Unit 4 - Infectious disease
37
Lecture 1+2+3 - Protozoal Infections
Systemic protozoal infections
Malaria
Malaria in humans is caused by Plasmodium falciparum,
P. vivax, P. ovale, P. malariae .
It is transmitted by the bite of female anopheline
mosquitoes and occurs throughout the tropics and
subtropics at altitudes below 1500 metres. Recent
estimates have put an episodes of clinical malaria at 515
million cases per year, with two-thirds of these occurring
in sub-Saharan Africa, especially amongst children and
pregnant women. P. falciparum has now become resistant
to chloroquine and sulfadoxine-pyrimethamine, initially in
South-east Asia and now throughout Africa.
Pathogenesis
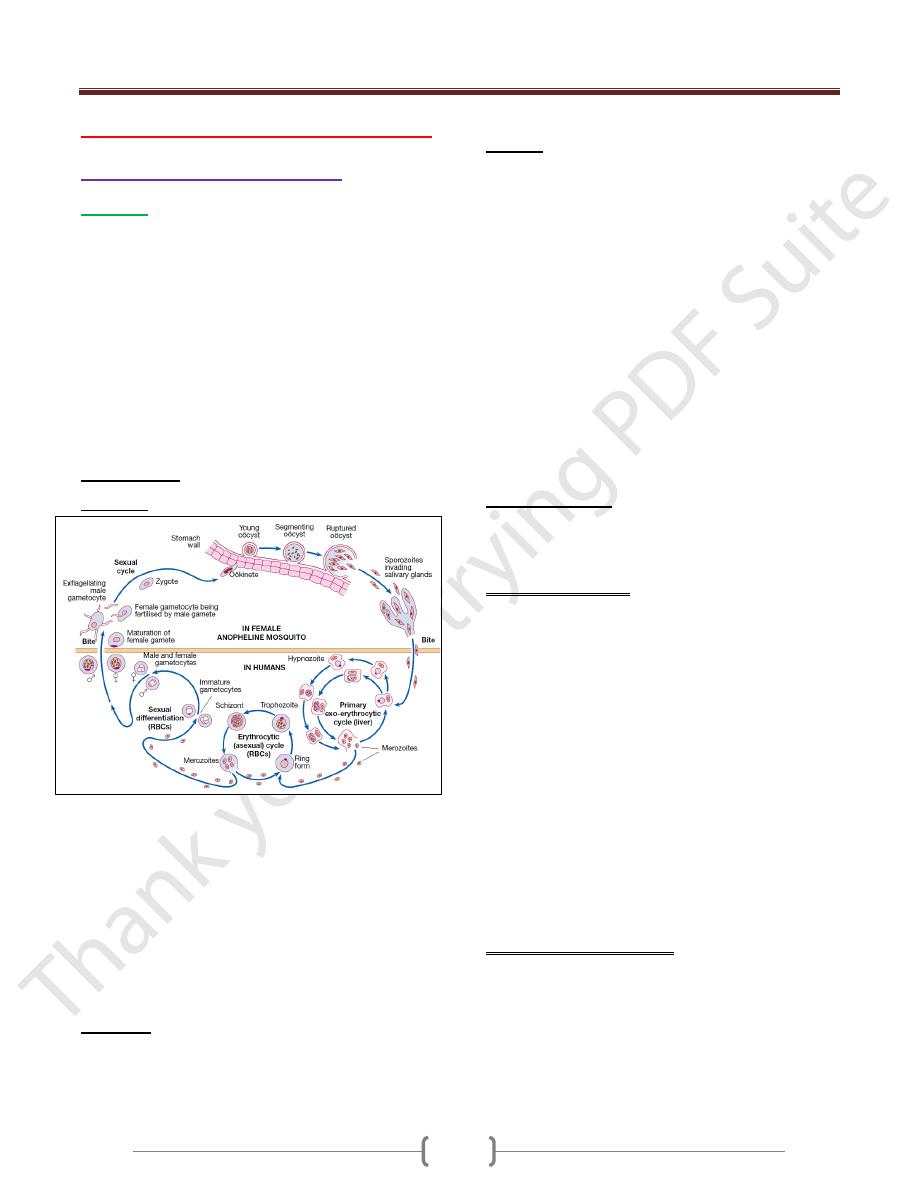
Life cycle
P. vivax and P. ovale may persist in liver cells as dormant
forms, hypnozoites, capable of developing into merozoites
months or years later. Thus the first attack of clinical
malaria may occur long after the patient has left the endemic
area & the disease may relapse after treatment if drugs that
kill only the erythrocytic stage of the parasite are given.
