Unit 1: Immunology
32
Lecture 8+9+10 - Immune response to
infectious diseases
(Viral infection)
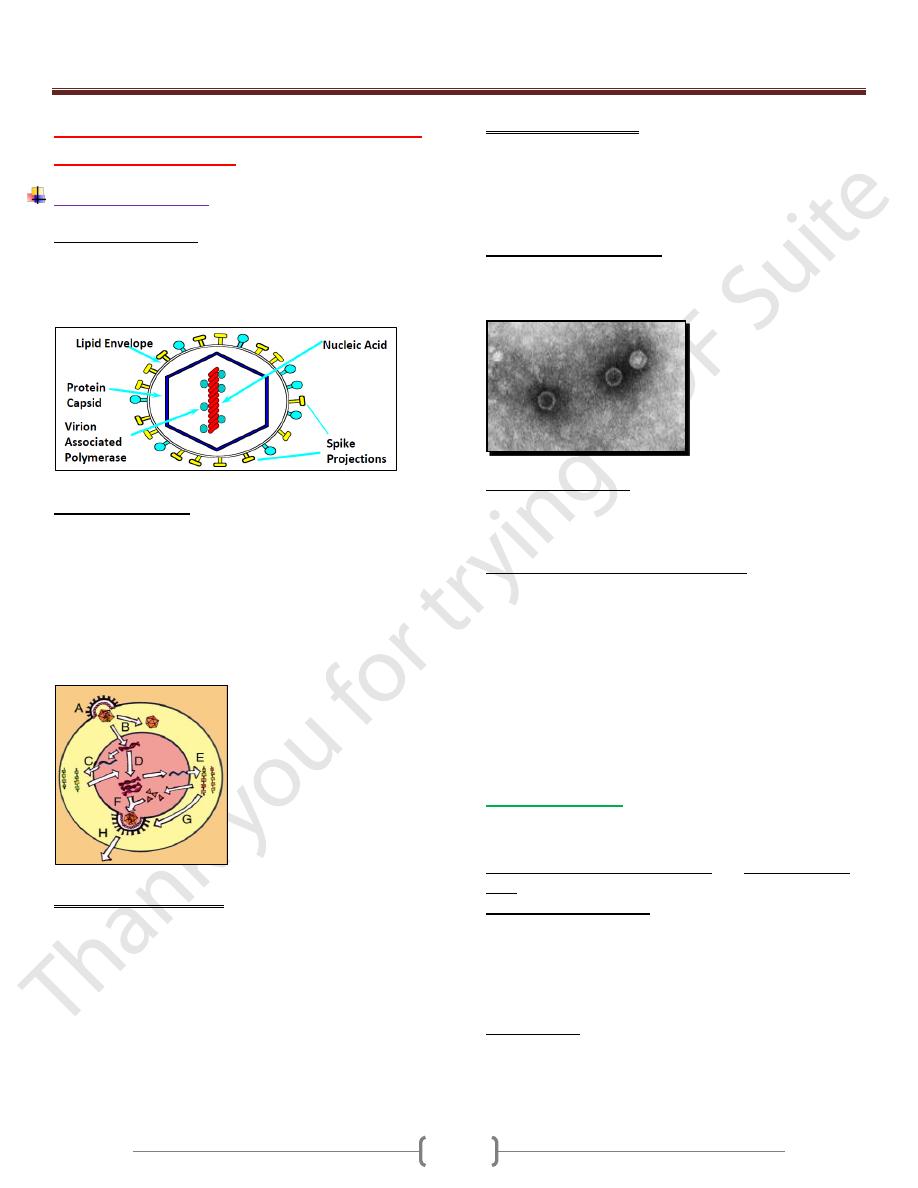
Definition of a Virus
Sub microscopic organism consisting of a single nucleic acid
surrounded by a protein coat and capable of replication only
within the living cells of bacteria, human, animals or plants.
It is obligate Intracellular Parasite
Virus Replication
A. Virus attachment and entry
B. Uncoating of virion
C. Migration of genomic nucleic acid to nucleus, and
Transcrirption
D. Genomic replication
E. Translation of viral mRNA
F and G. Viroin assembly
H. Release of new virus particles
Transmission of Viruses
Respiratory transmission
Influenza A virus
Faecal-oral transmission
Enterovirus
Blood-borne transmission
Hepatitis B virus
Sexual Transmission
HIV
Animal or insect vectors
Rabies virus
Virus Tissue Tropism
Targeting of the virus to specific tissue and cell types
Receptor Recognition
CD4+ cells infected by HIV
CD155 acts as the receptor for poliovirus
In vivo Disease Processes
Cell destruction
Virus-induced changes to gene expression
Immunopathogenic disease
Acute Virus Infections
Localised to specific site of body
Development of viraemia with widespread infection of tissues
Immunity against Viral Infections
Within viruses life cycle they have a relatively short
extracellular period, prior to infecting the cells, and a longer
intracellular period during which they undergo replication.
