
Unit 2: Protozoa
30
Lecture 12+13+14 - Suborder:
Haemosporina (Genus Plasmodium)
Subkingdom: Protozoa.
Phylum: Apicomplexa.
Class: Coccidia.
Order: Eucoccidia.
Suborder: Haemosporina.
Genus: Plasmodium.
There are 4 species of the genus plasmodium that infect
human, these are:
1) Plasmodium vivax (benign tertian malaria)
2) Plasmodium ovale (ovale tertian malaria)
3) Plasmodium falciparum (malignant tertian malaria)
4) Plasmodium malariae (quartan malaria).
The disease caused by the genus plasmodium is called
Malaria.
Genus plasmodium require 2 hosts for the life cycle, the
intermediate host which is the vertebrate host where
asexual phase develop and the definitive host which is the
female of Anopheline mosquitoes where sexual phase
develop.
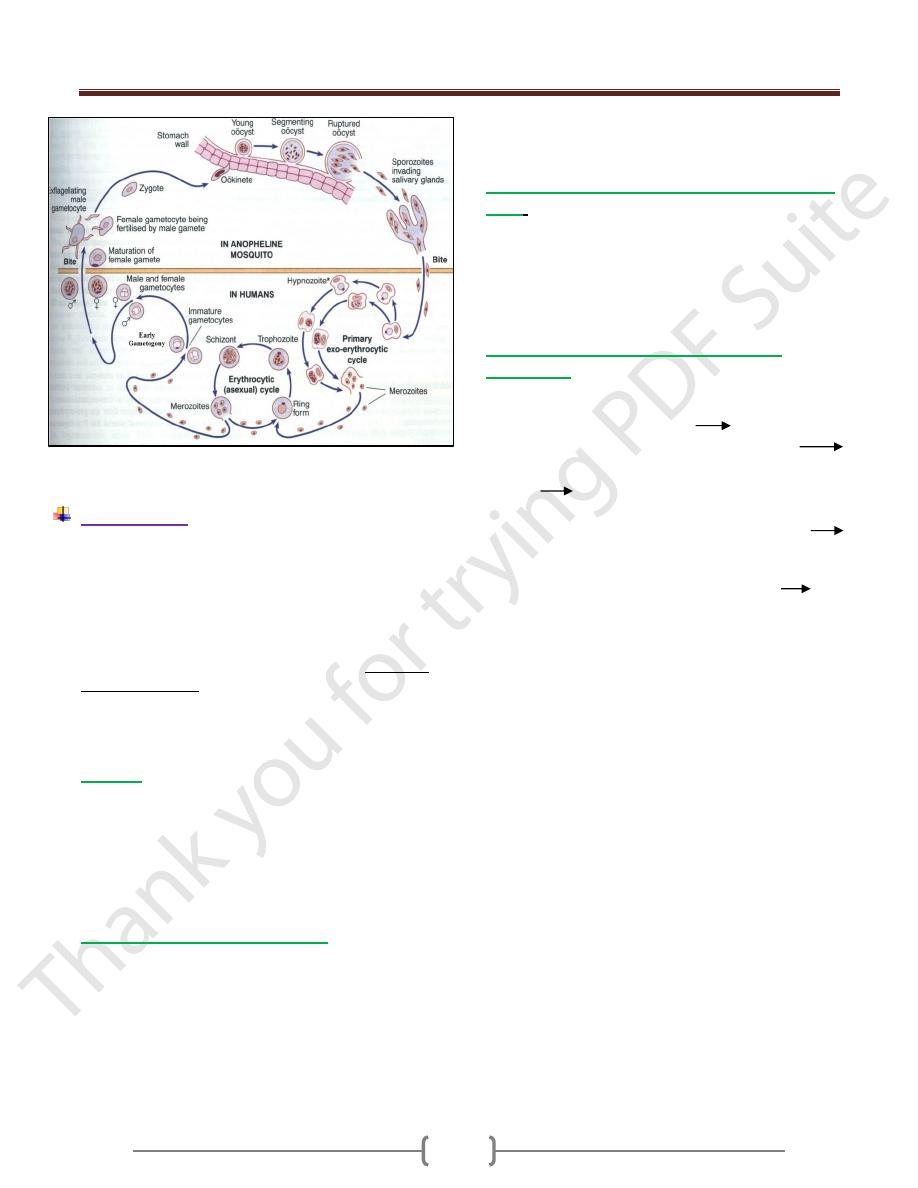
Life cycle (Figure 1)
1) In mosquitoes:
Once the ripe gametocytes are ingested by a female
anopheles in a blood meal and reach the midgut, they
transform into mature gametes. One macrogametocyte
develops a single macrogamete (oocyte or unfertilized
ova), and one microgametocyte produces several
flagellated microgametes. A microgamete then enters a
macrogamete, resulting in a zygote that becomes a motile
ookinete, migrates through the stomach wall, & becomes
an oocyst just under the outer membrane of the stomach.
The oocyst grows rapidly and develops internal nuclear
centers. Each center then produces a large number of
delicate, spindle – shaped sporozoites. By the time the
sporozoite become mature, the wall of the greatly enlarged
oocyst bursts, releasing the sporozoites into the hemocele of
the mosquito. The sporozoites then migrate to the salivary
glands, which they enter, and from there they pass down
through the salivary ducts into the median tube of the
mosquito's proboscis. When the mosquito next takes a
blood meal, sporozoites are injected into the cutaneous
blood vessels of the victim and initiate a new infection.
Time & temperature for complete sexual phases in mosquito:
Plasmodium falciparum 10 days, 30 C
o
Plasmodium vivax 11 days, 25 C
o
Plasmodium ovale 14 days.
Plasmodium malariae 18-21 days, 22 C
o
2)
In humans
:
Three phases
a) Exoerythrocytic or preerythrocytic schizogony.
Bite of the female of anopheline mosquito's
sporozoite to human blood circulation and remain
for 30 minutes liver parenchymal cells in which
Exoerythrocytic cycle occur and result in the production
of tiny merozoites in each schizont, rupture of the liver
cells merozoites released to the circulation.