P. falciparum and P. malariae have no persistent exo-
erythrocytic phase but recrudescence of fever may result
from multiplication of parasites in red cells which have
not been eliminated by treatment and immune processes.
Pathology
Red cells infected with malaria are prone to hemolysis.
This is most severe with P. falciparum, which invades red
cells of all ages but especially young cells.
P. vivax and P. ovale invade reticulocytes.
P. malariae normoblasts, so that infections remain lighter.
Anaemia may be profound and is worsened by dys-
erythropoiesis, splenomegaly and depletion of folate stores.
In P. falciparum malaria, red cells containing trophozoites
adhere to vascular endothelium in post-capillary venules
in brain, kidney, liver, lungs and gut. The vessels become
congested, resulting in widespread organ damage which is
exacerbated by rupture of schizonts, liberating toxic and
antigenic substances. P. falciparum has influenced human
evolution, with the appearance of protective mutations
such as sickle-cell, thalassaemia, G6PD deficiency and
HLA-B53. P. falciparum does not grow well in red cells
that contain haemoglobin F, C or especially S.
Haemoglobin S heterozygotes (AS) are protected against
the lethal complications of malaria.
P. vivax cannot enter red cells that lack the Duffy blood
group; therefore many West Africans and African-
Americans are protected.
Clinical features
The clinical features of malaria are non-specific and the
diagnosis must be suspected in anyone returning from an
endemic area that has features of infection.
P. falciparum infection: This is the most dangerous of the
malarias and patients are either ‘killed or cured’. The
onset is often insidious, with malaise, headache and
vomiting. Cough and mild diarrhea are also common. The
fever has no particular pattern. Jaundice is common due to
haemolysis and hepatic dysfunction. The liver and spleen
enlarge and may become tender. Anaemia develops
rapidly, as does thrombocytopenia. A patient with
falciparum malaria, apparently not seriously ill, may
rapidly develop dangerous complications.
Cerebral malaria is manifested by confusion, seizures or
coma, usually without localizing signs. Children die rapidly
without any special symptoms other than fever. Immunity
is impaired in pregnancy & the parasite can preferentially
bind to a placental protein known as chondroitin sulphate A.
Abortion and intrauterine growth retardation from
parasitisation of the maternal side of the placenta are frequent
Previous splenectomy increases the risk of severe malaria.
P. vivax and P. ovale infection
In many cases the illness starts with several days of
continued fever before the development of classical bouts
of fever on alternate days.
Fever starts with a rigor.
The patient feels cold & the temperature rises to about 40 °C.
After half an hour to an hour the hot or flush phase begins.
It lasts several hours and gives way to 3- 3- profuse
perspiration and a gradual fall in temperature. The cycle is
Unit 4 - Infectious disease
38
repeated 48 hours later. Gradually the spleen and liver
enlarge and may become tender. Anaemia develops slowly.
Relapses are frequent in the first 2 years after leaving the
malarious area & infection may be acquired from blood
transfusion.
P. malariae infection
This is usually associated with mild symptoms and bouts
of fever every third day. Parasitaemia may persist for
many years with the occasional recrudescence of fever, or
without producing any symptoms. Chronic P. malariae
infection causes glomerulonephritis and longterm
nephrotic syndrome in children.
Investigations
1) Giemsa-stained thick and thin blood films should be
examined whenever malaria is suspected.
In the thick film erythrocytes are lysed, releasing all
blood stages of the parasite. This, as well as the fact that
more blood is used in thick films, facilitates the diagnosis
of low level parasitaemia. A thin film is essential to
confirm the diagnosis, to identify the species of parasite
and, in P. falciparum infections, to quantify the parasite
load (by counting the percentage of infected erythrocytes).
P. falciparum parasites may be very scanty, especially in
patients who have been partially treated. With P.
falciparum, only ring forms are normally seen in the early
stages (see Fig. 13.31); with the other species all stages of
the erythrocytic cycle may be found.
Gametocytes appear after about 2 weeks, persist after
treatment and are harmless, except that they are the source
by which more mosquitoes become infected.
2) Immunochromatographic tests for malaria antigens,
such as OptiMal® (which detects the Plasmodium lactate
dehydrogenase of several species) and ParasightF®
(which detects the P. falciparum histidine-rich protein 2),
are extremely sensitive and specific for falciparum.
malaria but less so for other species. They should be used
in parallel with blood film examination but are especially
useful where the microscopist is less experienced in
examining blood films.