The immune system has mechanisms which can attack the
virus in both these phases of its life cycle, and which involve
both innate and adaptive effectors mechanisms.
Immunological reactions are thus of two kinds:
Those directed against the virion are predominantly humoral
Those that act upon the virus infected cell are T-cell-mediated.
1) Innate Immunity:
Involves skin, mucous membrane, HCL, enzymes in tears and
secretions, toll-like receptors, but primarily through the
induction of type I interferons (α,β) and activation of NK
cells.
Toll-like receptors (TLR):
TLR allow cells to “see” molecules, it signifying presence of
microbes outside the cell e.g. TLR2, TLR4 and found in
variety of cell types
Toll-like receptor 9 (TLR9) Intracellular sensing in
monocytes, dendritic cells and lymphocytes
NOD proteins:
Are dipeptides binding intracellular protein, signaling via NF-
κb. These receptors recognize bacterial cell wall components
within cytoplasm

Unit 1: Immunology
33
RIG-I:
Intracellular sensing of RNA viruses e.g. Hepatitis C.
It induces type I interferons
A. Induction of type I interferons (IFN-α and IFN-β).
Are one of the first lines of defense against viral infections. ds
RNA produced during the viral life cycle can induce the
expression of IFN- α and IFN- β by the infected cells leading
to induction of "antiviral response or resistant to viral
replication” by binding to IFN- α / β receptor. They activate
the (JAK-STAT) transcription pathway, which in turn induces
the activation of several genes. One of these genes encodes the
enzyme known oligo-adenylate synthetase which activates a
ribonuclease (RNAse) that degrades viral RNA. and also
blocking viral replication.
B. Natural Killer Cells (NK):
NK cells possess the ability to recognize and lyses virally
infected cells and certain tumor cells.
During the initial stages of infection, NK cells undergo non-
specific proliferation mediated by IFN- α and IFN- β and IL-
12. There is no "lag" phase of clone expansion for NK cells to
be active as effectors, as there is with antigen-specific T and B
lymphocytes. Thus NK cells may be effective within 2 days of
viral infection, and may limit the spread of infection during
this early stage.
2) Adaptive Immunity:
A. Viral neutralization by humoral antibodies
Antibodies specific for viral surface antigens are crucial in
preventing the spreading of the virus during the acute infection
and in protecting against reinfection.
Secretary IgA (sIgA): blocking viral attachment to mucosal
cells.
IgG, IgM, IgA: blocks fusion of viral envelop to host cell
plasma membrane.
IgG, IgM: enhance phagocytosis by opsonization.
IgM: agglutinates viral particles.
Complement activated by Ag-Ab immune complex leading to
lysis of virus by membrane attack complex and lysis of
enveloped viral particles.
B. Cell mediated immune response mediated by cytotoxic T
lymphocytes (CD8+) that kill virus infected cell, after
induction by activated Th1 cells which produce a number of
cytokines including IL-2, IFN-γ and TNF-α that defends
against viruses either directly or indirectly.
Mechanism of killing of MHC class I- restricted CD8+ CTLs
cells specific for the virus elimination through the release of
perforin and granzymes or through Fas-FasL interaction. CTL
activity arises within 3-4 days, peak by 7-10 days and then
decline.
But in case of persistent virus infection (e.g. hepatitis B
virus), CTLs release IFN-γ and TNF, resulting in clearance
of the virus without death of the cell.