Time for asexual cycle in the liver (Prepatent period):
Plasmodium falciparum 6days
Plasmodium vivax 8 days
Plasmodium ovale 9 days
Plasmodium malariae 13 days
In case of Plasmodium vivax and Plasmodium ovale, there
is a varying proportion of sporozoites enter a resting stage
before undergoing asexual multiplication, this stage is
known as hypnozoite. The hypnozoites may undergo
reactivation after weeks or months which bring about the
relapse which is the characteristic of these 2 species but
not for Plasmodium falciparum and Plasmodium
malariae.
b) Erythrocytic schizogony
Merozoites that have developed in preerythrocytic cycle
enter the RBC; they transform into trophozoites, which
grow and develop into schizonts, each producing a no. of
merozoite characteristic of the spp. of plasmodium. When
fully matured, the merozoites break out of the parasitized
RBC and soon actively enter other RBC to repeat the
asexual cycle. The time required to complete an asexual
cycle in the RBC depends on the species of the
plasmodium.
Plasmodium falciparum it is every 36 - 48 hours.
Plasmodium vivax it is every 48 hours.
Plasmodium ovale it is every 48 hours
Plasmodium malariae it is every 72 hours
c) Early gametogony.
In individual bitten by infected mosquitoes, gametocytes
begin to appear in circulating erythrocytes after a few to
several asexual multiplications, those of P.falciparum
usually appearing relatively late. These cells do not
multiply or develop further unless taken up by a suitable
mosquito host.
Unit 2: Protozoa
31
Figure 1 Life cycle of plasmodium
Pathogenesis
The pathogencity of malaria is related to RBC infection.
Tissue destruction in exoerythrocytic cycle appears not to
produce sign and symptoms. The plasmodia in RBC grow
and segment at the expense of the host cells and the
malarial parasite progressively consumes and degrades
intracellular proteins, the hemoglobin. The potentially
toxic heme is polymerized to biologically inert hemozines
or malarial pigment.
Plasmodium falciparum infects RBC at all ages.
Plasmodium vivax and ovale infects only reticulocytes.
Plasmodium malariae infects only mature RBC.
1) Anemia:
as the no. of parasites increase with each successive
schizogony, the no. of RBC decreases due to rupture of
parasitized and non-parasitized RBC.The mechanism by
which non parasitized RBC ruptured is through the
production of autoAb to the RBC during infection or
through the binding of soluble material Ag or the
circulating Ag-Ab complex to the cell surface.
2) Chills and fever of malarial attack:
When the debris of the ruptured cells, merozoites and
their metabolite by product is set free into the blood
stream, it stimulates chemoreceptor of the temperature
regulating mechanism of host to conserve heat. The
amount of pyrogen released initially is not enough to
produce a marked reaction, but they cause prodromal
symptoms. As the no. of the invaded RBC increases and
the asexual cycle of the parasites become more
synchronized, the quantities of the pyrogen become
sufficient to produce the characteristic chills and fever of
a malarial attack.
3)
Infection with P.falciparum differs from the other
types
:
a) It invades erythrocytes of all ages and thus causing
very extensive parasetemia.
b) The schizogonic cycle in the bloodstream requires not
more than 48 hrs but is frequently less synochronized.
c) There is a tendency for more than one parasite to
develop in a single RBC.
4)
cytoadherance phenomena in Plasmodium
falciparum :
it is the result of expression of knobs
(ligands) on the surface of the parasitized RBC which
adhere to specific receptors on the endothelial cell, the
RBC tend to adhere to each other rosetting and
agglutination, and to the lining of the blood vessel
capillary blockage in the vital organ e.g. brain, lungs,
kidneys. Tissue anoxia.
5)
Agglutination of RBC in Plasmodium falciparum + loss
of plasma from the blood vessel in all spp. of malaria
phenomena called
sludging
of the RBC in the vessel.
6)
The decrease in the no. of RBC + decrease in the quality
of the circulating RBC with decrease in oxygen
multiple thromboses
in the smaller blood vessel and
decrease in the circulating blood volume.
7)
Spleen is enlarged
, congested, soft and hemorrhagic in
acute stage and hard in chronic stage.
Splenomegaly is due to congested of sinusoids with
RBC+ hyperplasia of the lymphocyte and macrophage.
8)
Liver
is hypertrophic and congested.
9)
Kidneys
are congested, glomerular capillaries become
thrombotic with accumulation of parasitized RBC, free
hematin, macrophage.
Plasmodium malariae have been associated with
nephrotic syndrome (quartan nephrosis) in children with
peak incidence at the age of 5 years.
10)
Pulmonary capillaries
are congested.
11)
Brain
is edematous and grayish in color.
12)
All mucous membranes
show patechial hemorrhage
13)
Heart
shows fatty degeneration.

Unit 2: Protozoa
32
Clinical features
Prepatent period
It is defined as the time between sporozoite inoculation
and the appearance of parasites in the blood and
represents the duration of the liver stage and the number
of merozoites produced.
Incubation periods
It tends to be a little longer than prepatent period and
is defined as the time between sporozoite inoculation and
the onset of symptoms.
Incubation periods
= exoerythrocytic cycle (usually 2)
+1 or 2 erythrocytic cycle.
Plasmodium falciparum = 12 days.
Plasmodium vivax and P.ovale = 13-17 days.
Plasmodium malariae = 28-30 days
I. Nonspecific symptoms (prodromal stage)
Malaria begins with nonspecific initial symptoms that last
several days, including for instance headache, pain in limbs,
general fatigue, chills, and occasionally nausea as well as
intermittent fever, either continuous or at irregular intervals,
this is because the cycles are asynchronous. Several days to
a week after onset of parasitemia, the schizogonic cycle
synchronizes: in infections with P. vivax, P. ovale, and P.
falciparum, a cycle is completed within 48 hours, in
infections with P. malariae within 72 hours.