3) DNA detection (PCR) is used mainly in research and is
useful for determining whether a patient has a
recrudescence of the same malaria parasite or a
reinfection with a new parasite.
Management
Mild P. falciparum malaria
Since P. falciparum is now resistant to chloroquine and
sulfadoxine- pyrimethamine (Fansidar) almost worldwide,
an artemisinin-based treatment is recommended. Co-
artemether (CoArtem® or Riamet®) contains artemether
and lumefantrine and is given as 4 tablets at 0, 8, 24, 36,
48 and 60 hours. Alternatives are quinine by mouth (600
mg of quinine salt every 8 hours for 5–7 days), together
with or followed by either doxycycline (200 mg once
daily for 7 days) or clindamycin (450 mg every 8 hours
for 7 days) or atovaquone-proguanil (Malarone®, 4
tablets once daily for 3 days).
Doxycycline & artemether should be avoided in pregnancy.
Complicated P. falciparum malaria
Severe malaria should be considered as a medical emergency.
Management includes
1) early and appropriate antimalarial chemotherapy,
2) active treatment of complications,
3) Correction of fluid, electrolyte and acid–base balance,
and avoidance of harmful ancillary treatments.
4) The treatment of choice is intravenous artesunate , as
soon as the patient has recovered sufficiently to
swallow tablets, oral artesunate
5) Quinine salt can also be used.
Management of non-falciparum malaria
P. vivax, P. ovale and P. malariae infections should be
treated with oral chloroquine: 600 mg chloroquine base
followed by 300 mg base in 6 hours, then 150 mg base
12- hourly for 2 more days. ‘radical cure’ is now achieved
in most patients with P. vivax or P. ovale malaria using a
course of primaquine (15 mg daily for 14 days), which
destroys the hypnozoite phase in the liver.
Note:
Development of a fully protective malaria vaccine
is still some way off, which is not surprising considering
that natural immunity, is incomplete and not long-lived.
There is, however, some evidence that vaccination can
reduce the incidence of severe malaria in populations.
Trial vaccines are being evaluated in Africa.
African trypanosomiasis (sleeping sickness)
African sleeping sickness is caused by trypanosomes
conveyed to humans by the bites of infected tsetse flies,
and is unique to sub-Saharan Africa.
Trypanosoma brucei gambiense trypanosomiasis has a
wide distribution in West and Central Africa.
T. brucei rhodesiense trypanosomiasis is found in parts of
East and Central Africa, where it is currently on the increase.
Clinical features
A bite by a tsetse fly is painful and commonly becomes
inflamed, but if trypanosomes are introduced, the site may

Unit 4 - Infectious disease
39
again become painful and swollen about 10 days later
(‘trypanosomal chancre’) and the regional lymph nodes
enlarge (‘Winterbottom’s sign’). Within 2–3 weeks of
infection the trypanosomes invade the blood stream. The
disease is characterised by an early haematolymphatic
stage and a late encephalitic stage in which the parasite
crosses the blood–brain barrier and chronic
encephalopathy develops.
Rhodesiense infections
In these infections the disease is more acute and severe
than in gambiense infections, so that within days or a few
weeks the patient is usually severely ill and may have
developed pleural effusions and signs of myocarditis or
hepatitis. There may be a petechial rash. The patient may
die before there are signs of involvement of the CNS. If
the illness is less acute, drowsiness, tremors and coma
develop.
Gambiense infections
The distinction between early and late stages may not be
apparent in gambiense infections. The disease usually
runs a slow course over months or years, with irregular
bouts of fever and enlargement of lymph nodes. The
spleen and liver may become palpable. After some
months without treatment, the CNS is invaded. This is
shown clinically by headache and changed behaviour,
blunting of higher mental functions, insomnia by night
and sleepiness by day, mental confusion and eventually
tremors, pareses, wasting, coma and death.
Investigations
1) Thick & thin blood films stained will reveal trypanosomes.
2) The Concentration methods include buffy coat
microscopy & miniature anion exchange chromatography.
3) Rapid card agglutination trypanosomiasis test (CATT) for
antibody detection.
4) If the CNS is affected, CSF analysis which reveals
pleocytosis, increased protein, pressure and diminished
glucose. Presence of high level IgM in CSF is suggestive
for trypanosomiasis.
Management
Before CNS involvement, intravenous suramin, for
rhodesiense infections.