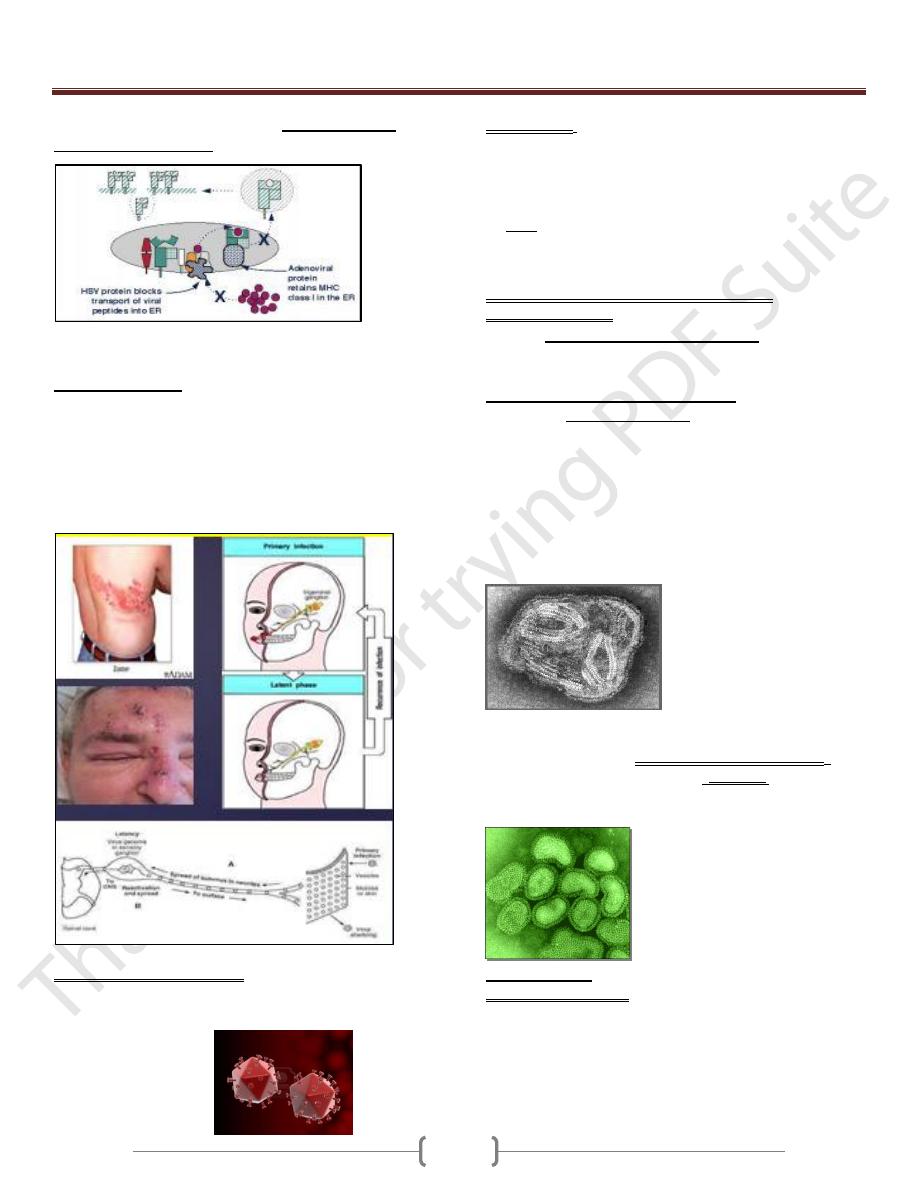
Viral evasion of host- defense mechanism
1)
Some viruses developed strategies to evade the action of IFN-
α/β. These include hepatitis C virus, which binds to IFN
receptor & blocking or inhibiting the action of protein kinase
(PKR).
2)
Herpes Simplex Viruses (HSV) inhibiting antigen
presentation by infected host cells.
HSV -1 and HSV-2 both express an immediate- early protein
that synthesized shortly after viral replication, they effectively
inhibits the human transporter protein TAP needed for
antigen processing, and thus blocks peptide association with
the class I MHC and effectively shut down a CD8+ T- cell
responses to HSV- infected cells.
Unit 1: Immunology
34
Other viruses used this strategies are: Adenoviruses and
Cytomegalovirus (CMV)
How can viruses interfere with endogenous Ag processing
as part of the active mechanism of evading immunity?
