Unit 1 - Immunology
7
Lecture 2 – Antibodies & Cytokines
Immunoglobulin Antibody (Ab)
1) Blood from an individual and put it in a plain tube
without anticoagulant and left it for half an hour.
2) Blood will coagulate and you will get serum.
Protein electrophoresis: Gamma globulin fraction of
protein has antibody activity.
Antibody
Is a specific glycoprotein developed for a specific
antigen.
Synthesized by:
B-Cells armed on its surface and act as a surface
molecule bind an antigen
plasma cell that secrets free specific antibodies
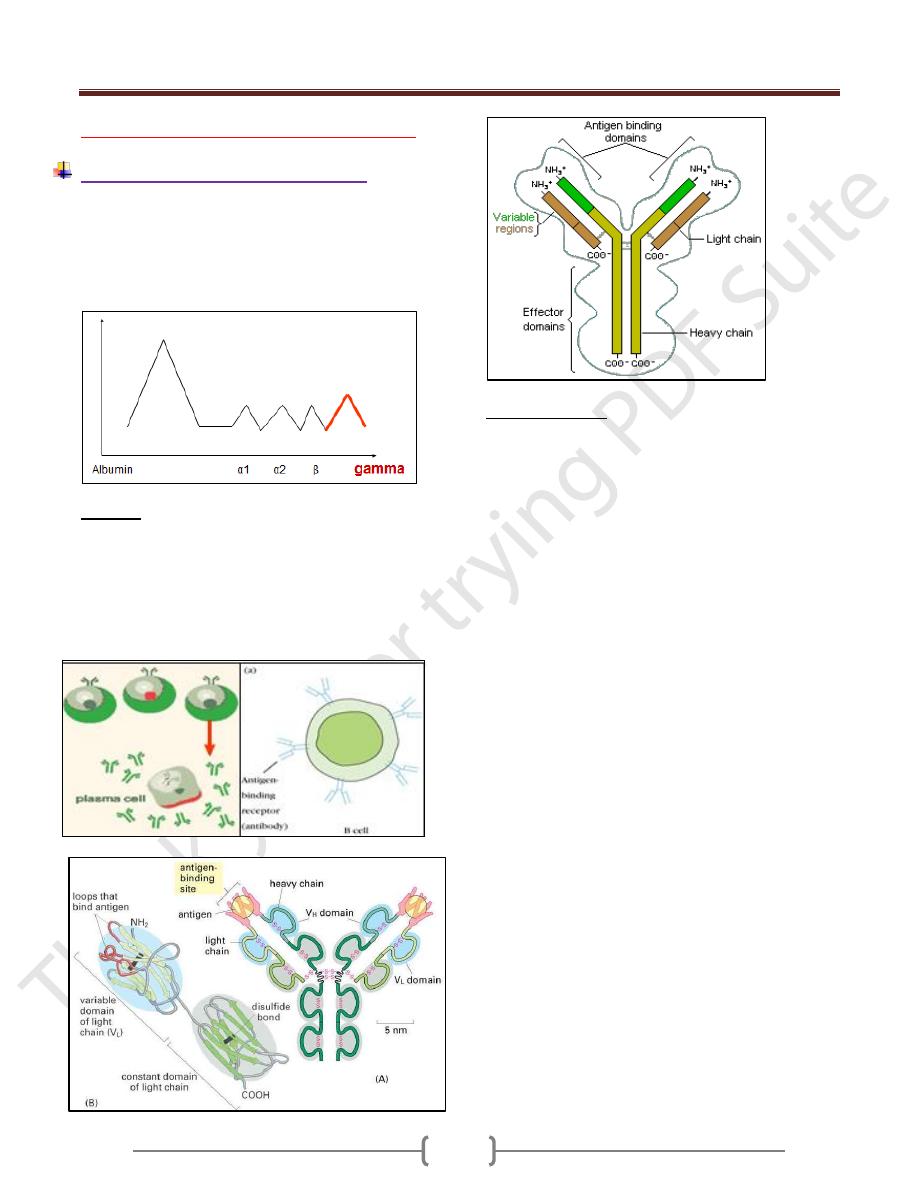
Structure of Ab
Each antibody is made up of two identical heavy
polypeptide chains and two identical light polypeptide
chains, shaped to form a Y Linked covalently bind by a
disulfide bounds
Heavy chain (H) has a molecular weight twice that of
light chain (L), so called heavy and light.
