) into the enterocytes, nicotinamide may be converted
to NAD or released into the portal circulation.
• Can be synthesized in the body from the essential amino acid tryptophan (not consider
as a vitamin, since it can be synthesized in the body )
Food content
1) In plant: Niacin is mainly in the form of nicotinic acid.
2) In animal: contain mainly NAD and NADP coenzymes.
Distribution and excretion
Liver plays an important role in the preparation of niacin for urinary excretion,
on the
products, depending
producing a variety of methylation and hydroxylation
.
niacin status
Metabolic function niacin
(NAD as cofactors in Redox reactions)
NAD coenzymes reactions are the basis for hydrogen transfer reactions (NAD+ is
oxidative decarboxylation
glycolytic reactions,
reduced to NADH), important in
.
of pyruvate & oxidation of acetate in the TCA cycle
11
Pellagra (disease of tryptophan & niacin deficiency).
• Characterized by a photosensitive dermatitis, like severe sunburn, typically with a
butterfly-like pattern of distribution over the face, affecting all parts of the skin that
are exposed to sunlight.
• Similar skin lesions may also occur in areas not exposed to sunlight, but subject to
pressure, such as the knees, elbows & ankles.
Food sources
• Liver, kidney, yeast, egg yolk .
• About 85% of dietary Pantothenic acid is as CoA.
Deficiency (Why its deficiency has not been reported?)
Because, its widely distributed in foods, and it is absorbed throughout the small
intestine so that deficiency has not been reported in human except in specific
depletion studies.
Metabolic role
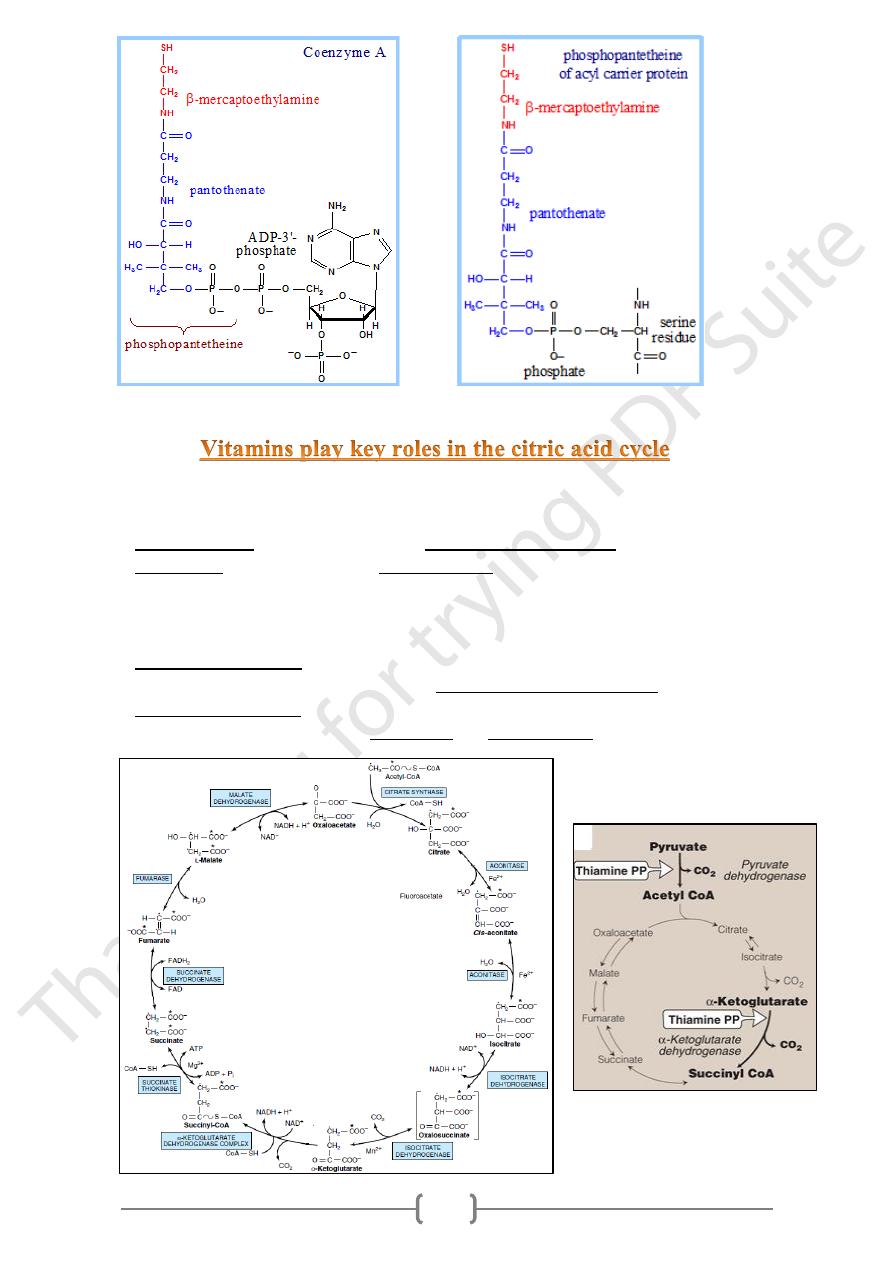
1) Part of Co-enzyme A:
• CoA is the major carrier of acyl groups in an acyl transfer reactions ex:
a) In ketogenesis. b) In β-oxidation of FFA.