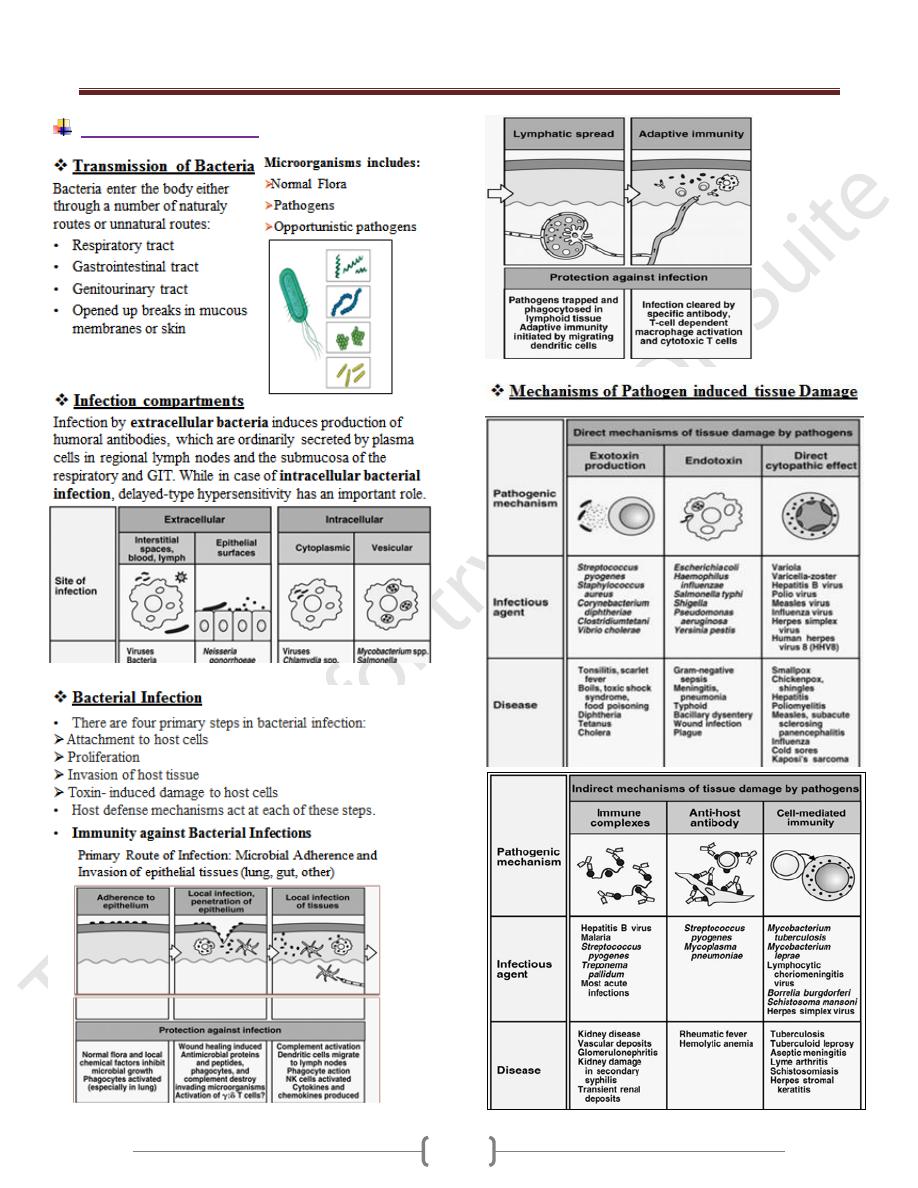
Herpes zoster virus can remain inactive or in latent state
inside the cell (latency virus genome in sensory ganglion) and
undergoing periodic cycles of activation and replication. The
viral genome remains within the host cell but no expression of
viral antigens occurs. When the host defense is upset perhaps
by other infections, the virus may be activated (it is the most
effective mechanism).
3)
CMV, Measles virus and HIV, have been shown to down
regulation of class II MHC on the cell surface, thus blocking
the function of antigen- specific anti- viral helper T cells.
HIV
4)
Vaccina virus is the prototypic member of the poxvirus
family of cytoplasmic DNA viruses have strategies for
evading complement- mediated destruction, by secretion a
protein that binds to the C4b complement component thus
inhibiting the classical pathway.
HSV have glycoprotein component that binds to C3b
complement components, and inhibiting both the classical
and alternative pathways.
5)
Large number of viruses causing generalized
immunosuppresion.
Either by direct infection of B lymphocytes (e.g. Epestien
Bar Virus), or macrophages (e.g. Measles virus) resulting in
direct lysis of immune cells or alter their function. HIV
destroy CD4+ T cells and macrophages
Some causes cytokine imbalance, (e.g. EBV) inhibits T
lymphocytes by producing a protein termed BCRF1 that is
homologous to IL-10 and thus suppress the cytokine
production by the Th 1 subset, resulting in decreased levels of
IL-2, TNF, and IFN-γ.
Measles Virus binds the complement regulatory protein CD46
(is a human cell receptor for measles virus ) on macrophages.
Example: paramyxoviruses that cause mumps, the measles
virus, epstein- barr virus (EBV), CMV and HIV.
6)
Some viruses escape immune attack by constantly changing
their antigens, as in the Influenza virus, Rhinoviruses, the
causative agent of the common cold, and HIV results in the
frequent emergence of new infectious strains due to mutation
and antigenic variation.