For gambiense infections, intramuscular or intravenous
pentamidine.
Once the nervous system is affected, treatment with
melarsoprol is effective for both East &West African
diseases.
American trypanosomiasis (Chagas’ disease)
The cause is Trypanosoma cruzi, transmitted to humans
from the faeces of a reduviid (triatomine) bug in which
the trypanosomes have a cycle of development before
becoming infective to humans.
There are 2 phases of the disease: acute & chronic phases.
Investigations
1) T. cruzi is easily detectable in a blood film in the acute illness
2) In chronic disease it may be recovered in up to 50% of
cases by xenodiagnosis,.
3) Parasite DNA detection by PCR in the patient’s blood is a
highly sensitive method for documentation of infection
and, in addition, can be employed in faeces of bugs used
in xenodiagnosis tests to improve sensitivity.
4) Antibody detection is also highly sensitive (99%).
Management
Parasiticidal agents are used to treat the acute phase,
congenital disease and early chronic phase (within 10
years of infection).
1) Nifurtimox is given orally.
2) Benznidazole is an alternative.
3) Specific drug treatment of the chronic form is now
increasingly favoured, but in the cardiac or digestive
‘mega’ diseases it does not reverse established tissue
damage. Surgery may be needed.
Toxoplasmosis
Toxoplasma gondii is an intracellular parasite
Transmission
1- Via oöcyst- contaminated soil, salads and vegetables.
2- Ingestion or tasting of raw or undercooked meats
containing tissue cysts. Sheep, pigs and rabbits are the
most common meat sources.
Outbreaks of toxoplasmosis have been linked to the
consumption of unfiltered water.
Clinical features
1) In most immunocompetent individuals, including children
and pregnant women, the infection goes unnoticed. In
approximately 10% of patients it causes a self-limiting
illness, most common in adults aged 25–35 years.
2) The most common presenting feature is painless
lymphadenopathy, either local or generalised. In
particular, the cervical nodes are involved, but
mediastinal, mesenteric or retroperitoneal groups may be

Unit 4 - Infectious disease
40
affected. The spleen is seldom palpable. Most patients
have no systemic symptoms, but some complain of
malaise, fever, fatigue, muscle pain, sore throat and
headache. Complete resolution usually occurs within a
few months, although symptoms and lymphadenopathy
tend to fluctuate unpredictably and some patients do not
recover completely for a year or more.
3) Very infrequently, patients may develop encephalitis,
myocarditis, polymyositis, pneumonitis or hepatitis.
4) Retinochoroiditis is nearly always the result of congenital
infection but has also been reported in acquired disease.
Congenital toxoplasmosis
Acute toxoplasmosis, mostly subclinical, affects 0.3– 1%
of pregnant women, with an approximately 60%
transmission rate to the fetus which increases with
increasing gestation.
Seropositive females infected 6 months before conception
have no risk of fetal transmission.
Congenital disease affects approximately 40% of infected
fetuses, and is more likely and more severe with infection
early in gestation. Many fetal infections are subclinical at
birth but long term sequelae include retinochoroiditis,
microcephaly and hydrocephalus.
Investigations
1) Immunocompromised patients diagnosis often requires
direct detection of parasites.
2) Serology is often used in immunocompetent individuals.
a. The Sabin–Feldman dye test (indirect fluorescent
antibody test), which detects IgG antibody. Recent
infection is indicated by a fourfold or greater increase in
titre when paired sera are tested in parallel. Peak titres of
1/1000 or more are reached within 1–2 months of the
onset of infection, and the dye test then becomes an
unreliable indicator of recent infection. The detection of
significant levels of Toxoplasma-specific IgM antibody
may be useful in confirming acute infection. False
positives or persistence of IgM antibodies for years after
infection make interpretation difficult; however, negative
IgM antibodies virtually rule out acute infection.
b. During pregnancy it is critical to differentiate between
recent & past infection; the presence of high-avidity IgG
antibodies excludes infection acquired in the preceding 3–
4 months.
3) If necessary, the presence of Toxoplasma organisms in a
lymph node biopsy can be sought by staining sections
histochemically with T. gondii antiserum, or by the use of
PCR to detect Toxoplasma-specific DNA.
Management
1) In immunocompetent subjects uncomplicated toxoplasmosis
is self-limiting and responds poorly to antimicrobial therapy.
2) Treatment with pyrimethamine, sulfadiazine and folinic
acid is therefore usually reserved for rare cases of severe
or progressive disease, and for infection in
immunocompromised patients.
