
1
Physiology
By dr. Nadeem
Lecture 1
Respiration
11/10/2015

2
Respiration
Pulmonary function
Introduction
Respiration, as term is generally used, includes two processes: external respiration, the
absorption of O
2
and removal of Co
2
from the body as a whole, and internal respiration,
the utilization of O
2
and production of Co
2
by cells and gaseous exchanges between the
cells and their fluid medium.
The respiratory system is made up of gas-exchanging organ (the lung) and pump that
ventilates the lungs. The pump consists of the chest wall, the respiratory muscles, which
increase and decrease the size of the thoracic cavity, the area in the brain that control the
muscles and the tracts and nerves that connect the brain to the muscles.
At rest, a normal human breathes 12-15 times a minute. About 500 mL of air per
breath, or 6-8 L/min, is inspired and expired. This air mixed with the gas in the alveoli,
and, by simple diffusion, O
2
enters the blood in the pulmonary capillaries while Co
2
enter
the alveoli. In this manner, 250 mL of O
2
enters the body per minute and 200 mL of Co
2
is excreted.
Properties of gases
The pressure of gas is proportionate to its temperature and number of moles per volume.
nRT
P= ------
V
P= pressure
n= number of moles
R= gas constant
T= Absolute temperature
V= volume
Partial Pressures
Unlike liquids, gas expand to fill the volume available to them, and the volume occupied
by a given number of gas molecules at a given temperature and pressure is the same

3
regardless of the composition of the gas. Therefore, the pressure exerted by any one gas
in a mixture of gases ( its partial pressure) is equal to the total pressure times the fraction
of the total amount of the gas it represent.
The composition of dry air is 20.98% O
2
, 0.04% Co
2
, 78.06% N
2
, and 0.92% other inert
constituents such as argon and helium. The barometric pressure (PB) at sea level is 760
mmHg (1 atmosphere). The partial pressure ( indicated by the symbol P) of O
2
in the dry
air is therefore 0.21 X 760, or 160 mmHg at sea level. And the Pco
2
is 0.0004 X 760, or
0.3 mmHg. The inspired air is saturated with water by the time it reaches the lungs. The
PH
2
O at the body temperature (37 C) is 47 mmHg. Therefore, the partial pressure at sea
level of the other gases in the air reaching the lung are PO
2
, 149 mmHg, Pco
2
, 0.3 mmHg,
and PN
2
(including the other inert gases), 564 mmHg.
Gas diffuses from area of high pressure to area of low pressure, the rate of diffusion
depending on the concentration gradient and the nature of the barrier between the two
areas. When a mixture of gases is in contact with liquid, each gas in the mixture dissolves
in the liquid to an extent determined by its partial pressure and its solubility in the fluid.
Methods of quantitating respiratory phenomena
1- spirometers: measures gas intake and output.
2- Pulse oximeter: noninvasive continuous assessment of oxygenation .
3- Blood gas analysis: small probe sensitive to O
2
or Co
2
can be inserted into blood
vessels.
4- cabnograph: to measure the Pco
2
in the expired air.
ANATOMY OF THE LUNG
Air Passages
After passing through the nasal passages and pharynx, where its warmed and takes up
water vapor, the inspired air passes down the trachea and through the bronchioles,
respiratory bronchioles, and alveolar ducts and alveoli.
Between the trachea and the alveolar sacs, the airways divided 23 times. The first 16
generations of passages form the conducting zone of the airways that transports gas from
and to the exterior. They are made up of bronchi, bronchioles, and terminal bronchioles.
The remaining seven generations forms the transitional and the respiratory zones where
gas exchange occurs and are made up of respiratory bronchioles, alveolar ducts, and
alveoli. These multiple divisions greatly increase the cross sectional area of the airways,
from 2.5 cm
2
in the trachea to 11,800 cm
2
in the alveoli. Consequently, the velocity of air
flow in the small air ways declines to very low values.
The alveoli are surrounded by pulmonary capillaries. In most areas air and blood are
separated only by the alveolar epithelium and the capillary epithelium, so they are about
70 nm apart. Human have 300 million alveoli, and the total area of the alveolar walls in
contact with the capillaries in both lungs is about 70 m
2
.

