
Unit 4: Virology
212
Lecture 9+10 - Retroviruses
The retroviridae family is divided into seven genera .The
genera which are important to human are delta retrovirus
and lentivirus.
Lentivirus
Human immunodeficiency virus (HIV)
Human immunodeficiency virus is a member of lentivrus
which cause slow infection with long incubation period
(AIDS).
The acquired immunodeficiency syndrome (AIDS) was
first recognized in 1981. It is caused by the human
immunodeficiency virus (HIV-1). HIV-2 causes a similar
illness to HIV-1 but is less aggressive and restricted
mainly to western Africa.
HIV is one of the two important human T cell
lymphotropic viruses. It preferentially infects and kills
helper CD-4
+
T cells leading to loss of cell mediated
immunity and high probability of opportunistic infection.
Other cells (macrophage and monocytes) can be infected
also.
General characteristic
HIV has a bar shaped core surrounded by an envelope
contain type specific glycoprotein (gp 120, gp 41).
The genome consists of 2 identical molecules of SS,
positive polarity RNA.
There are three typical retroviral genes gag, pol, and
env, (encode the structural proteins), and six regulatory
genes. Two of the regulatory genes, tat, and rev, are
required for replication, and the other four, nef, vif, vpr,
and vpu, are not required for replication and are termed
accessory genes.
The gag gene encodes the internal corer protein [(p24) (is
the most important used in serology)], and p17 (matrix).
The pol gene encodes:
1) Virion reverse transcriptase.
2) Integrase.
3) Protease
The env gene encodes for gp 160 that is cleaved to gp 120
(attachment to CD4) and gp 41(fusion with host cell).
Regulatory genes:
1) Tat for activation of transcription of viral genes.
2) Rev for transport of late mRNA from nucleus to
cytoplasm.
3) Nef .repress the synthesis of class I MHC proteins,
thereby reducing the ability of cytotoxicT cells to kill HIV
– infected cells.
4) Vif (viral infectivity) inhibit the action of APOBEC3G, an
enzyme that cause hypermutation in retroviral DNA.
5) Vpr transports viral core from cytoplasm into nucleus in
non-dividing cells.
6) Vpu enhances virion release from cell.
Important antigens of HIV:
1) gp 120 and gp 41 are type specific envelop gp. HIV
classified into 3 types (M, N, and O) .M is common types
and contain 10 subtypes (A-J). The gene that encodes gp
120 mutates rapidly, resulting in many antigenic variants.
Antibody against gp 120 neutralizes the infectivity of
HIV, but the rapid appearance of gp 120 variants will
make production of an effective vaccine difficult.
2) The group-specific antigen, p24, is located in the core and
antibody against p24 serves as important serologic
markers of infection.
Origion of HIV
HIV-1 in humans originated from cross-species transmission
of SIV
cpz
(simian immunodeficiency virus of chimpanzee)
while HIV-2 from SIV
sm
(sm= Sooty mangabey)
Disinfection and inactivation
HIV is completely inactivated by treatment for 10 minutes
at room temperature with any of the following: 10%
household bleach, 50% ethanol, 35% isopropanol, 0.5%
paraformaldehyde, 0.3 % H2O2.The virus inactivated by
extreme pH(1,13).If HIV present in clotted or unclotted
blood in a needle or syringe, exposure to undiluted bleach
for at least 30 seconds is necessary for inactivation. HIV
is readily inactivated in liquid or 10% serum by hearting
at 56 c for 10 minutes.
Unit 4: Virology
211
Virus receptors
Lentiviruses use CD4 molecule as a receptor, which is
expressed on macrophages and T lymphocytes. A second
core receptor in addition to CD4 is necessary for HIV-1 to
gain entry to cells (required for fusion of the virus with
cell membrane).The virus first bind to CD4 receptor and
then to core receptor. The core receptors are CXCR4 for
T-tropic strains and CCR5 for macrophage-tropic strains.