3) In a pregnant woman with an established recent infection,
spiramycin (3 g daily in divided doses) should be given
until term. Once fetal infection is established, treatment
with sulfadiazine and pyrimethamine plus calcium
folinate is recommended (spiramycin does not cross the
placental barrier).
Leishmaniasis
Leishmaniasis is caused by unicellular flagellate
intracellular protozoa belonging to the genus Leishmania
(order Kinetoplastidae). There are 21 leishmanial species
which cause several diverse clinical syndromes, which
can be placed into three broad groups:
• Visceral leishmaniasis (VL, kala-azar)
• Cutaneous leishmaniasis (CL)
• Mucosal leishmaniasis (ML).
Epidemiology and transmission
Zoonotic transmission of parasites from animals (chiefly
canine and rodent reservoirs) to humans through
phlebotomine sandfly vectors, Humans are the only
known reservoir (anthroponotic) in major VL foci in India
and for transmission of leishmaniasis between injection
drug-users.
Visceral leishmaniasis (VL, kala-azar)
VL is caused by the protozoon Leishmania donovani
complex including:
1- L. donovani, 2- L. infantum. 3- L. chagasi.
India, Sudan, Bangladesh and Brazil account for 90% of
cases of VL, while other affected regions include the
Mediterranean, East Africa, China, Arabia, and other
South American countries .
In addition to sandfly transmission,
VL has also been reported to follow blood transfusion and
disease can present unexpectedly in immunosuppressed
patients—for example, after renal transplantation and in
HIV infection. The great majority of people infected
remain asymptomatic.

Unit 4 - Infectious disease
41
In visceral diseases the spleen, liver, bone marrow and
lymph nodes are primarily involved.
Clinical features
On the Indian subcontinent adults and children are equally
affected; on other continents VL is predominantly a
disease of small children and infants, except in adults with
HIV co-infection.
The incubation period ranges from weeks to months
(occasionally several years).
1) The first sign of infection is High fever, usually
accompanied by rigor and chills. Fever intensity decreases
over time and patients may become afebrile for
intervening periods ranging from weeks to months. This is
followed by a relapse of fever, often of lesser intensity.
2) Splenomegaly develops quickly in the first few weeks and
becomes massive as the disease progresses.
3) Moderate hepatomegaly occurs later.
4) Lymphadenopathy is seen in the majority of cases in
Africa, the Mediterranean and South America, but is rare
on the Indian subcontinent.
5) Blackish discoloration of the skin, from which the disease
derived its name, kala-azar (the Hindi word for ‘black
fever’), is a feature of advanced illness & is now rarely seen
6) Pancytopenia is a common feature. Moderate to severe
anaemia develops rapidly, and can result in congestive
cardiac failure and associated clinical features.
Thrombocytopenia, often compounded by hepatic
dysfunction, may result in bleeding from the retina,
gastrointestinal tract and nose.
7) In advanced illness, hypoalbuminaemia may manifest as
pedal oedema, ascites and anasarca (gross generalised
oedema and swelling).
8) As the disease advances, there is profound
immunosuppression.and secondary infections are very
common. These include tuberculosis, pneumonia, severe
amoebic or bacillary dysentery, gastroenteritis, herpes
zoster and chickenpox. Skin infections, boils, cellulitis
and scabies are common.
9) Without adequate treatment most patients with clinical
VL die.
Investigations
1) Pancytopenia is the most dominant feature, with
granulocytopenia and monocytosis.
2) Polyclonal hypergammaglobulinaemia, chiefly IgG
followed by IgM, and hypoalbuminaemia are seen later.
3) Demonstration of amastigotes (Leishman–Donovan bodies)
in splenic smears is the most efficient means of diagnosis,
with 98% sensitivity); however, it carries a risk of serious
haemorrhage in inexperienced hands. Safer methods, such
as bone marrow or lymph node smears, are not as sensitive.
Parasites may be demonstrated in buffy coat smears,
especially in immunosuppressed patients. Sensitivity can be
improved by culturing the aspirate material or by PCR for
DNA detection and species identification, but these tests
can only be performed in specialised laboratories.
4) Serodiagnosis, by ELISA or immunofluorescence
antibody test, is employed in developed countries.
5) In endemic regions, a highly sensitive and specific direct
agglutination test using stained promastigotes and rapid
immunochromatographic k39 strip test have become
popular. These tests remain positive for several months
after cure has been achieved, so do not predict response to
treatment or relapse. A significant proportion of the
healthy population in an endemic region will be positive
for these tests due to past exposure.