4
The alveoli are lined by two types of epithelial cells. Type I cells are the primary
lining cells. Type II cells these cells secrete surfactant. The trachea and bronchi have
cartilage in their walls but relatively little smooth muscles. They are lined by ciliated
epithelium that contains mucous and serous glands. Cilia are present as far as the
respiratory bronchioles. The walls of the bronchioles contains more smooth muscle, of
which the largest amount relative to the thickness of the wall is present in the terminal
bronchioles.
The walls of the bronchi and the bronchioles are innervated by the autonomic nervous
system. Muscarinic receptors are abundant, and cholinergic discharge cause
Bronchoconstriction. The bronchial epithelium and smooth muscle contain B
2
-adrenergic
receptors. The B
2
receptors mediate bronchodilatation, they also increase bronchial
secretion, while a1 adrenergic receptors inhibit secretions. There is in addition a
noncholinergic , nonadrenergic innervation of the bronchioles that produces
bronchodilatation. And evidence suggest that VIP is mediator responsible for the
dilatation.
Pulmonary circulation
Almost all the blood in the body passes via the pulmonary artery to the pulmonary
capillary bed, where it is oxygenated and return to the left atrium via the pulmonary
veins. The separate and much smaller bronchial arteries come from systemic arteries.
They form capillaries which drain into bronchial veins or anastomose with pulmonary
capillaries or veins. The bronchial veins drain into the azygos vein. The bronchial
circulation supply the bronchi and the pleura. Lymphatic channels are more abundant in
the lungs than in any other organ.
MECHANICS OF RESPIRATION
Inspiration and expiration
The lungs and the chest wall are elastic structures. Normally, no more than a thin layer of
fluid are present between lungs and the chest wall (intrapleural space). The lungs slide
easily on the chest wall but resist being pulled away from it in the same way that two
moist pieces of glass slide on each other but resist separation. The pressure in the space
between the lung and the chest wall (intrapleural pressure) is subatmospheric. At the end
of quite expiration, the lungs tendency to recoil from the chest wall is just balanced by
the tendency of the chest wall to recoil in the opposite direction. If the chest wall is
opened, the lung collapse and if the lung lose their elasticity, the chest expands and
become barrel shaped.

5
Inspiration is an active process, the contraction of the inspiratory muscles increase
intrathoracic volume. The intrapleural pressure at the base of the lungs, which is normally
about -2.5 mm Hg (relative to the atmospheric) at the start of inspiration, decreases to
about -6 mm Hg . the lungs are pulled into a more expanded position. The pressure in the
airway become slightly negative, and the air flow into the lung. At the end of the
inspiration, the lung recoil begins to pull the chest back to expiratory position. Where the
recoil pressure of the lung and the chest wall balance, the pressure in the airway become
slightly positive and air flows out of the lungs. Expiration during quite breathing is
passive because no muscles that decrease intrathoracic volume is contract.
Strong inspiratory efforts reduce intrapleural pressure to value as low as -30mm Hg,
producing corresponding greater degrees of lung inflation. When ventilation is increased,
the extent of lung deflation is also increased by active contraction of expiratory muscles
that decrease intrathoracic volume.
Lung volumes
1- tidal volume: the amount of air that move into the lungs with each
inspiration or the amount that moves out with each expiration.
2- inspiratory reserve volume: the air inspired with a maximal inspiratory
effort in excess of the tidal volume.
3- expiratory reserve volume: the volume expelled by active expiratory effort
after passive expiration.
4- residual volume: the air left in the lungs after maximal expiratory effort.
5- Respiratory dead space: the space in the conducting zone of the airways
occupied by gas that does not exchange with the blood in the pulmonary
vessels.
6- Vital capacity: the largest amount of air that can be expired after a maximal
inspiratory effort, is frequently measured clinically as an index of pulmonary
function. It gives useful information about the strength of the respiratory
muscles and other aspects of pulmonary function.
7- FEV1: the fraction of the vital capacity expired during the first second of a
forced expiration.
8- Pulmonary ventilation, respiratory minute volume: the amount of air
inspired per minute is normally about 6L (500 mL / breath x 12 breath
/minute).

6
9- Maximal voluntary ventilation (MVV): is the largest volume of gas that can
be moved into and out of the lungs in one minute by voluntary effort, the
normal MVV is 125-170 L/min.
Respiratory muscles
1- diaphragm: is important inspiratory muscle account for 75% of the change in
intrathoracic volume during quite inspiration. Attached around the bottom of the
thoracic cage, this muscle arch around the liver and moves downward like a
piston when its contracts. The distance it moves ranges from1.5 cm to as much as
7cm with deep inspiration.
2- External intercostals muscles: are important inspiratory muscles, which run
obliquely downward and forward from rib to rib. When the external intercostals
contract they elevate the lower ribs. This pushes the sternum outward and
increases the anterioposterior diameter of the chest. The transverse diameter also
increases, but to a lesser degree. Either the diaphragm or the external intercostals
muscles alone can maintain adequate ventilation at rest.
3- The scalene and sternocleidomastoid muscles: are accessory inspiratory
muscles that help to elevate the thoracic cage during deep labored respiration.
4- The internal intercostals: are expiratory muscles, when contracts they decreases
the intrathoracic volume during the force expiration. They pass obliquely
downward and posteriorly from rib to rib and therefore pull the rib cage
downward when they contracts.
5- Muscles of the anterior abdominal wall: when contracts also aid expiration by
pulling the rib cage downward and inward and by increasing the intraabdominal
pressure, which pushes the diaphragm upward.
Glottis
The abductor muscles in the larynx contract early in inspiration, pulling the vocal
cord apart and opening the glottis. During swallowing or gagging, a reflex contraction
of the adductor muscles closes the glottis and prevents aspiration food, fluid, or
vomitus into the lung. In unconscious or anesthetized patients, glottic closure may be
incomplete and vomitus may enter the trachea, causing an inflammatory reaction in
the lung (aspiration pneumonia).
The laryngeal muscles are supplied by the vagus nerves. When the abductor are
paralyzed, there is inspirtory stridor. When the adductor are paralyzed, food and flood
enter the trachea, causing aspiration pneumonia.