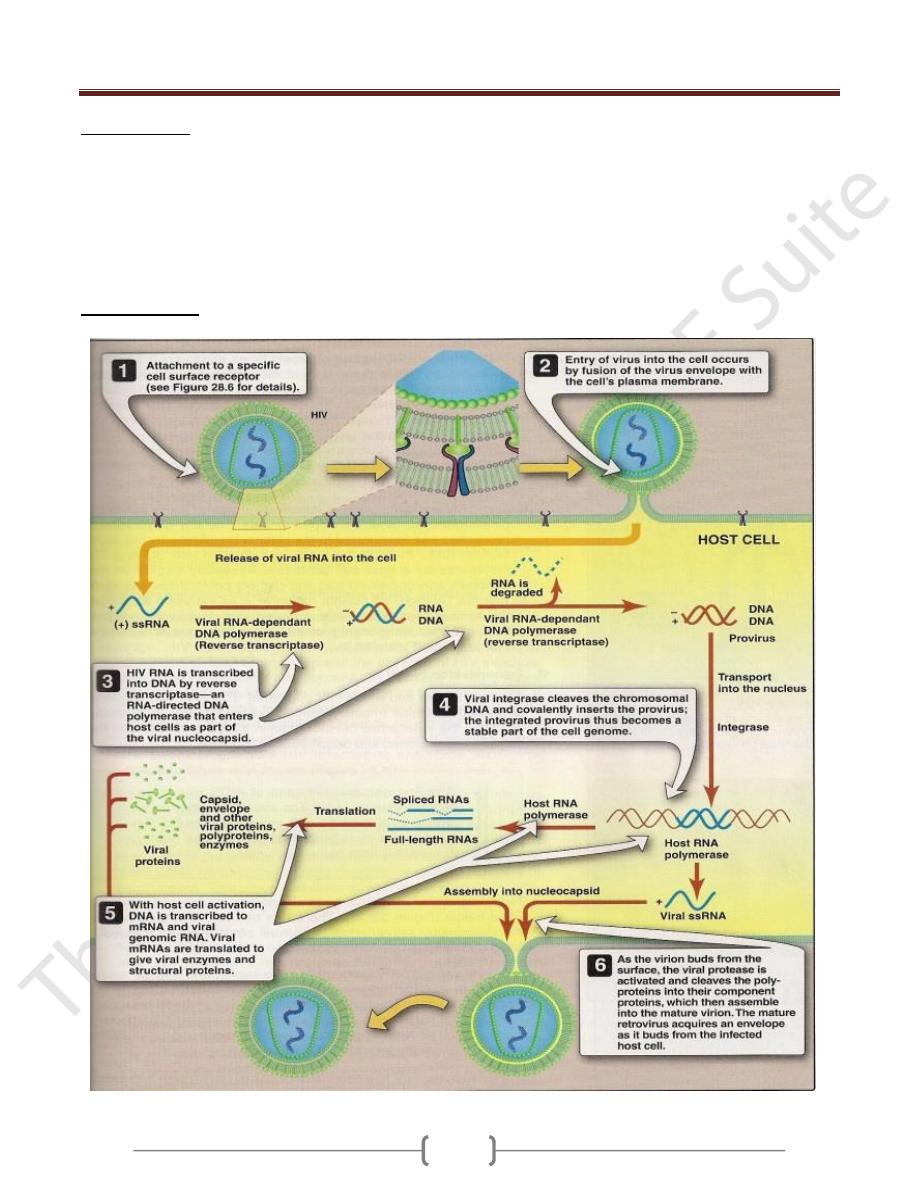
Replicative cycle

Unit 4: Virology
211
Transmission
1) Sexual contact
2) Transfer of infected blood
3) From infected mother to neonate.50% of neonatal
infection are acquired at time of birth.25% trancplacental
and 25% via breast feeding.
4) Sharing needles in I.V drug addicts.
5) Transfer through body fluid e.g. saliva, tear.
6) The risk of infection after percutaneous exposure and
needle- stick injuries is about 0.3% .This means that the
infectious dose of HIV is high.
Pathogenesis
HIV infects T
h
cells and kills them, resulting in
suppression of cell-mediated immunity. This predisposes
the host to various opportunistic infections and certain
cancers such as Kaposi ´s sarcoma and lymphoma. HIV
doesn’t directly cause these tumors because HIV genes
arte not found in these cancer cells.
Monocytes and macrophage play a major role in the
dissemination and pathogenesis of HIV infection.
Macrophage tropic strain HIV predominates in the early
infection and these strains responsible for initial
infection.T-tropic strain predominate later. It is believed
that the monocyte and macrophage serve as major
reservoirs for HIV in the body.
The initial infection of the genital tract occurs in dendritic
cells that line the mucosa, after which the local CD-4
+
Th
cells become infected .HIV,
is first found in the blood 4-11
days after infection.
Neurologic abnormalities are common in late stages of
infection and are AIDS-defining condition. The CNS
diseases are HIV encephalopathy, peripheral
neuropathies, and AIDS dementia complex. The
predominant cell types in the brain that are infected with
HIV are monocytes and macrophages.
B-cells abnormalities. Polyclonal activation of B cells is
seen, with resultant high Ig levels. Autoimmune diseases,
such as thrombocytopenia occur.
Mechanisms that explain the death of Th cells in HIV
infection.
1) The immunologic attack of the HIV infected cells by
Tc-CD-8 cells.
2) HIV acts as superantigen which activates many T
h
cells and leads to their death.
A group of HIV infected individuals has lived for
many years without opportunistic infection and
without a reduction in the number of their T
h
CD4 cells. Why?
1) The strain of HIV isolated from these individuals has
mutation in the Nef gene, indicating the importance of this
gene in pathogenesis.