6) Formal gel (aldehyde) for detection of raised globulin has
limited value and should not be employed for the
diagnosis of VL.
Management
1) Pentavalent antimonials
2) Amphotericin B, The antifungal drug,
3) Other drugs
a. The oral drug miltefosine, an alkyl phospholipid, has been
approved in several countries for the treatment of VL.
b. Paromomycin is an aminoglycoside that has undergone
trials in India and Africa India for the treatment of VL.
c. Pentamidine isetionate was used to treat Sb-refractory
patients with VL.
Post-kala-azar dermal leishmaniasis (PKDL)
After treatment and apparent recovery from the visceral
disease in India and Sudan, some patients develop
dermatological manifestations due to local parasitic infection.
Clinical features
In India dermatological changes occur in a small minority
of patients 6 months to ≥3 years after the initial infection.
They are seen as macules, papules, nodules (most
frequently) and plaques which have a predilection for the
face, especially the area around the chin. The face often
appears erythematous Hypopigmented macules can occur
over all parts of the body and are highly variable in extent
and location. There are no systemic symptoms and no
spontaneous healing.
Unit 4 - Infectious disease
42
Investigations and management
The diagnosis is clinical, supported by demonstration of
scanty parasites in lesions by slit-skin smear and culture.
Immunofluorescence and immunohistochemistry may
demonstrate the parasite in skin tissues.
In the majority of patients serological tests (direct
agglutination test or k39 strip tests) are positive.
Treatment of PKDL is difficult. In India, sodium
stiobogluconate for 120 days or several courses of
amphotericin B infusions are required.
Cutaneous and mucosal leishmaniasis
Cutaneous leishmaniasis (CL)
CL (oriental sore) occurs in both the Old World and the
New World (the Americas). Transmission is by sandfly .
In the Old World, CL is mild. It is found around the
Mediterranean basin, throughout the Middle East and
Central Asia as far as Pakistan, and in sub-Saharan West
Africa and Sudan .
The causative organisms for Old World zoonotic CL are
L. major, L. tropica and L. aethiopica .
Anthroponotic CL is caused by L. tropica, and is confined
to urban or suburban areas of the Old World. Afghanistan
is currently the biggest focus, but infection is endemic in
Pakistan, the western deserts of India, Iran, Iraq, Syria and
other areas of the Middle East.
New World CL is a more significant disease, which may
disfigure the nose, ears and mouth & is causedby the L.
mexicana complex (comprising L. mexicana, L. amazonensis
& L. venezuelensis) and by the Viannia subgenus L. (V.)
brasiliensis complex (comprising L. (V.) guyanensis, L.
(V.) panamensis, L. (V.) brasiliensis and L. (V.) peruviana).
CL is commonly imported and should be considered in
the differential diagnosis of an ulcerating skin lesion,
especially in travellers who have visited endemic areas of
the Old World or forests in Central and South America.
Clinical features
The incubation period is typically 2–3 months (range 2
weeks to 5 years).
In all types of CL, the common feature is development of
a papule followed by ulceration of the skin with raised
borders, usually at the site of the bite of the vector.
Lesions, single or multiple, start as small red papules that
increase gradually in size, reaching 2–10 cm in diameter.
A crust forms, overlying an ulcer with a granular base .
These ulcers develop a few weeks or months after the
bite. There can be satellite lesions, especially in L. major
and occasionally in L. tropica infections.
Regional lymphadenopathy, pain, pruritus and secondary
bacterial infections may occur.
Clinically, lesions of L. mexicana and L. peruviana closely
resemble those seen in the Old World, but lesions on the
pinna of the ear are common & are chronic & destructive.
L. mexicana is responsible for chiclero ulcers, the self-
healing sores of Mexico.
If immunity is good, there is usually spontaneous healing
in L. tropica, L. major and L. mexicana lesions. In some
patients with anergy to Leishmania, the skin lesions of L.
aethiopica, L. mexicana and L. amazonensis infections
progress to the development of diffuse CL; this is
characterized by spread of the infection from the initial
ulcer, usually on the face, to involve the whole body in
the form of non-ulcerative nodules. Occasionally, in L.
tropica infections, sores that have apparently healed
relapse persistently (recidivans or lupoid leishmaniasis).
Mucosal leishmaniasis (ML)
The Viannia subgenus extends widely from the Amazon
basin as far as Paraguay and Costa Rica, and is
responsible for deep sores and ML.