II. Malarial paroxysms or febrile attack.
The Classic malarial paroxysm started suddenly with:
a) Shaking chills (cold stage)
Lasting 15min-1 hr, begins as the dividing generations of
parasites rupture their RBC and escape into the blood. The
patient complains of extreme cold, although the temperature
is elevated at the onset and rises during the period of chill.
The skin is pale and cyanotic and the patient huddled under
a pile of blankets.
Nausea and vomiting also occur at this stage.
b) Febrile stage (hot stage)
Lasting several hrs, characterized by a spiking fever that
reaches 40 C or more. The skin becomes flushed, the patient
is agitated, restless, disoriented or even delirious. Severe
frontal headache and pains in the limbs and back. It lasts 2-6
hours in vivax and ovale malaria, 6 hoursor more in
quarten, and longer in falciparum.
During this stage, the parasite invades their RBC.
c) Sweating phase.
The fever subsides and the patient falls asleep and later
awakes feeling well.
Note:
1) In P.falciparum infection the initial chill is usually less
pronounced and the fever more prolonged.
2) In mixed infections with 2 or more species, or in the
early stages of infection with one species there may be
daily (quotidian) paroxysms or even double paroxysms in
one day. Occasionally, two 48- hour parasite broods may
be asynchronous by 24 hour , producing regular , daily
fever in tertian species.
Following an essentially symptomless remission which
varies with the species, there is a 2
nd
paroxysms followed by
several additional one over a period of up to 3 weeks or
more before the symptoms terminate. This series of
paroxysms constitutes the primary attacks.
The malarial paroxysms will become less severe and
irregular in periodicity as the host develops immunity.
Relapse:
Following the termination of the primary attack, either
naturally or following treatment, parasites are depressed
or may be completely disappear from the blood. In
P.vivax and p.ovale, one to several more attacks occur due
to hypnozoite in the liver.Relapses of P.vivax malaria
usually continue over a period of 2-3 years before the
infection is terminated, those of p.ovale malaria occur
infrequently and rarely persist longer than 1 year.
Recrudescence:
In P.falciparum and quartan malaria a renewal of clinical
manifestations after weeks, months, or years without re-
exposure is attributed to the persistence of parasites in the
blood at levels too low to be detected or to produce
symptoms. Such parasitemias may persist for up to one
year in P.falciparum infections and for many years in
quartan infection.
Note:
Only the sporozoites (introduced by the mosquitoes
themselves) can penetrate the liver cells. Thus, if malaria
is acquired by blood transfusion or transplacentally, no
infection of the liver occurs and relapses do not occur.
Primary vivax attacks if untreated, last 3 weeks – 2
months or longer. Relapse extending over a period of 5-8
years.
In ovale malaria, early spontaneous recovery after no
more than 5-10 paroxysms. Relapse no longer than a year
after the initial attack.
In quartan malaria, 3weeks- 24 weeks.

Unit 2: Protozoa
33
Un treated primary attack of plasmodium falciparum
tends to run its course quickly and seldom exceeds 2-3
weeks duration but coma or death occurs within this
period.
Complications of malaria
P.vivax, ovale is benign.
P.falciparum cause severe infection (severe malaria).
Complications of severe malaria include hypoglycemia,
severe anemia, renal failure and metabolic acidosis
Severe malaria should be considered in any non-immune
patient with a parasite count greater than 2%.
1) Cerebral malaria.
Severe complication of Plasmodium falciparum.it starts
suddenly as severe headache, drowsiness, confusion and
coma.
Pathogenicity: cytoadherance phenomena + decrease in
deformability of P.falciparum infected RBC, once the
parasite has matured beyond the ring stage.
Sequele: cortical blindness, hemiparesis, cerebellar ataxia
and severe headache.
Cerebral malaria is assumed when asexual parasites are
present in the blood film and the patient has impaired
consciousness and other encephalopathies have been
excluded, particularly bacterial meningitis and locally
occurring viral encephalitis.
2)
Anemia
which is more severe in Plasmodium
falciparum. What are the causes of anemia?
3)
Renal disease
occurs in severe Plasmodium falciparum
infection and in chronic Plasmodium malariae infection.
In Plasmodium falciparum there is acute tubular necrosis
and tissue anoxia because of RBC sludging.
Plasmodium malariae associated with nephrotic
syndrome due to acute glomerulonephritis due to
deposition of immune complex in the glomeruli. Seen in
children < 5 years associated with odema, proteinuria.
4) Black water fever (Hemoglobinuric fever)
It is seen in severe Plasmodium falciparum infection with
irregular treatment with quinine.
Pathogenesis: Massive intravascular hemolysis
haemoglobinuria.
It occurs in persons who have lived in areas where
P.falciparum infections abounds. The hemolysis is caused
by erythrocytic AutoAb derived from previous infections
and reacting with autoantigens initiated by fresh RBC
infection with the same strain.
Clinically: chills, rigor, high fever, jaundice, vomiting,
anemia and passage of dark red or black urine.
5)
Dysenteric malaria
: uncommon complication of
Plasmodium falciparum characterized by abdominal pain,
nausea and vomiting, upper GIT bleeding.
6) Algid malaria (shock).
Also seen in severe Plasmodium falciparum infection
characterized by hypotension, decrease temperature and
impairment of vascular perfusion. This is due to gram
negative septicemia, GIT bleeding, splenic rupture or
incorrect dehydration.
7) Tropical splenomegaly syndrome.
Seen in some hyperendemic area in which exaggerated
immune response to malaria is seen. There is high level of
IgM. IgM aggregated with other Ig or complement and
precipitate in cold. IgM aggregate phagocytosed by RE
cell in the spleen and liver leading to enlargement of the
spleen, reticulocytosis, thrombocytopenia.
Patients with Tropical splenomegaly syndrome present
with an abdominal mass or a dragging sensation in the
abdomen and occasional sharp abdominal pains suggesting
perisplenitis. Anemia and some degree of pancytopenia are
usually evident, but in many cases malarial parasites cannot
be found in peripheral- blood smears.