• Coenzyme A is synthesized in a 5-step process
from Pantothenic acid & cysteine.
• Coenzyme A contains a thiol group that carries
acyl compounds as activated thiol esters
2) Prosthetic group of Fatty acid Synthase :
Phosphopantetheine is covalently linked via a phosphate ester to a serine OH of
the acyl carrier protein domain of Fatty Acid Synthase.
Fatty acid synthase is a multi-enzyme protein that catalyzes
fatty acid synthesis
.
It is not a single
enzyme
but a whole enzymatic system composed of two
identical multifunctional
polypeptides
, in which
substrates
are handed from one
functional domain to the next
11
Four of the B vitamins are essential in the citric acid cycle and therefore in
energy-yielding metabolism:
1) Riboflavin (B2): FAD a cofactor in the succinate dehydrogenase;
2) Niacin (B3): NAD coenzyme for 3 dehydrogenas
1. isocitrate dehydrogenase,
2. α-ketoglutarate dehydrogenase,
3. malate dehydrogenase;
3) Thiamin (vitamin B1):
reaction.
ketoglutarate dehydrogenase
-
α
in the
coenzyme for decarboxylation
The
4) Pantothenic acid (B5): as part of coenzyme A, the cofactor attached to “active”
carboxylic acid residues such as acetyl-CoA and succinyl-CoA.

12
Food source
• The major source of folate is green vegetables, bread, citrus fruits and juices, meat,
and fish.
• Reduced folates are less stable than folic acid and large losses in food folate can occur
during food preparation such as heating.
• Most dietary folate is metabolized to 5-methyl-tetrahydrofolate mono glutamate
during its passage through the intestinal mucosa.
• This metabolism in depend on the amount of folate ingested, so that when dose of
folic acid is high , most of the folate appears unchanged in the portal circulation.
• A folate binding receptor (folate receptor) has been detected in the intestine, which
has been shown to mediate endocytosis of folate in the kidney and other tissues.
Transport
Methyl-tetrahydrofolate (the main vitamer) from the intestinal mucosa circulates
in plasma protein bound to albumin for uptake by extrahepatic tissues.
Source of folate in RBCs ?
• RBC s contain higher levels of folate (a several-hundred-fold) higher than plasma,
incorporated during erythropoeisis rather than taken up from the circulation, as
polyglutamates bound to hemoglobin because mature red cells do not transport folate
and their folate stores are formed during erythropoiesis and are retained due to
binding to hemoglobin, through the life span of the human RBC.
• Red cell folate levels are often used as a measure of long-term folate status.