Each polypeptide chain is not linear but folded to form
domes or loops by intrachain disulfide bonds (-s-s) and
called domains.
Light chain had one VL and CL domain
Heavy chain had one VH an CH 1,2,3,
Hinge region: area of heavy chain between CH1 and CH2
domains where the disulfide bond is present. It is a
flexible area permits the movement of Ab binding
fragment (Fab) from 30-180
o
.
Each chain has two regions:
1) The Variable Region: makes up the tips of the Y's arms,
represent the amino terminal of polypeptide chain, varies
greatly in shape from one antibody to another.
This variation is due to change in aa sequence for this
reason called the variable region. it has unique shape
that "match" antigen to antibody., such as a lock
matches a key
2) The Constant Region: The stem of the Y activates the
complement system and encourages phagocytosis
Its amino acid content and sequence is relatively
constant and identical in all antibodies of the same
class and it's called the constant region. It represents
the carboxy terminal of polypeptide chain. This region
of the antibody molecule is called the Fc
region because it can be crystallized.
Unit 1 - Immunology
8
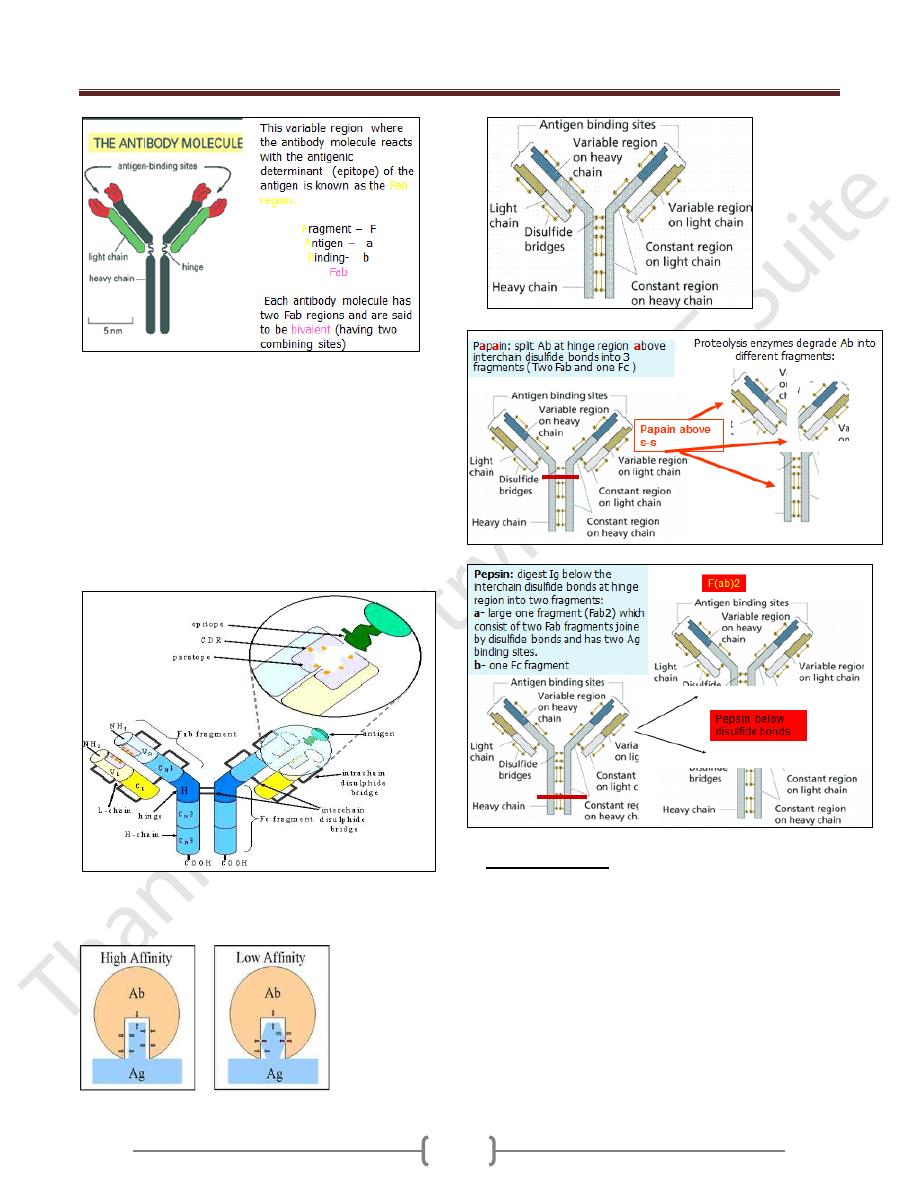
In this variable region (heavy and light) , there is a three
area called a hypervariable region in that area the aa
sequence is highly variable and called the
Complemantarity Determining Region (CDR). This binds
epitope of Ag.
The regions between the complementarity determining
regions are called the framework regions
Paratope: It is a small region (of 15–22 amino acids) of
the antibody's Fv region and contains parts of the
antibody's heavy and light chains
Affinity: Strength of interaction between single epitope
and single paratope.
Functions of Igs
1) Activation of complement
2) Opsonization
3) Ab dependent cell mediated cytotoxicity