8)
Hypoglycemia
a) Hyperinsulinaemia due to treatment with quinine or quinidine
b) Impairment of hepatic gluconeogenesis
9)
Pulmonary edema:
Due to fluid overload in oliguric or anuric patient or due
to disseminated intravascular coagulation.
Malaria in hyperendemic area:
In population living in highly malarious area, they are
subjected to periodic re-exposure throughout the life and
the typical overt manifestations are observed in young
only. Deaths occur commonly in children. Older child and
adults who have survived the earlier attacks have
developed tolerance to the disease.
Unit 2: Protozoa
34
Diagnosis
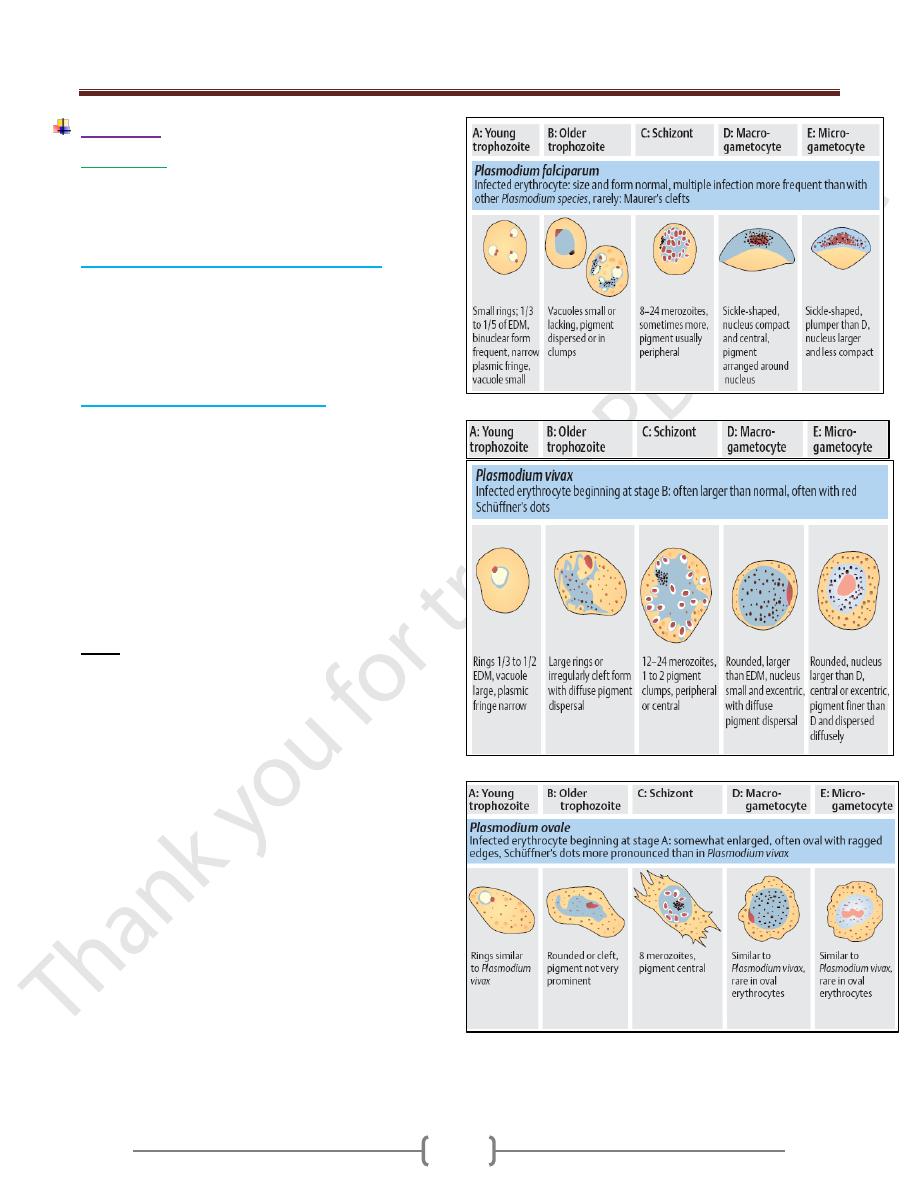
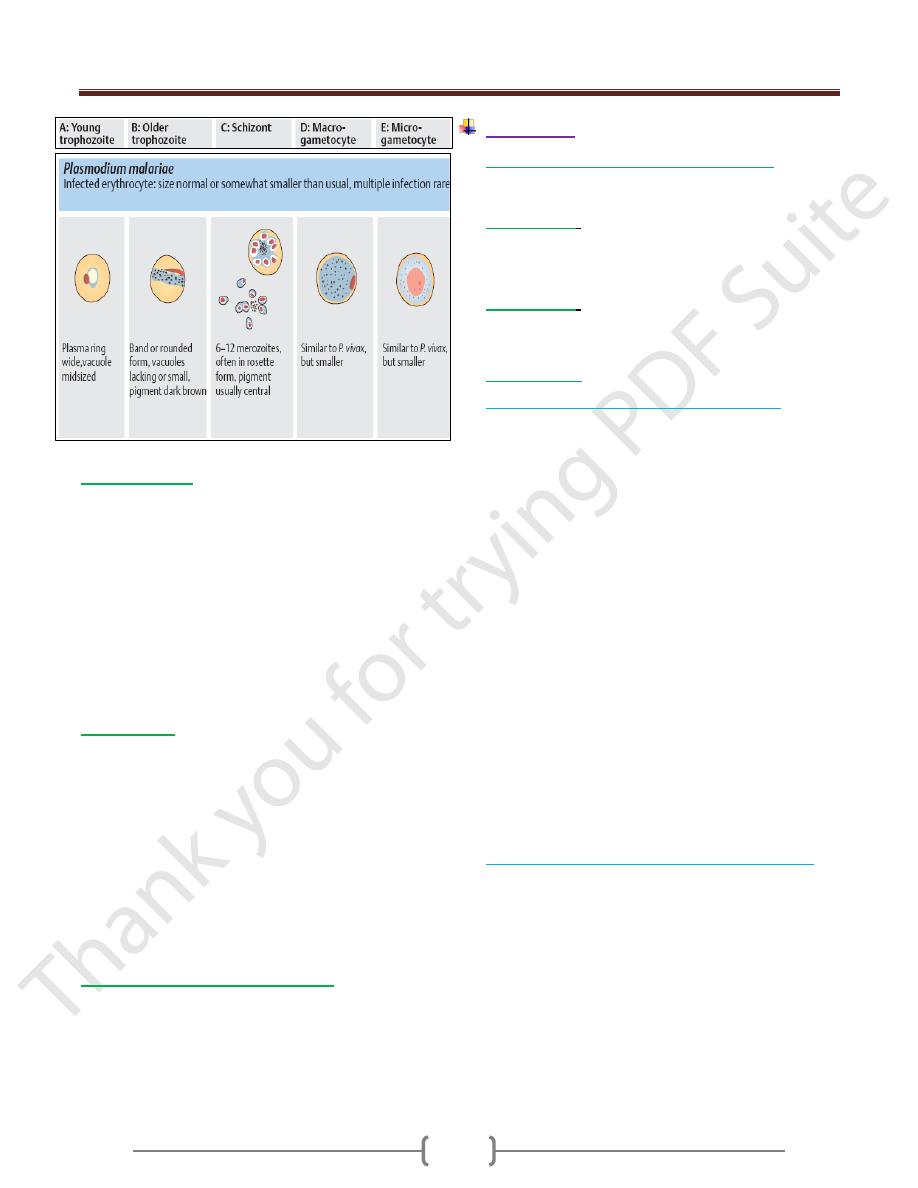
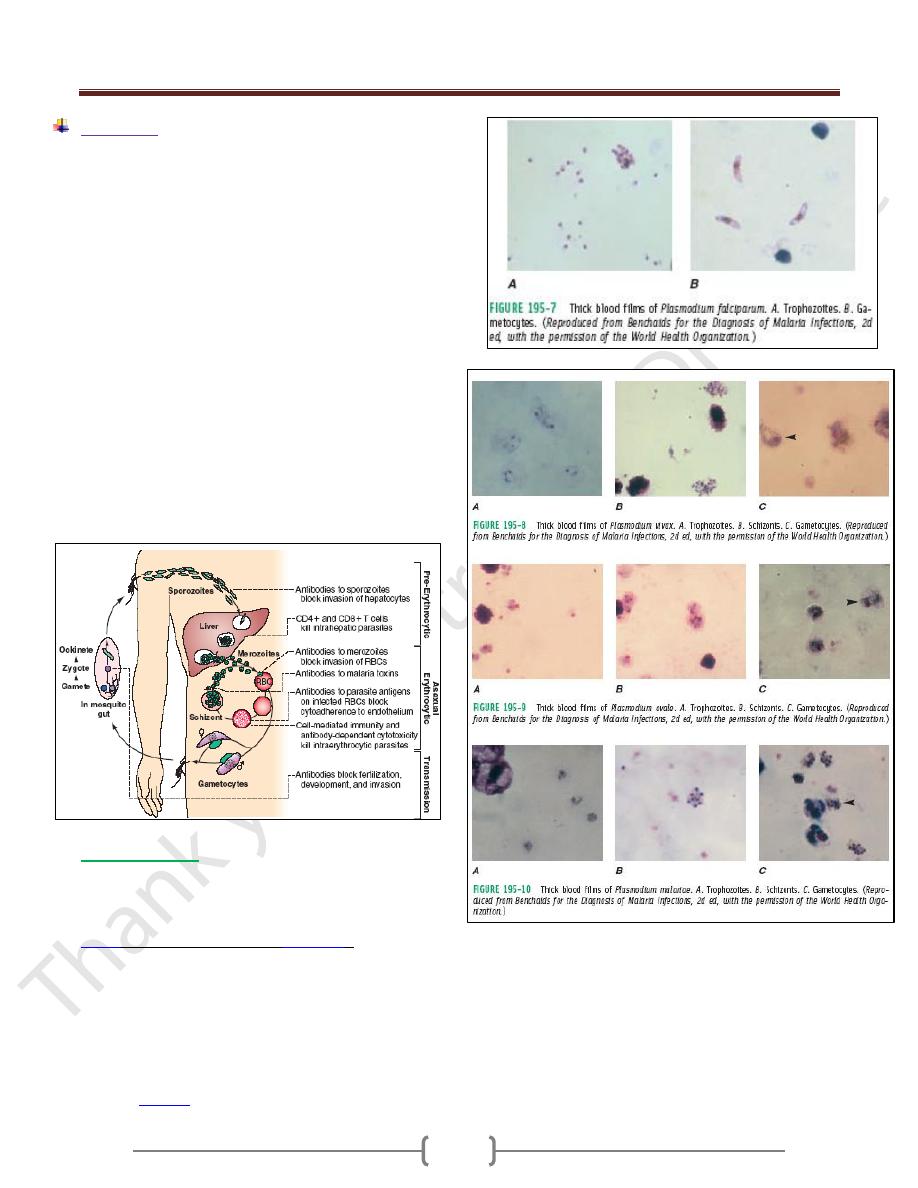
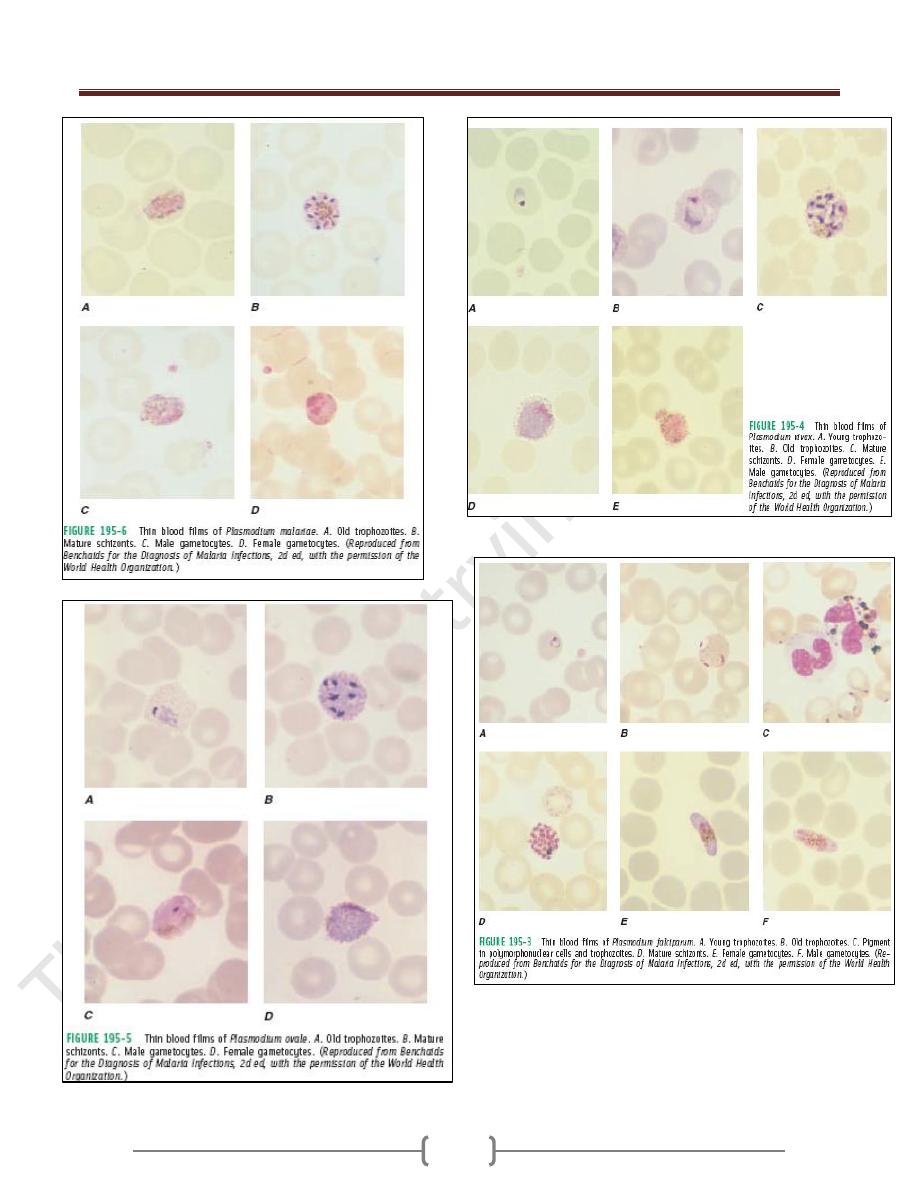
1) Microscopic
Thick and thin blood films.
The careful examination of a well-prepared and well-
stained blood film currently remains the "gold standard"
for malaria diagnosis.
A. Thick blood film for screening of the organism.
Thick films allow the examiner to screen a larger volume
of blood and are about eleven times more sensitive than
the thin film, so picking up low levels of infection is
easier on the thick film, but the appearance of the parasite
is much more distorted and therefore distinguishing
between the different species can be much more difficult.
B. Thin blood film for spp. identification.
The timing of blood examination is important; blood film