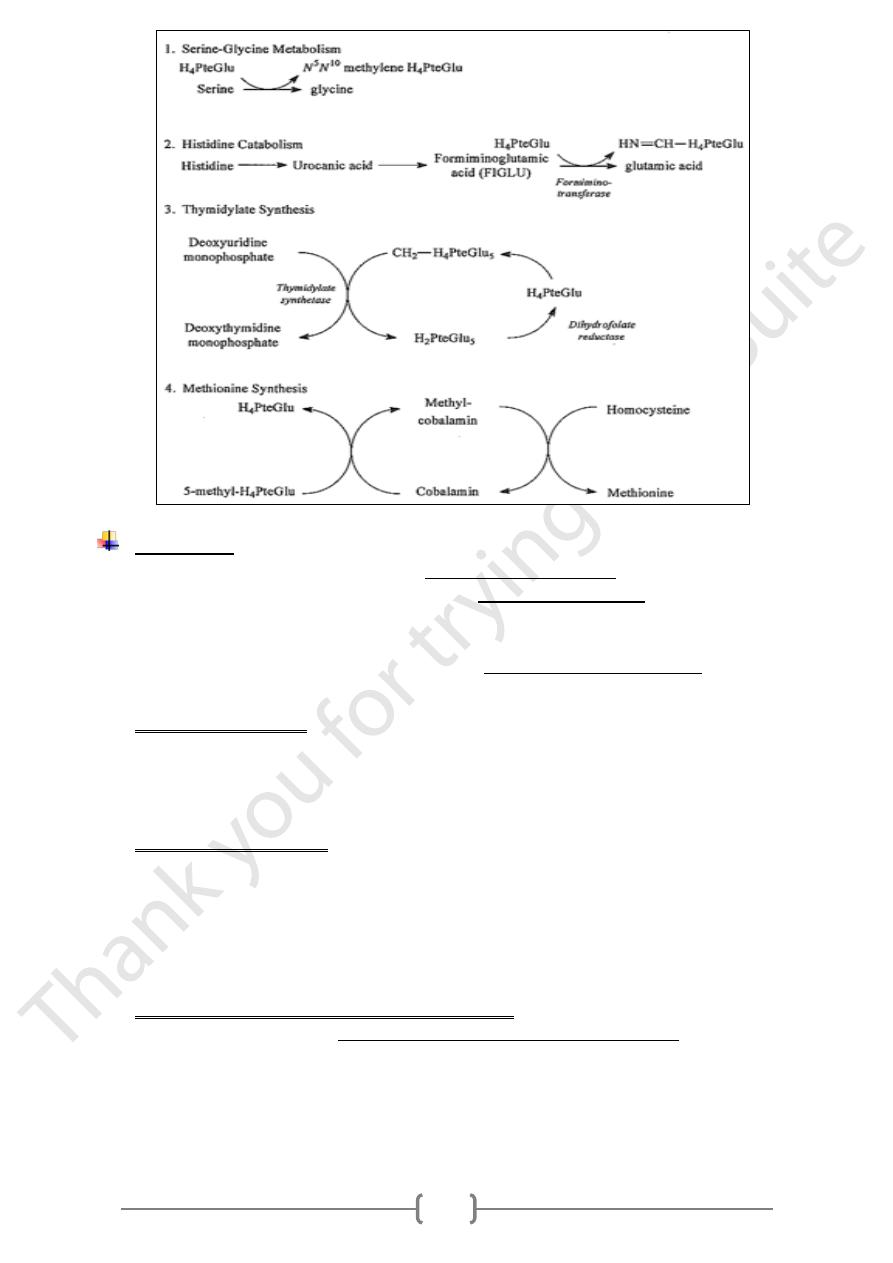
Biochemical roles:
• Folic acid is itself not biologically active, but its biological importance is due to
tetrahydrofolate
and other derivatives after its conversion to dihydrofolate in the liver.
• Folates act serve as acceptors and donors of one-carbon units in a variety of
reactions in amino acid and nucleotide metabolism.
• The C-3 of serine is the major source of one-carbon units for folate metabolism.
• Other sources include formate, which is derived from serine metabolism in the
mitochondria, and the C-2 of histidine.
11
Deficiency:
• Folate deficiency symptoms are usually due to a dietary deficiency.
• The classical symptom of folate deficiency is a Megaloblastic anemia that cannot be
distinguished from that caused by vit. B12 deficiency. WHY?
• Many of the clinical effects of folate deficiency can be explained by the metabolic
role of folate coenzymes in pathways leading to nucleotide synthesis of DNA .
• Because of this role, symptoms of deficiency are expressed in rapidly growing tissues.
1) Megaloblastic Anemia
Characterized by large immature red blood cells, it is a reflection of disturbed
DNA synthesis in blood cells characterized by enlarged red cells and
hypersegmentation of the nuclei with reduced cell number, the condition is usually
detected clinically by the anemia.
2) Vitamin B12 interactions
• Vitamin B12 deficiency is identical to that observed in folate deficiency, these
vitamins are cofactors for the methionine synthase reaction [a block in this enzyme
lead to accumulation of folate in form of 5-CH3- THF which cannot be
metabolized by other mechanism].
• this would result in the trapping of folate in a nonfunctional form with a associated
reduction in the level of other folate coenzymes required for other reactions.
Hyperhomocysteinemia and Folate concentration
Genetic disease includes methylenetetrahydrofolate reductase deficiency.