The Nef protein decreases class I MHC protein synthesis,
and the inability of the mutant virus to produce functional
Nef protein allows the cytotoxic T cells to retain their
activity.
2) Production of large amounts of ALPHA- defensins which
has an antiviral activity in addition to its antibacterial
activity.
ALPHA-Defensins interfere with HIV binding to the
CXCR4 receptor and block entry of the virus into the cell.
Immunity
1) The main immune response to HIV infection consists of
cytotoxic CD8-positive lymphocytes. These cells respond
to the initial infection and control it for many years. It is
the ultimate failure of these cytotoxic T cells that results
in the clinical picture of AIDS.Cytotoxic T cells lose their
effectiveness because so many CD4-helper T cells have
died; thus , the supply of lymphokines, such as IL-2,
required to activate the cytotoxic T cells is no longer
sufficient.
2) HIV has three main mechanisms by which it evades the
immune system:
A. Integration of viral DNA into host cell DNA, resulting in
a persistent infection.
B. A high rate of mutation of the env gene.
C. The production of Tat and Nef proteins that downregulate
class I MHC proteins required for cytotoxic T cells to
recognize and kill HIV-infected cells. The ability of HIV
to infect and kill CD4-positive helper T cells further
enhances its capacity to avoid destruction by the immune
system.
Clinical finding
The clinical picture of HIV infection can be divided into
three stages: an early acute stage, a middle latent stage
and a late immunodeficiency stage.
Acute stage:
Begins 2- 4 weeks after infection with mononucleosis –
like picture of fever, lethargy, sore throat, and generalized
lymphadenoppathy occurs .Maculopapular rash on the
trunk, arms, and legs (sparing the palms and soles).
Leukopenia with normal number of CD-4
+
T cells.

Unit 4: Virology
211
High level of viremia and the infection is highly
transmissible.
This stage resolves spontaneously in about 2 weeks
accompanied by a lower level of viremia and a rise in the
number of CD-8
+
T cells directed against HIV.
Abs against HIV typically appears 10-14 days after
infection, and most will have seroconverted by 3-4 weeks
after infection. Window period prior to this period in
which the patient is infected with HIV but antibodies are
not detected leading to false negative results and 87% of
infected patients are asymptomatic.
After initial viremia a virus set point can occur which
represents the amount of virus produced (viral load) and
tend to remain constant for years.
Middle latent stage:
In untreated patient, this period lasts for 7-11 years and
the patient is a symptomatic and viremia is low or absent.
A large amount of HIV is being produced by lymph node
cells but remains sequestered within lymph nodes
A syndrome called AIDS-related complex (ARC) can
occur during this stage and manifested by persistent fever,
weight loss, fatigue and lymphadenopathy.ARC often
progress to AIDS.
Late stage:
The late stage of HIV infection is AIDS , manifested by
decline in the number of CD-4 cells to below 400/ µl
An increase in the frequency and severity of opportunistic
infection. The two most important is pneumocystis
pneumonia and Kaposi's sarcoma in addition to viral,
fungal , and bacterial infections.Neurologic problems also
occur e.g. dementia and neuropathy.
Laboratory diagnosis:
1) The presumptive diagnosis of HIV is made by the
detection of antibodies by ELISA.
2) Because there are some false –positive results with this test,
the definitive diagnosis is made by Western blot analysis in
which the viral proteins are displayed by acrylamide gel
electrophoresis, transferred to nitrocellulose paper and react
with patient´ s serum. If antibodies are present, they will
bind to the viral proteins. Enzymatically labeled Ab to
human IgG is then added .A color reaction reveals the
presence of HIV Ab in the serum.
3) Ora Quick is a rapid screening immunoassay for HIV Ab
detection within 20 minutes that uses a blood sample
obtained by finger prick. Positive Results confirmed by
western blot test.
4) Tissue culture. To assess drug resistance and for the
diagnosis of HIV infection in newborns whose mothers
are HIV
+
.
5) PCR to detect HIV DNA within infected cells and for
detection of viral load.PCR also can be used in the
diagnosis of HIV infection in neonate of HIV
+
mothers.
During the first month after infection, Ab tests may be
negative (window periods).The diagnosis of acute HIV
infection may not be made by using serologic tests. The
presence of HIV can be detected either by viral culture,
p24 antigen test, or PCR assay. Approximately 10-20
days after infection, an increase in HIV RNA can be
detected by PCR assay and by 30 days after infection, an
increase in p24 antigen can be seen in patients whose Ab
test results are negative.
Treatment
Management of HIV involves both treatment of the virus
and prevention of opportunistic infections. The aims of
HIV treatment are to:
reduce the viral load to an undetectable level (< 50
copies/ml) for as long as possible
improve the CD4 count (above 200 cells/mm
3
significant
HIV-related events rarely occur)
increase the quantity and improve the quality of life
without unacceptable drug-related side-effects or lifestyle
alteration
Reduce transmission (mother-to-child & person-to-person).