taken just before or at the height of the malarial paroxysm
will contain a detectable number of parasites.
If the parasites cannot be detected in the 1
st
sample, so it
is advisable to do thick and thin blood films every 6-12
hrs. as long as 48 hrs.
The aim of the diagnosis is the presumptive
differentiation of Plasmodium falciparum from other
spp. and the diagnosis of plasmodium falciparum is based
on the detection of ring forms with or without
gametocytes and > 5% of the RBC are parasitized.
Note:
1) Parasites are not likely to be circulating in the blood
during suppressive therapy, immediately following
curative treatment, or soon after self-medication with
antimalarial drugs.
2) When two species of malaria are present, there appeaers
to be antagonism between them, Plasmodium falciparum
is predominantes over P.vivax which in turn
predominates over p.ovale and p.malariae . Thus in a
mixed infection with Plasmodium falciparum and
P.vivax, the latter would be initially suppressed and not
diagnosed. This could produce relapse later.
Unit 2: Protozoa
35
2) Serological test:
Serological tests provide confirmation of past malaria in
patients and are valuable for epidemiological studies.
These tests are also useful for screening donated blood
and diagnosing hyperactive malarial splenomegaly.
Among the tests used are the:
a) Indirect fluorescent antibody test (IFAT),
b) Indirect hemagglutination antibody (IHA) test,
c) Enzyme-linked immunosorbent assay (ELISA).
All these tests produce positive results several days after
malaria parasites appear in the blood and so do not help in
the diagnosis of the acute infection for treatment purposes.
3) Dipstick tests
Dipstick tests based on the detection of P. falciparum
histidine-rich protein-2 (PfHRP-2) antigen are specific for
P. falciparum infections and do not detect the other 3
species. Dipstick tests based on the detection of parasite
lactate dehydrogenase are now available; these tests can
detect both P falciparum and P vivax.
These tests have high sensitivity and specificity, require
no special equipment or training, and produce results
rapidly. However, they remain positive for a week or
more after the treatment and cure, and, in this situation,
can yield false-positive results.
4) Molecular biological detection tests:
DNA and RNA probes and polymerase chain reaction
(PCR) have good sensitivity and specificity but require
sophisticated expensive equipment.
Treatment
Suppressive therapy (chemoprophylaxis)
Kills the parasites as it enter the blood stream with small
doses of drugs effective against erythrocytic stage.
Clinical cure
:
Larger doses of the same type of the drug used in
suppressive therapy or different ones to eliminate the
large no. of erythrocytic parasites.
Radical cure
:
Elimination of not only the blood stream infection but the
tissue stage in the liver.