Fasting homocysteine levels have been inversely correlated with both plasma
folate levels and food folate intake.
Increased folate intake lowers the mean homocysteine of groups, and the lowering
effect is greatest in subjects with the highest plasma homocysteine levels.
11
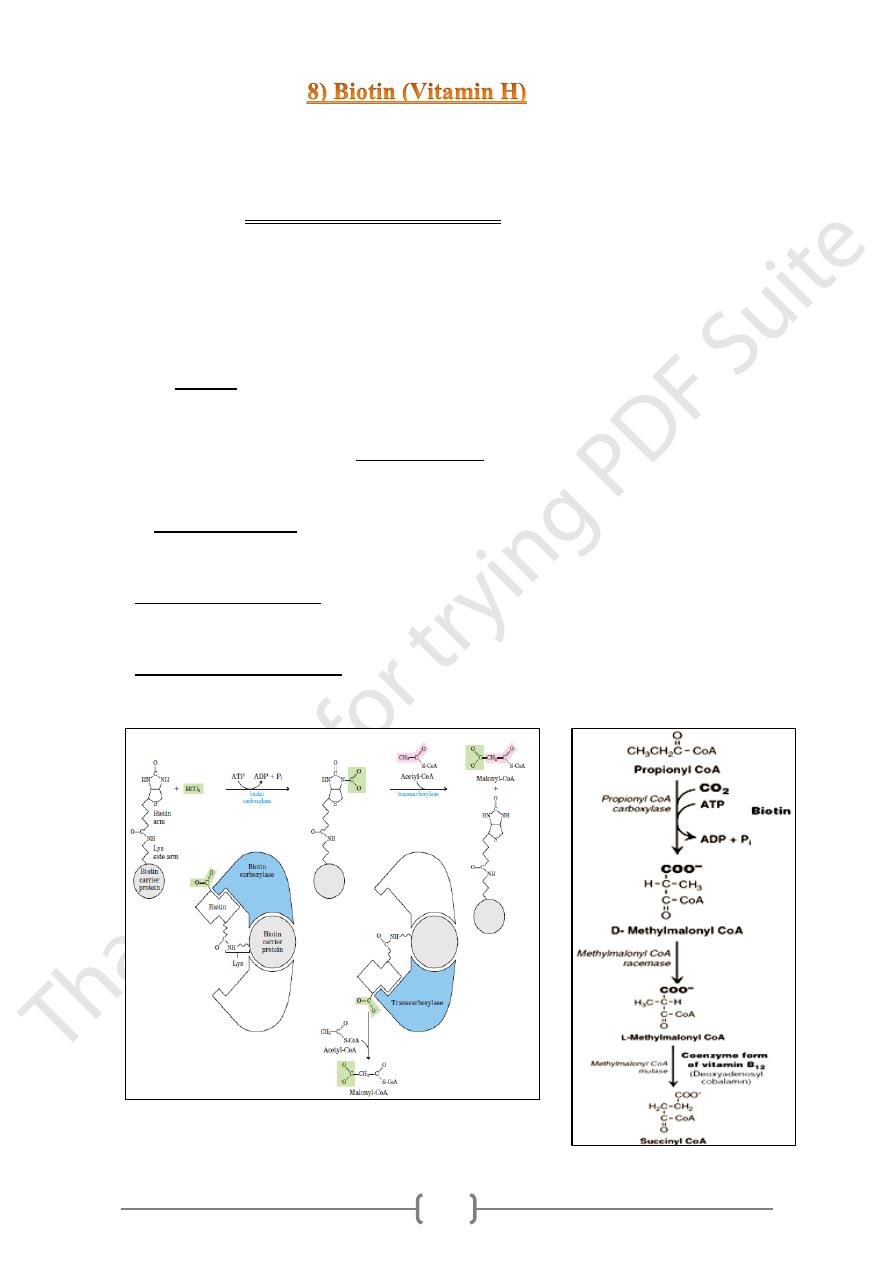
• Biotin is a coenzyme in carboxylation reactions, in which it serves as a carrier of
activated carbon dioxide.
• The biotin-dependent enzymes (biotin-dependent carboxylase) are cytosolic and
mitochondrial. They catalyze a two-step reaction:
1) enzyme-biotin + ATP + HCO3− →enzyme-biotin-COOH + ADP + Pi
2) Enzyme-biotin-COOH+ acceptor → enzyme-biotin + acceptor-COOH.