Influenza virus
Properties of the virus
o Myxovirus
o Enveloped virus with a segmented RNA genome
o Infects a wide range of animals other than humans
o Undergoes extensive antigenic variation
o Major cause of respiratory infections

Unit 1: Immunology
35
o Influenza viral particles or virions are spherical in shape
surrounded by an outer envelope, a lipid bilayer. Two
glycoproteins particles inserted into the envelope,
hemagglutinin (HA) and neuraminidase (NA), which form
radiating projections.
Hemagglutinin are responsible for the attachment of the
virus to host cells.
Neuraminidase facilitates viral budding from the infected
host cell.
Each virus strain is defined by its animal host of origin or
human, strain number, year of isolation, antigenic
description of HA and NA
o Two different mechanisms generate antigenic variation in HA
and NA:
1. Antigenic drift involves a series of spontaneous point
mutations that occur gradually, resulting in minor changes in
HA and NA.
2. Antigenic shift results in the sudden emergence of a new
subtype of influenza whose HA and possibly also NA are
different from that of the virus present in a preceding
epidemic.
Generation of Novel Influenza A Viruses
o Host response to influenza infection
Humoral antibody specific for the HA molecule is produced
during an influenza infection.
The antibody protects against influenza infection, but its
specificity is strain- specific and is readily bypassed by
antigenic drift.
CTLs can play a role in immune response to influenza.
Unit 1 - Immunology
36
Bacterial infection
Unit 1 - Immunology
37

Unit 1 - Immunology
38

Unit 1 - Immunology
39
Parasitic Infection
Immunity against parasites
Parasites of major medical important successfully adapted
to innate & acquired immune responses of host.
Parasites can cause direct damage to host by:
Competing for nutrients (e.g. tapeworms).
Disrupting tissues (e.g. Hydatid cyst) or destroying
cells (e.g. malaria, hookworm, schistosomiasis; feeding
on or causing destruction of cells causing anaemia).
Mechanical blockage (e.g. Ascaris in intestine).
However, severe disease often has a specific immune or
inflammatory component.
Protozoan Diseases
Protozoans are unicellular eukaryotic organisms. They are
responsible for several serious diseases in humans,
including amoebiasis, African sleeping sickness,
malaria, leishmaniasis, and toxoplasmosis.
Parasitic protozoa may live:
In the gut (e.g. amoebae)
In the blood (e.g. African trypanosomes)
Within erythrocytes (e.g. Plasmodium spp.)
In macrophages (e.g. leishmania spp., Trypanosomes)
In liver and spleen (e.g. leishmania spp.)
Immune responses against protozoan infection
The types of immune response that develop depend on
the location of the parasite within the host.
Many protozoans have life-cycle stages in which they are
free within the blood stream , the humoral antibody is
most effective (e.g. T. brucei)
Unit 1 - Immunology
40
Many of these same pathogens are also capable of
intracellular growth, during these stages, cell mediated
immune reaction are more effective in host defense.
(e.g. Plasmodium malariae (liver and blood stages),
T. cruzi and Leishmamia ( inside macrophages)
Role of Abs in protozoan infection
Antibody responses.
Extracellular protozoa are eliminated by:
Opsonisation and enhance phagocytosis.
Has direct damage, lysis of protozoa by Ag-Ab
immune complex and complement activation.
Intracellular protozoa are
Prevented from entering the host cells by a process of
neutralizing attachment sites, e.g. neutralising
antibody against malaria sporozoites, blocks cell
receptor for entry into liver cells.