4) (ADCC)
5) 4- Neutralization of toxins
6) 5- Agglutination of RBC
7) 6- Blocking the reaction
Unit 1 - Immunology
9
Immunoglobulin Classes (ISOTYPES)
Immunoglobulin classes
The immunoglobulins can be divided into five different
classes, based on differences in the amino acid
sequences in the constant region of the heavy chains.
1. IgG - Gamma heavy chains
2. IgM - Mu heavy chains
3. IgA - Alpha heavy chains
4. IgD - Delta heavy chains
5. IgE - Epsilon heavy chains
Immunoglobulin Subclasses
The classes of immunoglobulins can be divided into
subclasses based on small differences in the amino acid
sequences in the constant region of the heavy chains. All
immunoglobulins within a subclass will have very similar
heavy chain constant region amino acid sequences.
1. IgG Subclasses
a) IgG1 - Gamma 1 heavy chains
b) IgG2 - Gamma 2 heavy chains
c) IgG3 - Gamma 3 heavy chains
d) IgG4 - Gamma 4 heavy chains
2. IgA Subclasses
a) IgA1 - Alpha 1 heavy chains
b) IgA2 - Alpha 2 heavy chains
Immunoglobulin Types
Immunoglobulins can also be classified by the type of light
chain that they have. This based on differences in the amino
acid sequence in the constant region of the light chain
1-Kappa light chains
2- Lambda light chains
Immunoglobulin Subtypes
The light chains can also be divided into subtypes based
on differences in the amino acid sequences in the constant
region of the light chain.
1- Lambda 1 2- Lambda 2 3- Lambda 3 4- Lambda 4
Nomenclature
Immunoglobulins are named based on the class, or
subclass of the heavy chain & type or subtype of light
chain.
IgG = Immunoglobulin Gamma .
IgM - Immunoglobulin Mu
IgA - Immunoglobulin Alpha
IgD - Immunoglobulin Delta
IgE - Immunoglobulin Epsilon
IgG
Structure
The structures of the IgG are made up of two identical
heavy chains and two identical light chains.