7
Bronchial tone
Bronchoconstriction
bronchodilatation
1- during expiration during inspiration
2- parasympathetic discharge sympathetic discharge
3- irritants and chemicals
4- cool air
5- Exercise
6- Maximal at 6 AM maximal at 6 PM
7- Substance P VIP
Compliance of the lung and the chest wall
The pressure in the airways is zero at a lung volume that is corresponds to the volume
of gas in the lungs at the end of quite expiration (relaxation volume, which is equal
the functional residual capacity). It is positive at greater volumes and negative at
smaller volumes. The change in the lung volume per unite change in the airway
pressure (V/P) is the strechability (compliance) of the lung and the chest wall. The
normal value is approximately 0.2 L/cm H
2
O. however, compliance depends on the
lung volume, an individual with only one lung has approximately half the V for a
given P. the compliance is decrease by pulmonary congestion and interstitial lung
fibrosis. In pulmonary fibrosis there is stiffness and scaring of the lung. The
compliance is increased in emphysema.
Alveolar surface tension:
An important factor affecting the compliance of the lungs is the surface tension of the
film of fluid that lines the alveoli. The low surface tension when the alveoli are small
is due to the presence of the fluid that lining the alveoli (surfactant). It is a lipid
surface-tension-lowering agent. Surfactant is a
mixturedipalmitoylphosphatidylcholine (DPPC), other lipids, and proteins. it is
produced by type II alveolar epithelial cells. If the surface tension is not kept low
when the alveoli become smaller during expiration , they collapse in accordance with
the law of Laplace. In spherical structures like the alveoli, the distending pressure
equals two times the tension divided by the radius (P = 2T/r) if T is not reduced as r is
reduced, tension overcome the distending pressure P. surfactant also helps to prevents

8
pulmonary edema. It has been calculated that if it were not present, unopposed
surface tension in the alveoli would produce a 20 mm Hg force favoring transudation
of fluid from the blood into the alveoli.
Phospholipids, which has hydrophilic “head” and two parallel hydrophobic fatty
acid “tail”, line up in the alveoli with their tail facing the alveolar lumen. The surface
tension inversely proportionate to their concentration per unite area. They move
further apart as the alveoli enlarge during inspiration, and surface tension decreases,
where as it decreases when they move closure during expiration.
Surfactant is important at birth, the lung remain collapsed until birth. After birth,
the infant makes several strong inspiratory movements and the lung expand.
Surfactant keeps them from collapsing again. Surfactant deficiency is an important
cause of infant respiratory distress syndrome (IRDS, also known as Hyaline
membrane disease), the serious pulmonary disease that develops in infant born
before their surfactant system is functional. Surface tension in the lungs of these
infants is high, and the alveoli are collapsed in many areas (atelectasis). An additional
factor in IRDS is retention of fluid in the lungs. Maturation of surfactant in the lung is
accelerated by glucocorticoid hormones. Fetal and maternal cortisol increase near
term, and the lung are rich in glucocorticoid receptors.
Patchy atelectasis is also associated with surfactant deficiency in patient who have
undergone cardiac surgery involving use of pump oxygenator and interruption of
pulmonary circulation. In addition, surfactant deficiency may play a role in some of
the abnormalities that developed following occlusion of the main bronchus, occlusion
of one pulmonary artery, or long term inhalation of 100% oxygen. Cigarette smoking
also decrease lung surfactants.
Differences in ventilation and blood flow in different part of the lung
In upright position, ventilation per unite lung volume is greater at the base of the lung
than at the apex. The reason for this is at start of inspiration, intrapleural pressure is
less negative at the base than at the apex, since itrapulmonayintrapleural pressure
difference is less than at the apex, the lung is less expanded. Conversely, at the apex,
the lung is more expanded. Because of the stiffness of the lung, the increase in lung
volume per unite increase in the pressure is smaller when the lung is initially more
expanded, and ventilation is consequently greater at the base than the apex. Blood
flow is also greater at the base than the apex. The relative change in blood flow from
the apex to the base is greater than the relative change in the ventilation , so the
ventilation/ perfusion ratio is low at the base and high at the apex. The gravity and
other unknown factors play a role in this differences. They disappear in supine
position, but it persist to a remarkable degree in weightlessness in the space.
In older people and in those with chronic lung diseases, some of the elastic recoil is
lost, with resulting in decrease in the intrapleural pressure. Consequently, airway
closure may occur at the base of the lung in the upright position without force
expiration.
Dead space and uneven ventilation