Prevents escape from lysosomal vacuoles
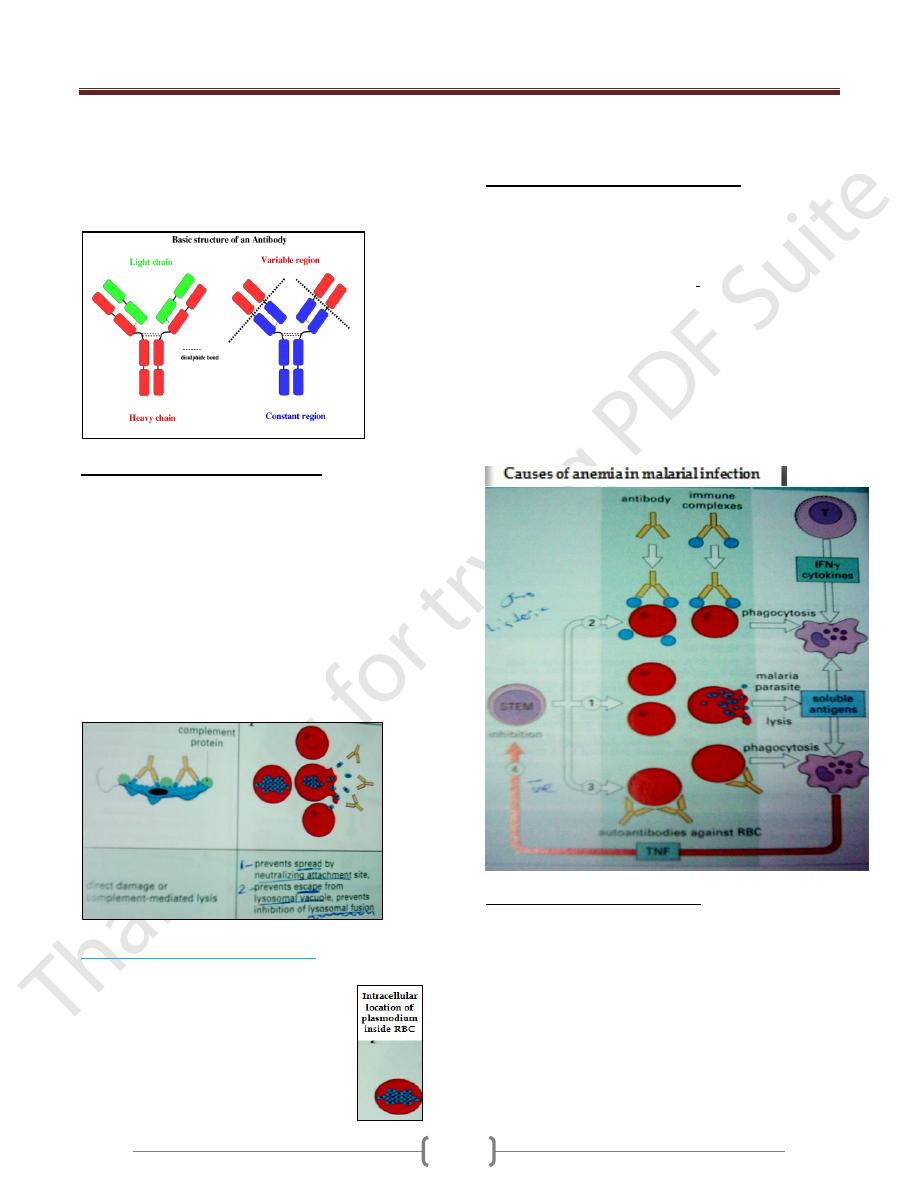
Malaria (Plasmodium Species)
It is caused by various spp. of genus Plasmodium, of
which the P. falciparum is the most
virulent. Human infection begins when
sporozoites are introduced into
individual's blood stream as an infected
mosquito takes a blood meal. Then they
migrate to the liver, after that the released
merozoites infect RBCs initiating the symptoms and
pathology of malaria.
Host Response to Plasmodium Infection
In regions where malaria is endemic, the immune
response to plasmodium infection is poor with low
antibody titer.
The type of T cells responsible for controlling an infection
varies with the stage of infection, and depends upon the
kinds of cytokine they produce.
B cells mediate immunity against blood stage
CD8 T cells protect against the liver stage
The action of CD8+ T cells is two folds:
1) They secrete IFN-γ which inhibits the multiplication of
parasites within hepatocytes.
2) They are able to kill infected hepatocytes, but not
infected erythrocytes.
Evasion strategies of Plasmodium
Plasmodium needs time in host to complete complex
development, to sexually reproduce & to ensure vector
transmission.
Chronic infections (from a few months to many years) are
normal; therefore parasite needs to avoid immune
elimination.
Plasmodium has evolved a way of overcoming the
immune response by sloughing off the surface CS-
antigen coat, thus rendering the antibodies ineffective
(immunosupression)
Unit 1 - Immunology
41
The maturational changes from sporoziote to merozoite
to gametocyte allow the organism to keep changing its
surface molecule resulting in continual changes in the
antigen seen by the immune system.
African sleeping sickness (Trypanosoma
Species)
Two spp. of African trypanosomes (Trypanosoma brucei,
Trypanosoma cruzi), which are flagellated protozoan,
can cause sleeping sickness, it transmitted to humans and
cattle by the bite of tsetse fly.
The disease beginning with an early (systemic) stage in
which trypanosomes multiply in the blood and
progressing to a neurologic stage in which the parasite
infects the CNS causing meningeoecephalitis.