Drug therapy
1) Chemotherapy of mild P. falciparum malaria .
P. falciparum is now resistant to chloroquine almost
world-wide, so quinine is the drug of choice.
Quinine dihydrochloride or sulphate 600 mg salt 8-hourly
by mouth is given until the patient is clinically better and
the blood is free of parasites (usually 3-5 days).
This regimen should be followed by a single dose of
sulfadoxine 1.5 g combined with pyrimethamine 75 mg,
i.e. 3 tablets of Fansidar®.
If sulphonamide sensitivity is suspected, quinine may be
followed by doxycycline 100 mg daily for 7 days.
Alternatives to quinine plus Fansidar are atovaquone 250
mg plus proguanil 100 mg (Malarone), or artemether
orally for 5 days then mefloquine.
Mefloquine may occasionally cause alarming
neuropsychiatric side-effects which can persist for several
days due to its plasma half-life of 14 days.
artemisinin combination therapy. This combines an
astemisinin drug (artemether or artesunate) with another
drug. Currently co-artemether (artemether-lumefantrine)
and artesunate+amodiaquine are the most widely used
In pregnancy a 7-day course of quinine alone should be
given.
2) Management of complicated P. falciparum malaria.
Severe malaria is a medical emergency and cerebral
malaria is the most common presentation and cause of
death in adults with malaria.
The management of severe malaria should include:
A) Early and appropriate antimalarial chemotherapy,
B) Active treatment of complications,
C) Correction of fluid,
D) Electrolyte and acid-base balance.
E) Quinine is indicated if a chloroquine-resistant infection
is at all likely.

Unit 2: Protozoa
36
3) P. vivax, P. ovale and P. malariae infections should be
treated with chloroquine: 600 mg chloroquine base
followed by 300 mg base in 6 hours, then 150 mg base
12-hourly for 2 more days.
4) In case of p.vivax and p.ovale, primaquine (15 mg daily
for 14 days), which destroys the hypnozoite phase in the
liver should follow the chloroquione.
Side effect: hemolytic anemia so one should do the G6PD
enzyme assay.
Prevention and control
1) Chemoprophylaxis
Choice of regimen is determined by
a) Area to be visited.
b) Length of stay.
c) Level of malaria transmission.
d) Level of drug resistance.
e) Presence of underlying disease in the traveler and
concomitant medication taken.
I. Chloroquine remains the drug of choice for the
prevention of infection with drug-sensitive P. falciparum
and with the other human malarial species (although
chloroquine-resistant P. vivax has been reported from
parts of eastern Asia, Oceania, and Central and South
America). Unfortunately, there are now few areas of the
world with Chloroquine-sensitive P. falciparum.
Chloroquine is considered safe in pregnancy. Chronic
administration for >5 years, a characteristic dose-related
retinopathy may develop, but this condition is rare at the
doses used for antimalarial prophylaxis.
Primaquine (0.5 mg of base/kg or 30 mg, daily adult
dose) has proved safe and effective in the prevention of
drug-resistant falciparum and vivax malaria in adults.
II. In areas where Chloroquine resistant P.falciparum
(CRPF) is endemic, drugs effective against resistant P.
falciparum should be used [mefloquine, atovaquone-
proguanil (Malarone), doxycycline or primaquine].
Atovaquone-proguanil (Malarone; 3.75/1.5 mg per kg or
250/100 mg, daily adult dose) is a fixed-combination
once-daily prophylactic agent that is very well tolerated
by adults and children, with fewer adverse gastrointestinal
effects than chloroquine-proguanil and fewer adverse
central nervous system effects than mefloquine.
Daily administration of doxycycline (100 mg daily, adult
dose) is an effective alternative to mefloquine.
The regime of chemoprophylaxis
1-2 weeks before traveling to the endemic area +during
the stay in the area + 4 weeks after leaving the area
Primquine can be used for 2 weeks after the above regime
to kill the hypnozoite.
2) Mosquitoes nets, windows screen, protective clothes and
insects repellents.
3) Drainage of stagnant water in swamps and ditches
decreasing the breeding areas.
Epidemiology
Plasmodium falciparum and Plasmodium ovale are
diseases of the tropics
Plasmodium malariae seen in subtropics and temperate zone
Plasmodium vivax in all endemic area.
The transmission of all species depends on the presence of
suitable spp. of anopheline mosquitoes and infected
gametocytes bearing humans.
Transmission can occur also by
1) Blood transfusion.
2) organ transplantation and in
3) Drug addicts.
Carriers are Peoples in whom gametocytes are
commonly circulating in peripheral blood over a
considerable period of time.
Autochthonous malaria: malaria which is acquired
locally by mosquito bite.
Imported malaria: malaria which is contracted outside
the area and brought in.
Introduced malaria: malaria which is acquired from an
imported case.
Induced malaria: malaria which is contracted by
parenteral inoculation (e.g., by blood transfusion). It is
also called transfusion malaria .The incubation period is
short because there is no preerythrocytic phase.
Individuals with sickle cell trait (heterozygote) are
protected against malaria because their RBC has little
ATPase activity and cannot produce sufficient energy to
support the growth of the parasites. Homozygous sickle
cell anemia is also protected.
The receptor for Plasmodium vivax is the Duffy blood
group antigen. People who are homozygous recessive for
the genes that encode this protein are resistant to infection
by Plasmodium vivax. More than 90% of black West
Africans and many of their American descendants do not
produce the Duffy antigen, so they resist Plasmodium
vivax infection.
People with G6PD deficiency are also protected against
the severe effects of Plasmodium falciparum.
Unit 2: Protozoa
37
Immunity
The immunity against malaria is slow to develop and
requires multiple exposures. In highly endemic areas only
young children are at a high risk of developing severe
falciparum malaria whereas older children and adults are
essentially protected from severe disease and death.