9
Since gaseous exchange in the respiratory system occurs only in the terminal portions
of the airways, the gas that occupies the rest of the respiratory system is not available
for gas exchange with pulmonary capillary blood. In man who weights 70 kg only the
first 350 mL of the 500 mL inspired with each with each breath at rest mixes with air
at the alveoli, the volume of the anatomic dead space is 150 mL . Conversely, with
each expiration, the first 150 mL expired is gas that occupies the dead space, and only
the last 350 mL is gas from the alveoli. Consequently, the alveolar ventilation, ie,
the amount of air reaching the alveoli per minute, less than the respiratory minute
volume. For this reson, rapid shallow breathing produces much less alveolar
ventilation than slow deep breathing at the same respiratory minute volume.
It is important to distinguish between:
Anatomic dead space: the volume of the respiratory system exclusive of the alveoli.
Total (physiological) dead space: volume of gas not equilibrating with blood.
In healthy individuals, the two dead spaces are identical, but in disease status, no
exchange may take place between the gas in some of the alveoli and the blood .
Although it is possible to stand under the water and breathe through a tube that
projects above the surface, such a tube is in effect an extension of respiratory dead
space. For each milliliter of tube volume, the depth of the respiration would have to
increase 1 mL to supply the same volume of air to the alveoli. Thus, if the volume of
the tube were at all large, breathing would be very laborious. Additional effort is also
required to expand the chest against the pressure of the surrounding water.
GAS EXCHANGE IN THE LUNGS
Composition of alveolar air
Oxygen continuously diffuses out of the gas in the alveoli into blood stream, and CO
2
continuously diffuse into alveoli from the blood. In steady state, inspired air mixes
with the alveolar gas , replacing the O
2
that has entered the blood and diluting the CO
2
that has entered the alveoli. Part of this mixture is expired. The O2 content of the
alveolar gas then falls and its CO2 contents rises until the next inspiration. Since the
volume of gas in the alveoli is about 2 L at the end of the expiration (functional
residual capacity), each 350 mL increment of inspired and expired air has relatively
little effect on PO2 and PCO2. indeed, the composition of the alveolar gas remains
remarkably constant, not only at rest but under variety of other conditions.
Diffusion across the alveolocapillary membrane
Gas diffuse from the from the alveoli to the blood in the pulmonary capillaries or vice
versa across the thin alveolocapillary membrane made up of pulmonary epithelium,
the capillary endothelium, and their fused basement membrane. The blood takes 0.75
sec to traverse the pulmonary capillary at rest.
Substances pass from the alveoli into the capillaries depends on their reaction with
substances in the blood. These substances two types:
Flow-limited for substances that not react with blood substances, for example N
2
O,
the anesthetic gas.
Diffusion- limited for substances that react with blood substances at high rate , for
example CO gas.

10
O2 is intermediate between N2O an CO it is taken up by the hemoglobin, but much
less avidly the CO, and it reach equilibrium with capillary blood in about 0.3 sec.
The diffusion capacity of the lung for a given gas is directly proportionate to the
surface area of the alveolocapillary membrane and inversely proportionate to its
thickness.
The PO
2
of the alveolar air is normally 100 mm Hg, and the PO2 of the blood
entering the pulmonary capillaries is 40 mm Hg. The diffusion capacity for O2
(DLO2) at rest , is about 25mL/min/mm Hg, and the PO2 of blood is raised to 97mm
Hg, a value just under the alveolar PO2. DLO2 increases to 65 mL/min/mm Hg or
more during exercise and is reduces during diseases like sarcoidosis.
The PCO2 of the venous blood is 46 mm Hg, whereas that of the alveolar air is
40 mm Hg, and CO2 diffuses from the blood into the alveoli along this gradient. CO2
passess through all the biological membrane with ease, and the diffusion capacity of
the lung for CO2 is much greater than the capacity for O2. it is for this reason the
CO2 retension is rarely a problem in patient with alveolar fibrosis even when the
reduction in the diffusion capacity for O2 is sever.
PULMONARY CIRCULATION
Pulmonary blood vessels
The pulmonary vascular bed resembles the systemic, except that the walls of the
pulmonary artery and its large branches are about 30% as thick as the wall of the
aorta. The small arterial vessels has relatively little muscle in their walls. The
pulmonary capillaries are large, and there are multiple anastmosises, so that each
alveolus sits in a capillary basket.
Pressure , volume and flow
1- flow: the blood put out by the left ventricle returns to the right atrium and is
ejected by the right ventricle. Two exceptions are presents:
a- There is anastomoses between the bronchial capillaries and the pulmonary
capillaries, bypassing the right ventricle.
b- there is blood that flows from the coronary arteries into the chambers of the left
side of the heart.
Because of the small physiologic shunt created by those two ways, the blood in
the systemic arteries has a PO2 about 2 mm Hg lower than that of blood
equilibrated with the alveolar air, and the saturation of hemoglobin 0.5% less.
2- pressure: the pressure in the various parts of the pulmonary portions of the
pulmonary circulation are as follows :
1-main pulmonary artery 24/9 mm Hg
2-right and left pulmonary arteries 14 mm Hg
3-inside the lungs 12 mm Hg
4-pulmonary veins 8 mm Hg
5-right atrium 2 mm Hg
6-right ventricle 25/0 mm Hg
The pressure gradient in the pulmonary system is about 7 mm Hg, compared with
gradient of about 90 mm Hg in the systemic circulation.