All IgG's are monomer. MW=150 000 d.
called so because of its gamma heavy chain
The subclasses (IgG1, IgG2, IgG3, IgG4) differ in the
number of disulfide bonds and length of the hinge region.
Properties:
a) IgG is the major Ig in serum - 75% of serum Ig
b) IgG is the major Ig in extra vascular spaces
c) Placental transfer - IgG is the only class of Ig that crosses
the placenta. IgG2 does not cross well.
d) Fixes complement - Not all subclasses fix equally well;
IgG4 does not fix complement
e) Binding to cells - Macrophages, PMN IgG2 and IgG4 do
not bind to Fc receptors. A consequence of binding to the
Fc receptors on PMNs, monocytes and macrophages. The
antibody has prepared the antigen for eating by the
phagocytic cells. The term opsonin is used to describe
substances that enhance phagocytosis.
f) main Ig in the secondary immune response
IgA
Structure
1- Serum IgA is a monomer
2- IgA found in secretions is a dimer
When IgA exits as a dimer, a J chain is associated with it &
another protein associated with it called the secretory piece
J chain: small glycoprotein that are covalently linked to
the carboxy terminal portions of heavy chains.
Secretary component: is a polypeptide chain synthesized
by exocrine epithelial cells that enable IgA to pass
through mucosal tissues into secretions and protect IgA
from protease enzymes.
Properties
a) IgA is the 2nd most common serum Ig.
b) IgA is the major class of Ig in secretions - tears, saliva,
colostrum, mucus called secretory IgA
c) IgA activates the alternative pathway of complement
d) IgA can bind to some cells - PMN's and some
lymphocytes.
e) E) MW=150 000- 600 000 d
f) F) constitutes 10-15 % of serum Ig
Unit 1 - Immunology
10
g) It is called so because of its alpha heavy chain
components and of two subclasses:
1- Alpha 1-----IgA1
2- alpha 2 -----IgA2
IgM
Structure
1) IgM normally exists as a pentamer but it can also exist as
a monomer on B cell. In the pentameric form all heavy
chains are identical and all light chains are identical.
Thus, the valence is theoretically 10 times
2) IgM did not has a hing region and replaced by an extra
domain on the mu chain (CH4) , so it has 4 constant
heavy domains
3) It has another protein covalently bound via a S-S bond
called the J chain. This chain functions in polymerization
of the molecule into a pentamer.
Properties:
a. IgM is the third most common serum Ig. Constitute 5-
10% of total serum Ig .
MW=900 000 dalton
b. IgM is the first Ig to be made by the fetus and the first Ig
to be made by a virgin B cells as an Ag receptor.
c. As a consequence of its pentameric structure, IgM is a
good complement fixing
d. As a consequence of its structure, IgM is also a good
hemagglutinating Ig
e. IgM binds to some cells via Fc receptors.
f. Called so because of Mu heavy chain
IgE
Structure
IgE exists as a monomer and has an extra domain in the
constant region had four CH domains.
Properties
a) IgE is the least common serum Ig since it binds very
tightly to Fc receptors on basophils and mast cells
b) Involved in allergic reactions - Binding of the allergen to
the IgE on the cells results in the release of various
pharmacological mediators that result in allergic
symptoms.
It is called homocytotropic (bind cell) & called reagenic Ab
c) IgE also plays a role in parasitic helminth diseases. Since
serum IgE levels rise in parasitic diseases, measuring IgE
levels is helpful in diagnosing parasitic infections.
Eosinophils have Fc receptors for IgE and binding of
eosinophils to IgE-coated helminths results in killing of
the parasite.
d) IgE does not fix complement.
e) MW=190 000 d
f) constitutes about 0.002% of total serum Ig
g) called IgE because of its epsilon ε heavy chain
components
IgD
Structure
IgD exists only as a monomer.
Properties
a) IgD is found in low levels in serum; constitutes about
0.2% of total serum Ig
its role in serum uncertain.