However, this immunity is not a sterilizing immunity in
that persons can still become infected. In addition, the
immunity is short lived and in the absence of repeated
exposure the level of immunity decreases. For example,
previously semi-immune adults will often develop severe
malaria upon returning to an endemic area after being in a
non-endemic area for 1-2 years. This state of partial
immunity in which parasitemia is lowered, but not
eliminated, and parasitemia is better tolerated is
sometimes referred to as premunition. Premunition
refers to an immunity that is contingent upon the pathogen
being present. In the endemic area, a low level of
parasitemia and low grade symptoms results; this is called
the premmunition or concomitant immunity.
Malarial vaccine
• Malaria vaccines are an area of intensive research.
However, there is no effective vaccine that has been
introduced into clinical practice.
•
/AS01 (commercial name:
), which
started Pivotal Phase III evaluation in May 2009 and is
designed not for travelers but for children resident in
malaria-endemic areas who suffer the burden of disease
and death related to malaria.
• The RTS,S vaccine was engineered using genes from the
outer protein of Plasmodium falciparum malaria parasite
and a portion of a hepatitis B virus plus a
chemical
to boost the immune system response.
Unit 2: Protozoa
38
Unit 2: Protozoa
39
Babesia
Babesia species produce fulminating malaria-like disease
known as babesiosis, piroplasmosis, or red water fever.
The disease is important in cattle, dogs and rodents, cats
and horses.
The parasite requires a tick as a vector.
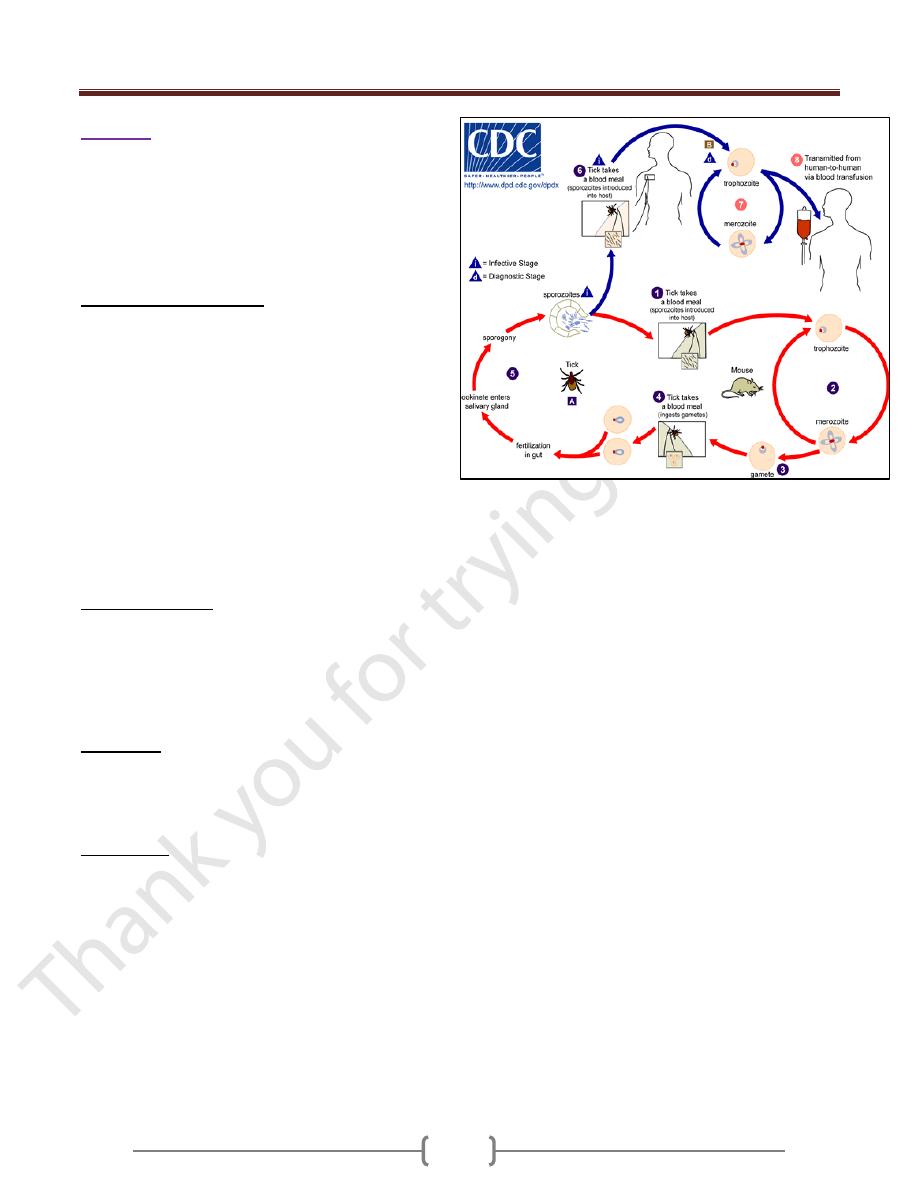
Life cycle :( Figure 1-B)
Ticks become infected by ingesting infected erythrocytes
but don’t themselves transmit the infection latter during
feeding. The organisms penetrate the developing ova of
the tick, and infecting the embryo which hatch from the
eggs, then the parasite penetrate the salivary glands of the
embryo where they continue reproduction and are
available for transmission to the mammalian host when
the tick feeds.
In man, Babesia occurs in the trophozoite stage- which may
be pear -shaped, spherical, ovoid, spindle-shaped, or
amoeboid – and only in erythrocytes. It undergoes asexual
reproduction, forming pairs, or tetrad groups within the cell,
organisms rupture the cell and infect other erythrocyte.
Clinical features:
The disease presented with acute onset of shaking chills,
headache, fever (40C
o
) and pain in the abdomen, muscles,
and back. Rapid onset of anemia and jaundice may occur.
The disease is more severe in splenctomized patient, but it
also occurs in normal patient with normal spleen.
Diagnosis:
Thin blood film to demonstrate a pale area in the RBC
that represents a vacuole. Babesia infection is more likely
to be confused with P. falciparum.
Treatment:
Quinine and clindamycin.
Figure 1-B - Life cycle of Babesia