Treatment with single drugs is liable to develop resistance
to that drug because of high mutation rate in HIV.
The current treatment of choice for advanced disease is a
regimen consisting of two nucleoside (-tide) reverse
transcriptase inhibitors plus a protease inhibitor. This
combination is known as HAART (Highly Active
Antiretroviral Therapy).
In 2003, a new drug emerged, it is fusion inhibitors, and
i.e. they prevent the fusion of the viral envelope with the
cell membrane. The drug is called enfuvertide (Fuzeon).It
is a synthetic peptide that binds to gp 41 on the viral
envelope, thereby blocking the entry of HIV into the cell.
It is given by injection.
Nucleoside (tide) reverse transcriptase inhibitors:
1) Abacavir 2) Didanosine (dideoxyinosine, ddI)
3) Lamivudine 4) Stavudine 5) Tenofovir
6) Zalcitabine 7) Zidovudine (ZDV,AZT)
Non-nucleoside reverse transcriptase inhibitors (NNRTI):
1) Nevirapine 2) Delavirdine 3) Efavirenz
Protease inhibitors:
1- Saquinavir 2- Ritonavir
3- Nelfinavir 4- Indinavir

Unit 4: Virology
215
Human T-cell lymphotropic virus (HTLV-1)
Human T-cell lymphotropic virus -1(HTLV-1) causes 2
distinctly disease:
a) A cancer called Adult T-cell leukemia /lymphoma
(ATL).
b) A neurologic disease called HTLV-associated
myelopathy (HAM) (spastic paraperesis, chronic
progressive myelopathy).
HTLV-2 also appears to cause these diseases, but the
association is less clearly documented.
Important properties
Two copies of SS positive polarity RNA enveloped virus
with reverse transcriptase in the virion.HTLV infects T
cell lymphocyte and cause malignant transformation of
the infected T cell but don’t kill them.
HTLV genome
HTLV genome is more stable than that of HIV.
Three structural genes common to all retroviruses (gag,
pol, env (gp 46, gp21)) plus two regulatory genes, tax
and rex (similar in action to tat and rev).
HTLV don’t possess an oncogene in its genome and does
not integrate its proviral DNA at a specific site near a
cellular oncogene, but it activate transcription of both
cellular and viral mRNA synthesis by the Tax protein that
initiates oncogenesis.The tax protein activates the
synthesis of IL-2 and of the IL-2 receptors.IL-2 promotes
rapid T-cell growth and eventually malignant
transformation of the T-cell.
Replicative cycle
As for HIV.
Transmission and epidemiology
1) Intravenous drug use.
2) Sexual contact.
3) Breast feeding
4) Blood transfusion. This can greatly reduced by screening
of donated blood for antibodies to HTLV and discarding
those that are positive.
5) Transmission by processed blood products, such as
immunglobulins has not occurred.
6) Trancplacental transmission has been rarely documented.
7) Transmission is thought to occur primarily by the transfer
of infected cells rather than free extracellular virus.
8) HTLV is endemic in certain geographic areas, The
Caribbean region including southern fluoride, eastern
South America, western Africa and Southern Japan.
Pathogenesis
ATL in which HTLV infection of CD4-positive T
lymphocytes induce malignant transformation. HTLV
remain latent within malignant cells.
HAM is a demylinating disease of the brain and spinal
cord, especially of the motor neuron in the spinal cord.
HAM is caused either by an autoimmune cross-reaction in
which the immune response against HTLV damage the
neurons or by cytotoxic T cells that kill HTLV-infected
neurons.
Clinical feature
1) ATL
It is characterized by lymphadenopathy,
hepatosplenomegaly, lytic bone lesion, and skin lesions,
hypocalcaemia due to increase osteoclast activity,
opportunistic infections.
2) HAM
Gait disturbance, weakness of the lower limbs, and low
back pain, loss of bowel and bladder control, loss of
motor function is much greater than sensory loss. HAM
occurs primarily in women of middle age. HAM
resembles multiple sclerosis but without the remission
characteristic of multiple sclerosis.
Laboratory diagnosis:
1) ELISA test for Ab detection in patient s serum. Positive
test is confirmed by western blot assay
2) PCR assay for HTLV RNA or DNA within infected cells.
3) ATL is diagnosed by finding malignant T cells in the
lesion.
4) HAM is diagnosed by HTLV Ab in the spinal fluid or
finding HTLV nucleic acid in cells in spinal fluid.
Treatment
There is no specific antiviral treatment for HTLV
infection, and no antiviral drug will cure latent infections
by HTLV.
ATL is treated with anti-cancer chemotherapy regimens
Corticosteroids and danazol have produced improvement
in some patient with HAM.