11
3- volume: the volume of the blood in the pulmonary vessels at any time is about 1 L,
of which less than 100 mL is in the capillaries. The red blood cell takes about 0.75
sec to transverse the pulmonary capillaries at rest.
Capillary pressure
Pulmonary capillary pressure is about 10 mm Hg, whereas the oncotic pressure is about
25 mm hg, so that an pressure gradient of about 15 mm Hg toward the capillaries keeps
the alveoli free of all but a thin film of fluid. When the pulmonary capillary pressure is
more than 25 mm Hg as in backward failure of the left ventricle pulmonary congestion
and edema results.
Ventilation/perfusion ratios
The ratio of pulmonary ventilation to pulmonary blood flow for the whole lung at rest is
about 0.8 (4.2 L/min ventilation divided by 5.5 L/min blood flow) however, relative
marked differences occur in this ventilation /perfusion ratio in various parts of the normal
lung as a result of the effect of the gravity, and local changes in the ventilation/perfusion
ratio are common in diseases.
Ventilation as well as perfusion, in the upright position declines in a liner fashion from
the base to the apices of the lung. However, the ventilation/perfusion ratios are high in
the upper portions of the lungs. It is said the high ventilation/perfusion ratios at the apices
account for the predilection of the tuberculosis for this area because the relatively high
alveolar PO2 that results provides a favorable environment for the growth of tuberculosis
bacteria.
When wide spread, nonuniformity of ventilation and perfusion in the lung an cause
CO2 retention and decline in systemic arterial PO2.
Regulation of pulmonary blood flow
Active factors affecting pulmonary blood flow:
1- adrenergic a1 contraction
a 2 relaxation
B2 relaxation
2-muscarenic relaxation
3-adenosine A1 contraction
A2 relaxation
4-angiotensin II contraction
5-histamine H1 relaxation
H2 relaxation
Passive factors:

12
1- cardiac output: with exercise cardiac output increases and pulmonary arterial
pressure rises proportionately with little or no vasodilatation. More red blood cells
moves through the lungs without any reduction in the O2 saturation of the
hemoglobin in them and consequently, the total amount of O2 delivered to the
systemic circulation is increased. Capillaries dilate, and previously underperfused
capillaries are recruited to carry blood. The net effect is a marked increase in the
pulmonary blood flow with few if any alteration in the autonomic outflow to the
pulmonary vessels.
2- Bronchus or bronchiole obstruction: hypoxia developed in the underventilated
alveoli beyond the obstruction. The o2 deficiency apparently acts directly on the
vascular smooth muscles in the area to produce constriction , shunting the blood
away from the hypoxic area.
3- Accumulation of CO2: lead to drop in pH in the area, and a decline in the pH also
produces vasoconstriction in the lungs, as oppose to the vasodilatation it produces
in other tissues.
Other functions of the respiratory system
Lung defense mechanisms
The respiratory passages that lead from the exterior to the alveoli do more than serve
as gas conduits:
1- they humidify and cool or warm the inspired air so that even in very hot or very
cold air is at or near body temperature by the time it reaches the alveoli.
2- The bronchial secretions contain secretory immunoglobulins (IgA) and other
substances that help resist infections, in addition, the epithelium of the paranasal
sinuses appears to produce NO, which is bacteriostatic and help to prevent
infection.
3- The pulmonary alveolar macrophage (PAMs “dust cells”) ingest inhaled bacteria
and small particles. The also help process inhaled antigen for immunological
attack.
4- The hair in the nostrils strain out many particles larger than 10 nm in diameter,
most of remaining particles of this size settle on the mucus membrane in the nose
and pharynx. They do not follow the air stream as it curves downward into the
lungs, and they impact on or near the tonsils and adenoids. Particles 2-10 nm in
diameter generally fall on the walls of the bronchi as the air flow slows in the
smaller passages, there they initiate reflex bronchial constriction and coughing.
They are also moved away from the lungs by the “ciliary escalator. “ the
epithelium of the respiratory passages from the anterior third of the nose to the
beginning of the respiratory bronchioles is ciliated, and the cilia which are
covered with mucus, beat in coordinated fashion at a frequency of 1000-1500
cycle per minute. The ciliary mechanism is capable of moving particles away
from the lungs at a rate of at least 16 mm per minute. Particles less than 2 nm in
diameter generally reach the alveoli, where they are ingested by the macrophages.
Cilia motility may produced by various air pollutants, or a may be congenital.
One congenital form is Kartagener’s syndrome, in which the axonemal dynein,