Host Response to Trypanosoma Infection
Humoral antibody
African Trypanosomes have one surface glycoprotein that
covers the parasite. This protein is immunodominant for
antibody responses .
The glycoprotein coat, called variant surface
glycoprotein (VSG).
These Abs eliminate most of the parasites from the blood
stream both by complement- mediated lysis and by
opsonization and subsequent phagocytosis.
However about 1% of the organisms which bear an
antigenically different VSG escape the initial Abs
response.
Evasion strategies of Trypanosoma
1) Antigenic variation
Trypanosoma brucei have “gene cassettes” of variant
surface glycoproteins (VSG’s) which allow them to
switch to different VSG.
Several unusual genetic processes generate the
extensive variation in trypanosomal VSG that enable
the organism to escape the immunological clearance.
VSG gene is switched regularly. The effect of this is
that host mounts immune response to current VSG
Abs but parasite is already switching VSG to another
type which is not recognised by the host. A parasite
expressing the new VSG will escape antibody
detection and replicate to continue the infection.
This allows the parasite to survive for months or years.
Up to 2000 genes involved in this process.
This type of antigenic variation is known as
phenotypic variation and is in contrast to genotypic
variation in the case of influenza virus in which a new
strain periodically results
2) Suppression of the immune response, e.g.
Trypanosoma cruzi produces molecules that either inhibit
the formation or accelerate the decay of C3 convertase, so
blocking complement activation on the parasite surface.
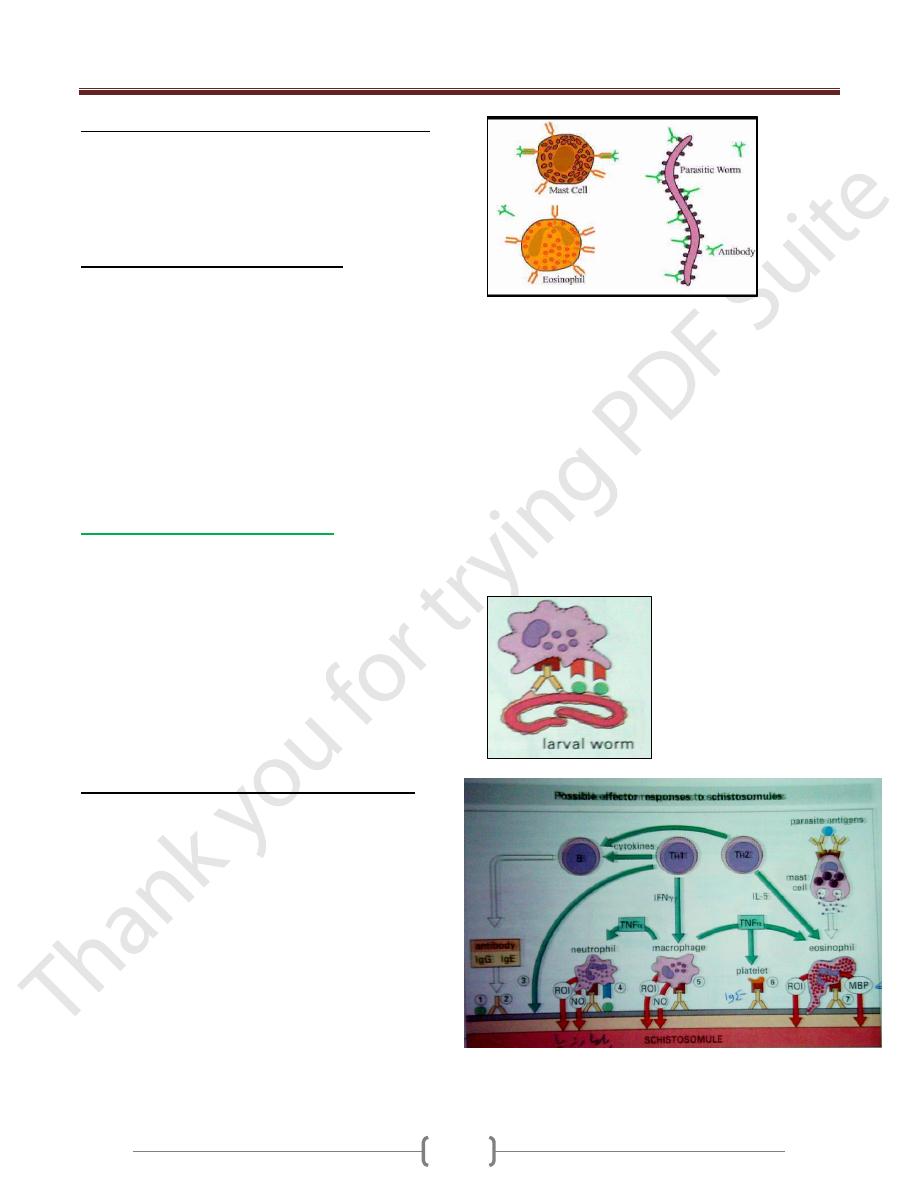
Leishmaniasis:
For Intra cellular protozoa
Leishmania major is a protozoan that
lives in the phagosomes of
macrophages.