In L. (V.) brasiliensis complex infections, cutaneous
lesions may be followed by mucosal spread of the disease
simultaneously or even years later. between 2% and 40%
of infected persons develop ‘espundia’, metastatic lesions
in the mucosa of the nose or mouth. This is characterised
by thickening and erythema of the nasal mucosa, typically
starting at the junction of the nose and upper lip. Later,
ulceration develops. The lips, soft palate, fauces and
larynx may also be invaded and destroyed, leading to
considerable suffering and deformity.
There is no spontaneous healing, and death may result
from severe respiratory tract infections due to massive
destruction of the pharynx.
Investigations in CL and ML
1) CL is often diagnosed on the basis of clinical
characteristics of the lesions
2) Parasitological confirmation to detect Amastigotes on a
slit-skin smear with Giemsa staining; is also important to
exclude other disease.
3) Cultured from the sores early during the infection.
Management of CL and ML
1) Small lesion: may a- self-heal or treated by b- freezing
with liquid nitrogen or curettage.
2) In CL, topical application of paromomycin 15% plus
methylbenzethonium chloride 12% is beneficial.

Unit 4 - Infectious disease
43
3) Intralesional antimony (Sb: 0.2–0.8 mL/lesion) up to 2 g
seems to be rapidly effective in suitable cases, well
tolerated and economic, and is safe in patients with
cardiac, liver or renal diseases.
4) In ML, and in( CL when the lesions are multiple or in a
disfiguring site),
a- parenteral Sb in a dose of 20 mg/kg/day (usually given
for 20 days for CL and 28 days for ML),
b- Conventional or liposomal amphotericin B (see
treatment of VL above).
5) Two to four doses (2–4 mg/kg) of alternate-day
administration of pentamidine are effective in New World
CL. In ML, 8 injections of pentamidine (4 mg/kg on
alternate days) cure the majority of patients.
6) Ketoconazole 600 mg daily for 4 weekshas shown some
potential against L. mexicana infection.
Gastrointestinal protozoal infections
Amoebiasis
Amoebiasis is caused by Entamoeba histolytica, which is
spread between humans by its cysts.
Two non-pathogenic Entamoeba species (E. dispar and E.
moshkovskii) are morphologically identical to E.
histolytica, and are distinguishable only by molecular
techniques, isoenzyme studies or monoclonal antibody
typing. However, only E. histolytica causes amoebic
dysentery or liver abscess.
Pathology
Cysts of E. histolytica are ingested in water or uncooked
foods contaminated by human faeces.
In the colon, vegetative trophozoite forms emerge from
the cysts. The parasite may invade the mucous membrane
of the large bowel, producing lesions that are maximal in
the caecum but found as far down as the anal canal. These
are flask-shaped ulcers, varying greatly in size and
surrounded by healthy mucosa. A localised granuloma
(amoeboma), presenting as a palpable mass in the rectum
or a filling defect in the colon on radiography, is a rare
complication which should be differentiated from colonic
carcinoma. Amoebic ulcers may cause severe
haemorrhage but rarely perforate the bowel wall.
Amoebic trophozoites can emerge from the vegetative
cyst from the bowel and be carried to the liver in a portal
venule. They can multiply rapidly and destroy the liver
parenchyma, causing an abscess.
The liquid contents at first have a characteristic pinkish
colour which may later change to chocolate brown (like
anchovy sauce).
Cutaneous amoebiasis, though rare, causes progressive
genital, perianal or peri-abdominal surgical wound ulceration.
Clinical features
Intestinal amoebiasis—amoebic dysentery
1) Most amoebic infections are asymptomatic.
2) The incubation period of amoebiasis ranges from 2 weeks
to many years, followed by a chronic course with
abdominal pains and two or more unformed stools a day.
3) Offensive diarrhoea alternating with constipation, and
blood or mucus in the stool, are common.
4) There may be abdominal pain, especially right lower
quadrant (which may simulate acute appendicitis).
5) A dysenteric presentation with passage of blood,
simulating bacillary dysentery or ulcerative colitis, occurs
particularly in older people, in the puerperium and with
superadded pyogenic infection of the ulcers.
Investigations
1) The stool & any exudate should be examined at once under
the microscope for motile trophozoites containing RBCs.
Movements cease rapidly as the stool preparation cools.
2) Several stools may need to be examined in chronic
amoebiasis before cysts are found. Sigmoidoscopy may
reveal typical flask-shaped ulcers, which should be
scraped and examined immediately for E. histolytica.