13
the ATPase molecular motor that produces ciliary beating , is absent. Patient with
this condition also are infertile because they lack motile sperm, and they often
have situs inversus, presumably because the cilia necessary for rotating the
viscera are nonfunctional during embryonic development.
Metabolic and endocrine function of the lung
In addition to their function in gas exchange, the lung have a number of metabolic
functions:
a. they manufacture surfactant.
b. They contain fibrenolytic system that lyses clots in the pulmonary
vessels.
c. Prostaglandins are removed from the circulation, but they are also
synthesized in the lungs and released into the blood when lung tissue is
stretched.
d. The inactive angiotensin I is converted to the pressor angiotensin II in
the pulmonary circulation.
e. Removal of serotonin and norepinephrine, reduces the amounts of
these vasoactive substances reaching the systemic circulation.
Gas Transport Between the Lungs and the Tissues
OXYGEN TRANSPORT
Oxygen Delivery to the Tissues
The O
2
delivery system in the body consists of the lungs and the cardiovascular system.
O
2
delivery to a particular tissue depends on the amount of O
2
entering the lungs, the
adequacy of pulmonary gas exchange, the blood flow to the tissue, and the capacity of the
blood to carry O
2
.The blood flow depends on the degree of constriction of the vascular
bed in the tissue and the cardiac output. The amount of O
2
in the blood is determined by
the amount of dissolved O
2
, the amount of hemoglobin in the blood, and the affinity of
hemoglobin for O
2.
Reaction of hemoglobin and Oxygen
The dynamic of the reaction of the hemoglobin with O
2
makes it a particularly suitable O
2
carrier. Hemoglobin is a protein made up of four subunits, each of which contains a heme
Moiety attached to a polypeptide chain. In normal adult, most of the hemoglobin
molecules contain two a and two B chains. Heme is a complex made up of a porphyrin
and one atom of ferrous. Each of the four iron atom, can bind reversibly one O2
molecule. The iron stays in the ferrous state. So the reaction is oxygenation, not an
oxidation.
The quaternary structure of the hemoglobin determines its affinity for O2. in
deoxygenation, the globin unites are tightly bound in a tense (T) configuration which
reduces the affinity of the molecule for O2. when O2 is first bound, the bonds holding the
globin unites are released, producing a relaxed (R) configuration which expose more O2
binding sites. The net result is 500 fold increase in O2 affinity. In the tissue this reaction
is revered releasing O2.

14
The oxygen-hemoglobin dissociation curve, the curve relating percentage saturation of
the O2-carrying power of the hemoglobin to the PO2. it has a characteristic sigmoid
shape due to the T-R interconversion. When blood is equilibrated with 100%
O2(po2=760 mm Hg), the normal hemoglobin become 100% saturated, when fully
saturated, each gram of normal of normal hemoglobin contain 1.39 mL of O2. however,
blood normally contains small amounts of inactive hemoglobin derivatives, and the
measured value in vivo is lower. The traditional figure is 1.34 mL of O2. The hemoglobin
concentration in normal blood is 15 g/dL. Therefore each dL of blood contains 20.1 mL
(1.34mL X 15)of O2 bound to hemoglobin when the hemoglobin 100% saturated. The
amount of dissolved O2 is a liner function of the PO2 (0.003 mL/dL blood / mm Hg
PO2).
In vivo the hemoglobin in the blood at the end of the pulmonary capillaries is about
97.5% saturated with O2(PO2=97 mm Hg). Because of a slight admixture with the
venous blood that bypasses the pulmonary capillaries (physiological shunt). The
hemoglobin in the systemic arterial blood is only 97% saturated. The arterial blood
therefore contain a total about 19.8 mL of O2 per dL. 0.29 mL in a solution and 19.5 mL
bound to hemoglobin. In venous blood at rest, the hemoglobin is 75% saturated and the
total O2 content is about 15.2 mL/dL. 0.12mL in a solution and 15.1 mL bound to
hemoglobin. Thus , at rest tissue removes about 4.6 mL of O2 from each dL of blood
passing through them. In this way, 250 mL of O2 per minute is transported from the
blood to the tissue at rest.
Factors Affecting the affinity of Hemoglobin For oxygen
Three important conditions affecting the oxygen-hemoglobin dissociation curve:
1- temperature: arise in temperature shift the curve to the right. When the curve
shift in this direction, a higher PO2 is required for hemoglobin to bind a given
amount of O2. conversely, a fall in temperature shifts the curve to the left, and a
lower PO2 is required to bind a given amount of O2. a convenient index of such a
shifts is the P
50
, the P
50
at which the hemoglobin is half saturated with O2. the
higher the P
50
, the lower the affinity of hemoglobin for O2.
2- PH: a fall in Ph shifts the curve to the right, and the rise in PH shifts the curve to
the left. The decrease in O2 affinity of hemoglobin when the PH of the blood falls
is called the Boher effect and is closely related to the fact that deoxygenated
hemoglobin bind H more actively than dose oxyhemoglobin. The PH of the blood
falls as its CO2 contents increases, so that when the PCO2 rises, the curve shift to
the right and the P
50
rises.
3- 2,3-BPG: is very plentiful in the red cells, it is formed from 3-
phosphoglyceraldehyde. It is a highly charged anion that bind to the B chains of
the deoxyhemoglobin. One molecule of deoxyhemoglobin bind one molecule 2,3-
BPG. In effects:
HbO2 + 2,3-BPG = Hb – 2,3-BPG + O2