Resistance to the infection correlates
with the cytokines secreted from TH1
and activation of Macrophages by
IFN-γ and TNF-α and killing by
Nitrous oxide and O2 metabolites
Unit 1 - Immunology
42
Immune evasion mechanisms of Intracellular protozoa
There are three main ways in which protozoa can evade or
modify the host's immunological attack:
1. Antigenic modulation
2. Resistance to macrophages killing
3. Suppression of the immune response
Protozoan immune evasion strategies
1. Leishmania can evade the immune surveillance by
Antigenic modulation, which can rapidly change their
surface coat (cap off) within minutes of exposure to
antibodies, so becoming refractory to the effects of
antibodies and complement.
2. Toxoplasma has evolved mechanisms which prevent
fusion of phagocytic vacuoles with lysosomes and resist
to macrophages killing.
3. Leishmania produce anti-oxidases to counter products of
macrophage oxidative burst, resist lysosomal enzymes
and suppressed the immune responses
Parasitic Worms (Helminthes)
Too large for phagocytosis BUT Immune response can
activate inflammation which results in expulsion of
worms.
Anti-worm IgE can activate degranulation of mast cells
and eosinophils leads to Type I hypersensitivity like
responses.
Initiation of response is poorly understood. Unusual
carbohydrates can be recognized by innate and adaptive
(antibody) responses. These responses are regulated by
the TH2 subsets of CD4 T lymphocytes.
The immunological responses in helminthes diseases
1) High titer of IgE that induced by a substance released
from the parasite acting as B cell mitogens.
2) Accumulation of mast cell and degranulation of these
cells releasing Eosinophils chemotactic factor (ECF),
Neutrophil chemotactic factor (NCF)
3) cytokines secretion by Th2 :
IL-4 induces B-cells to class switching to IgE
production ,
IL-5 induces bone marrow precursors to differentiate
into Eosinophils ,
IL-13 stimulates growth of mast cells
4) Ab- Ag complex activates complement ending in cell lysis
5) The eosinophils express Fc receptors for IgE and IgG and
bind to the Ab- coated parasite. Once bound to the
parasite, an eosinophil can participate in Ab- dependent
cell- mediated cytotoxicity (ADCC), releasing mediators
from its granules called Major basic protein (MBP) that
is toxic to helminthes causing small holes in the surface of
the helminth.
6) Neutrophils and macrophages act by releasing toxic O2
and N2 metabolites
7) In case of Nematodes, there is proliferation and
stimulation of goblet cells, increased mucus secretion
leads to expulsion of worm

Unit 1 - Immunology
43
Helminth immune evasion strategies
1) Antigenic disguise: (e.g. Adult Schistosoma)
Decrease expression of Ag on its outer surface.
Enclosed itself in a glycolipid and a glycoprotein coat
derived from the host , masking its own Ags Like
ABO blood group Ags
2) Suppression of T- & B- cell responses: young schistosomes
actively protect themselves by releasing peptidases that
cleave bound immunoglobulin & other factors that inhibit
both T-cell proliferation 7 release of IFN-γ or the mast cell
signal required for eosinophil activation.
3) Location inside the lumen of the gut like nematodes or
encysted inside protective cyst like Trichenella spirallis.
4) Presence of a thick extracellular cuticle like tegument
of Schistosomes which protect them from the immune
system
5) Molecules production that interfere with host immune
function e.g. Filarial worms secrete a protease inhibitor
Filarial worm evasion of immune responses
Compare between immune response to protozoa
and helminthes
Fungal Infection
Fungi are eukaryotes with a rigid cell wall enriched in
complex polysaccharides such as chitin, glucans. Among
the 70000 species of fungi, only a small number are
pathogenic for humans.
Fungal infections are regularly seen in:
* Patients with untreated AIDS.
* Patients with cancer and undergoing chemotherapy.
* Patients with transplants on immunosuppressive agents.
* Some patients taking long-term corticosteroids.
Degree of fungal infection can range from cutaneous to
deep and systemic