3) In endemic areas one-third of the population are
symptomless passers of amoebic cysts.
4) Serum antibodies are detectable by immunofluorescence in
over 95% of patients with hepatic amoebiasis & intestinal
amoeboma, but in only about 60% of dysenteric amoebiasis
5) DNA detection by PCR shown to be useful in diagnosis of
E. histolytica infections but is not generally available.

Unit 4 - Infectious disease
44
Amoebic liver abscess
1) The abscess is usually found in the right hepatic lobe.
2) There may not be associated diarrhoea. Early symptoms
may be local discomfort only and malaise; later, a
swinging temperature and sweating may develop, usually
without marked systemic symptoms or associated
cardiovascular signs.
3) An enlarged, tender liver, cough and pain in the right
shoulder are characteristic, but symptoms may remain
vague and signs minimal.
4) A large abscess may penetrate the diaphragm and rupture
into the lung, from where its contents may be coughed up.
5) Rupture into the pleural cavity, the peritoneal cavity or
pericardial sac is less common but more serious.
Investigations
1) An amoebic abscess of the liver is suspected on clinical
grounds; there is often a neutrophil leucocytosis and a
raised right hemidiaphragm on chest X-ray.
2) Confirmation is by ultrasonic scanning.
3) Aspirated pus from an amoebic abscess has the
characteristic anchovy sauce or chocolate brown
appearance but only rarely contains free amoebae .
Management
1) Intestinal and early hepatic amoebiasis responds quickly
to oral metronidazole (800 mg 8-hourly for 5–10 days) or
other long-acting nitroimidazoles like tinidazole or
ornidazole (both in doses of 2 g daily for 3 days).
Nitaxozanide (500 mg 12-hourly for 3 days) is an
alternative drug.
2) Either diloxanide furoate or paromomycin, in doses of
500 mg orally 8-hourly for 10 days after treatment, should
be given to eliminate luminal cysts.
3) If a liver abscess is large or threatens to burst, or if the
response to chemotherapy is not prompt, aspiration is
required and is repeated if necessary.
Rupture of an abscess into the pleural cavity, pericardial
sac or peritoneal cavity necessitates immediate aspiration
or surgical drainage. Small serous effusions resolve
without drainage.
Giardiasis
Infection with Giardia lamblia is found world-wide and is
common in the tropics.
In cystic form it remains viable in water for up to 3 months
and infection usually occurs by ingesting contaminated
water. Its flagellar trophozoite form attaches to the
duodenal and jejunal mucosa, causing inflammation.
Incubation period of 1–3 weeks,
Clinical features
There is diarrhoea, abdominal pain, weakness, anorexia,
nausea and vomiting.
Investigations
1) On examination there may be abdominal distension and
tenderness. Stools obtained at 2–3-day intervals should be
examined for cysts.
2) Duodenal or jejunal aspiration by endoscopy gives a
higher diagnostic yield.
3) The ‘string test’ may be used, in which one end of a piece
of string is passed into the duodenum by swallowing,
retrieved after an overnight fast, and expressed fluid
examined for the presence of G. lamblia trophozoites.
4) A number of stool antigen detection tests are available.
Jejunal biopsy specimens may show G. lamblia on the
epithelial surface.
Management
Treatment is with a single dose of tinidazole 2 g, or
metronidazole 400 mg 8-hourly for 10 days.
Cryptosporidiosis
Cryptosporidium spp. are coccidian protozoal parasites of
humans and domestic animals.
Infection is acquired by the faecal–oral route through
contaminated water supplies.
The incubation period is approximately 7–10 days, and
is followed by watery diarrhoea and abdominal cramps.
The illness is usually self-limiting, but in
immunocompromised patients, especially those with HIV,
the illness can be devastating, with persistent severe
diarrhea and substantial weight loss.
Cyclosporiasis
Cyclospora cayetanensis is a globally distributed
coccidian protozoal parasite of humans.
Infection is acquired by ingestion of contaminated water.
The incubation period is approximately 2–11 days, and
is followed by acute onset of diarrhoea with abdominal
cramps, which may remit and relapse.
Although usually self-limiting, the illness may last as long
as 6 weeks with significant associated weight loss and
malabsorption, and is more severe in
immunocompromised individuals.
Diagnosis is by detection of oöcysts on faecal
microscopy.
Treatment may be necessary in a few cases & the agent
of choice is co-trimoxazole 960 mg 12-hourly for 7 days.