15
In this equilibrium, an increase in the concentration of 2,3-BPG shifts the reaction
to the right. Causing more O2 to liberate.
Factors affecting the concentration of 2,3-BPG in the red cells include:
a- PH: the 2,3-BPG concentration falls when the PH is low.
b- Thyroid hormones, growth hormone, and androgens increase the
concentration of 2,3-BPG and the P
50
.
c- Exercise has been reported to produce an increase in 2,3-BPG within 60
minutes, although the rise may not occur in trained athletes. The P
50
is also
increased during exercise, because the temperature rises in active tissues and
the CO2 and the metabolites accumulate, lowering the PH.
d- Ascend to high altitude trigger a substantial rise in 2,3-BPG concentration in
red cells, with a consequent increases in P
50
and increase in the availability of
O2 to the tissue.
e- The affinity of fetal hemoglobin (hemoglobin F) for O2 which is greater than
that for adult hemoglobin (hemoglobin A).facilitates the movement of O2
from the mother to the fetus. The cause of this greater affinity is the poor
binding of 2,3-BPG by the Y chains that replace B chains in fetal hemoglobin.
f- Red blood cells 2,3-BPG concentration is increased in anemia and in Varity of
diseases in which there is chronic hypoxia. This facilitates the delivery of O2
to the tissues by raising the PO2 at which O2 is released in the peripheral
capillaries.
g- In bank blood that stored, the 2,3-BPG level falls and the ability of this blood
to release O2 to the tissue is reduced.
Myoglobin
Myoglobin is an iron-containing pigment found in skeletal muscles. It
resembles hemoglobin but bind 1 rather than 4 mol of O2 per mole. Its
dissociation curve is rectangular rather than sigmoid curve. Because its curve is to
the left of the hemoglobin curve, its take up O2 from hemoglobin in the blood. It
releases O2 only at low PO2 values, put the PO2 in exercising muscle is close to
zero. the myoglobin content is greatest in muscles specialized for sustained
contractions. The muscles blood supply is compressed during such contractions,
and myoglobin may provide O2 when blood flow is cut off.
CARBON DIOXIDE TRANSPORT
Fate of carbon Dioxide in Blood
The solubility of CO2 in blood is about 20 times that of O2, therefore,
considerably more CO2 than O2 is present in simple solution at equal partial
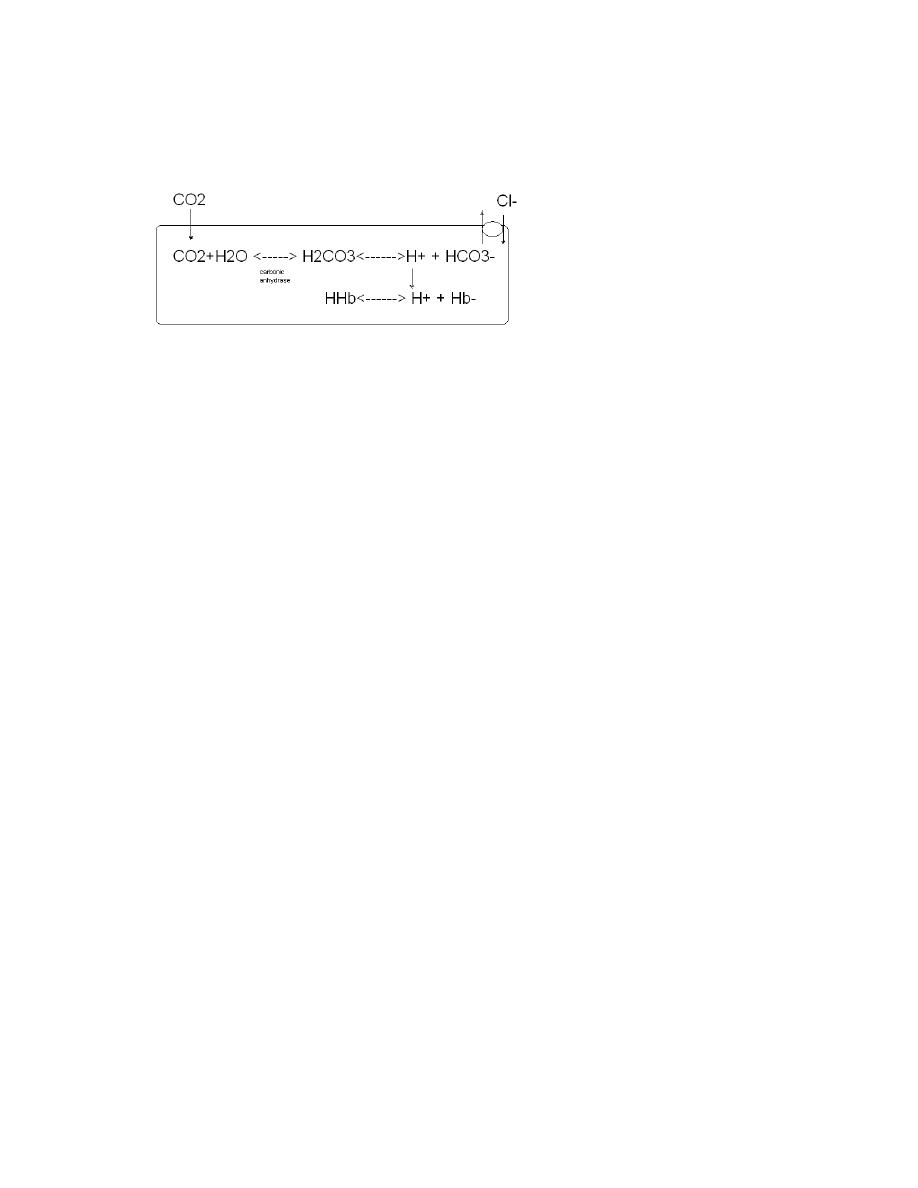
pressure. The CO2 that diffuses into red blood cells is rapidly hydrated to H2CO3
because of the presence of carbonic anhydrase. The H2CO3 dissociates to H
+
and
HCO3- and the H
+
is buffered, primarily by hemoglobin, while the HCO3- enters
the plasma. Some of the CO2 in the red cells reacts with amino groups of the
hemoglobin and other proteins ( R ), forming carbamino compounds.