• Biotin is linked to the enzyme by an amide bond between the terminal carboxyl of the
biotin side chain and the Enz-amino group of a lysine residue.
• This combination act as a long flexible arm that allows the biotin ring to translocate
between the 2 active sites.
• Only d-biotin is enzymatically active.
o It's bound covalently to enzymes by the formation of a peptide bond between the
carboxyl group of the side chain and the ε-amino group of a lysine residue.
o This combination act as a long flexible arm that allows the biotin ring to
translocate between the 2 active sites.
1- Biotin carboxylase,
Which activates CO2 by attaching it to a nitrogen in the biotin ring in an ATP-
dependent reaction.
2- Biotin carrier protein:
Has the long, flexible biotin arm carries the activated CO2 from the biotin
carboxylase site to the transcarboxylase active site.
3- Biotin transcarboxylase, which transfers activated CO2 from biotin to acetyl-
CoA, producing malonyl-CoA
11
Defeciency
• Biotin deficiency does not occur naturally because the vitamin is widely distributed in
food.
• Also, a large percentage of the biotin requirement in humans is supplied by intestinal
bacteria.
• Consequences of def.
:
all in deficiency, resulting in
f
dependent carboxylases
-
biotin
The activities of
1) Impaired gluconeogenesis, with accumulation of lactate, pyruvate, and alanine.
2) Impaired lipogenesis, with accumulation of acetyl CoA.
Dehydroascorbic acid and dehydroascorbate are the oxidized form of ascorbic acid,
both of which have vitamin activity because they can be reduced to ascorbate.
Sources
Ascorbic acid occurs in significant amounts.
1) In plants: In vegetables, and fruits.
2) In animal organs: such as liver, kidney, and brain.
Absorption &Tissue uptake of Vitamin C
• Ascorbic acid absorbed by
1) Sodium-dependent active transport at low concentration.
2) Simple diffusion at high concentrations.
• Absorption of vitamin C depend largely on the amount ingested, so that 90% of
dietary ascorbate is absorbed at intakes up to about 100 mg / day; the absorption
decreased to 50% of a 1.5-g dose & to 25% of a 6-g dose, and 16% of a 12g dose .
• The absorbed ascorbic acid moves rapidly from the intestinal cell into blood by
facilitated diffusion.
• Ascorbate uptake by cells is mediated by sodium-dependent transporters SVCT
1& SVCT 2.
• Dehydroascorbate uptake mediated by glucose transportes GLUT 1, 2 &3.
• Many cells accumulate ascorbic acid against a concentration gradient (up to 40-fold
higher than plasma concentrations).
• The normal physiological conc. of glucose will inhibit uptake of dehydroascorbate.
• Functional signs of deficiency may develop in poorly controlled diabetes mellitus,
despite an adequate intake and adequate plasma concentrations, suggesting that
11
hyperglycemia and insulin insensitivity and thus uptake of dehydroascorbate in
competition with glucose.
• Some of the adverse effects of poor glycemic control in diabetes mellitus may be
related to this impairment of vitamin C uptake, and supplements of vitamin C maybe
beneficial to decrease diabetic complication.
• There is no specific storage organ for ascorbate; the only tissues showing a significant
conc. of the vitamin are the adrenal and pituitary glands.
Metabolic functions
A. Ascorbate involved in
1) Synthesis of adrenal hormones via dopamine B-hydroxylase.
2) Biosynthesis of corticosteroids & aldosterone.
3) Folate metabolism & leukocyte functions.
4) Hydroxylation of cholesterol in the formation of bile acids.
B. Role of Ascorbate in iron absorption & metabolism
Ascorbic acid in the intestinal lumen will both maintain iron in the reduced state
and also chelate it, thus increasing absorption.
A dose of vitamin C taken together with a meal increases the absorption of iron
65%.
Ascorbate is also active in the reduction of Fe3+ in the plasma transport
protein (transferrin) to Fe2+ for storage in the liver or for heme synthesis.
C. Antioxidant roles of Ascorbate
1) A radical-trapping antioxidant, reacting with superoxide and a proton to yield
hydrogen peroxide.
2) With the hydroxyl radical to yield water.
Deficiency – (Scurvy)
Most of the clinical signs of scurvy can be accounted by effects of deficiency on
.
impaired proline and lysine hydroxylase activity
collagen synthesis as a result of
Characterized by hypercholesterolemia & fatigue.
Clinical signs of scurvy such as delayed in wound healing.