MW=150 000 D
b) IgD is primarily found on B cell surfaces where it
functions as a receptor for antigen.
c) IgD does not bind complement
d) D) called IgD because of its delta δ heavy chain
components.
Variation of Igs
1) Isotypes: All classes and subclasses of Ig that are present
in normal individuals (IgG,IgM,IgA,IgE,IgD)
2) Allotype: That there is a single aa ifference in the
peptide chain in CH and CL chain
3) Idiotype: represents the antigen binding specificities of
Igs. The unique aa sequence of VH and VL can function
as antigenic determinants.

Unit 1 - Immunology
11
Immune response
primary humoral immune response. The first contact of
an exogenous Ag with an individual leads to generation a
Characteristics:
1- longer lag phase: during this period , the naive B cells
undergo clonal selection, clonal expansion and
differentiation into memory and plasma cells
2- Log phase (logarithmic): increase in IgM
concentration.
Secondary immune response
Second contact with same exogenous antigen, generates
secondary humoral immune response.
Characterization:
1-shorter lag phase
2-Rapid reaches a greater magnitude of IgG and last for
longer time. This is because of memory B-cells specific
for this Ag is existed. The processes of affinity maturation
and class switching are responsible for higher affinity to
Ag and different isotype
Vaccination (immunization)
Used to provoke a positive immune response by an
individual to various pathogenic microorganisms to
confer protection.
Polyclonal antibody
Most Ags possess multiple epitopes and each one of them
induce different B cells to proliferate into many clones of
cells that recognize different epitopes, these B cells secret
Abs, resulting into a mixture of Abs called polyclonal Abs
Monoclonal antibody
A clone of single B-cells that recognize a single epitope
that secret Abs spesific to a single epitope so it’s called
monoclonal Abs. It’s used for diagnostic and theraputic
purposes.
Unit 1 - Immunology
12
Cytokines
Are regulatory proteins or glycoproteins of low molecular
weight secreted by white blood cells and other cells in
response to a number of stimuli.
Function as intercellular messenger that evoke particular
biological activity after binding to a specific receptor.
Nomenclature
Lymphokines: cytokines secreted by lymphocytes.
Monokines: cytokines secreted by monocytes and
macrophages.
Interlukines
: cytokines are secreted by some leukocytes
and act upon other leukocytes.
IL-1
o Secreted by macrophages
o Act on lymphocytes
o Induce lymphocytes maturation , activation and clonal
expansion
o Acts on hypothalamus inducing fever
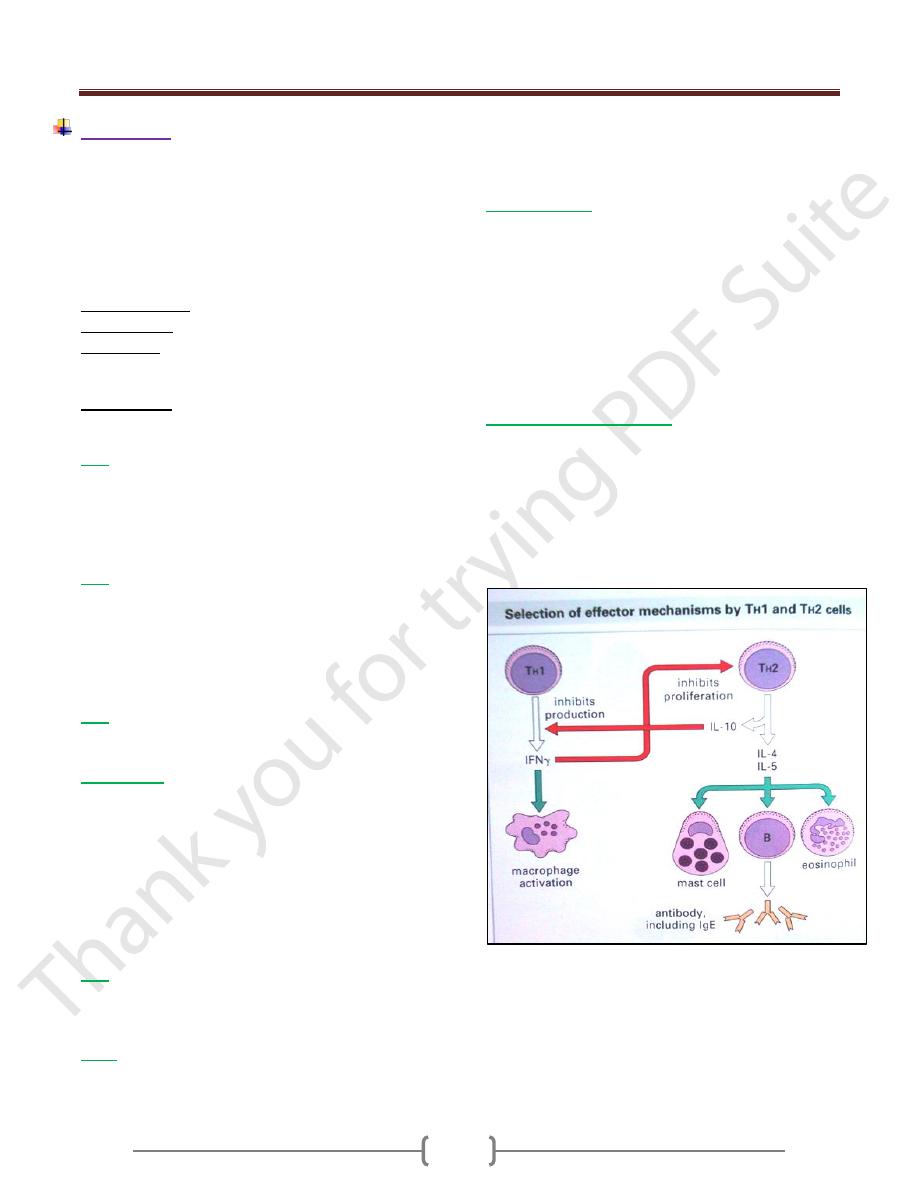
IL-2
o Secreted from Th1
o Acts on Ag specific T-cell supporting its growth
o Acts on NK cell increasing activity
o Acts on Tc cell increasing cytotoxicity
o Leads to development cell mediated immunity
o Suppress cytokines secreted from Th2 cells.
IL-3
o Secreted from Th2
o Supports growth and differentiation of hematopoietic cells
IL4 and IL-5
o Secreted from TH2
o Its up-regulate classII MHC expression
o Stimulate growth of mast cell
o Stimulate proliferation of activated B-cell
o Stimulate Abs secretions from plasma cell
o Stimulates humoral immune response
o Down regulates Th1
o IL-4 promotes class switch to IgE
o IL-5 promotes Eosinophil activation and generation
IL-6
o Secreted by macrophages and endothelial cells.
o Effect liver induces acute phase protein synthesis and
proliferation and antibody secretion of B-cells.
IL-10
o Secreted from Th2
o Antagonizes generation of Th1 subsets and cytokines
production by TH cell
o Mediate regulation of the immune system
Interferon (IFN)
o IFN α:secreted from leukocytes and inhibit viral
replication
o IFN β:secreted from fibroblasts and inhibit viral
replication
o IFN γ:secreted from Th1, Tc, NK cell and inhibit viral
replication,
Enhance activity of macrophages,
Increase MHC class-II expression,
Inhibits Th2 proliferation
Tumor necrosis factor (TNF)
o TNF :secreted from macrophages and act on tumor cells
o Had direct cytotoxic effect on tumor cells and tumor
undergoes visible hemorrhagic necrosis and regression by
inhibition angiogenesis, thereby decreasing the flow of
blood that is necessary for progressive tumor growth.
o Causes extensive loss weight (cachexia) by suppression
lipogenetic metabolism.