16
Since deoxygenated hemoglobin binds more H
+
than oxyhemoglobin does and
forms carbamino compounds more readily, binding of O2 to hemoglobin reduces
its affinity for CO2 (Haldane effect). Consequently venous blood carry more
CO2 than arterial blood, CO2 uptake is facilitated in the tissue, and CO2 release is
facilitated in the lung. About 11 % of the CO2 added to the blood in the systemic
capillaries is carried to the lungs as carbamino-CO2.
In the plasma, CO2 reacts with the plasma proteins to form small amounts of
carbamino compounds, and small amount of CO2 are hydrated but the hydration
reaction is slow in the absence of carbonic anhydrase.
Chloride Shift
Since the rise in HCO3- content of red cells is much greater than that in the
plasmas blood passes in the capillaries, about 70% of the HCO3- formed in the
red cells enters the plasma. The excess HCO3- leaves the red cells in exchange for
Cl-. This process is mediated by Band 3, a major membrane protein. This
exchange is called the chloride shift. Because of it, the Cl- content of the red cell
in venous blood is therefore significantly greater than in arterial blood.
Note that for each CO2 molecule added to a red cell, there is an increase of one
osmotically active particle in the cell-either an HCO3- or Cl- in the red cell,
consequently, the red cells take up water and increase in size. For this reason, plus
the fact that a small amount of fluid in the arterial blood return via the lymphatics
rather than veins, the hematocrit of venous blood is normally 3% greater than that
of the arterial blood. In the lungs, the Cl- moves out of the cells and they shrinks.
Summary of Carbon Dioxide Transport
In plasma 1. dissolved
2. formation of carbamino compounds with plasma proteins
3. hydration, H+ buffered, HCO3 in plasma
In red blood cells
1. dissolved
2. formation of carbamino-Hb
3. Hydration, H+ buffered, 70% of HCO3 enters the plasma
4. Cl- shifts into cells, mosm in cells increases.
In the tissue, CO2 is added to the blood, the PH of the blood drops from 7.40 to 7.36. in
the lungs the process are reversed, and the CO2 is discharged into the alveoli, in this
fashion, 200 mL of CO2 per minute at rest and much larger amounts during exercise are
transported from the tissue to the lungs and excreted.
17
Regulation of Respiration
INTRODUCTION
Spontaneous respiration is produced by rhythmic discharge of motor neurons that
innervate the respiratory muscles. This discharge is totally dependent on nerve impulses

18
from the brain, breathing stops if the spinal cord is transected above the origin of the
phrenic nerves.
The rhythmic discharge from the brain that produces spontaneous respiration are
regulated by alteration in arterial PO2, PCO2 and H+ concentration, and this chemical
control of the breathing is supplemented by a number of nonchemical influences.
NEURAL CONTROL OF BREATHING
Control systems
Two separate neural mechanisms regulates respiration, one responsible for voluntary
control and the other for autonomic control:
1- The voluntary system is located in the cerebral cortex and sends impulses to the
respiratory motor neurons via corticospinal tracts.
2- The autonomic system is driven by a group of pacemakers cells in the medulla.
Impulses from these cells activate motor neurons in the cervical and the thoracic
spinal cord that innervate inspiratory muscles. Those in the cervical cord activate
the diaphragm via phrenic nerves, and those in the thoracic spinal cord activate
the external intercostals muscles, the impulses also reaches the internal
intercostals muscles and other expiratory muscles.
The motor neurons to the expiratory muscles are inhibited when those supplying the
inspiratory muscles are activated, and vice versa.
Medullary systems
The main components of the respiratory control pattern generator responsible for
autonomic respiration are located in the medulla. Rhythmic respiration is initiated by
A small group of synapticlly coupled pacemaker cells in the pre-botzinger complex
on either sides of the medulla between the nucleus ambiguus and the lateral reticular
nucleus. These neurons discharge rhythmically, and they produces rhythmic
discharges in the phrenic motor neurons. They also contact the hypoglossal nuclei,
and the tongue is involved in the regulation of the airway resistance.
In vivo-substance P stimulates and opioids inhibit respiration. Depression of
respiration is a side effect that limit the use of opioids in the treatment of pain.
However, it is now known that 5HT4 receptors are present in the pre-Botzenger
complex and treatment with 5HT4 agonists blocks the inhibitory effects of opiates on
respiration in experimental animals without inhibiting their analgesic effects.
Pontine and Vagal Influences
Although the rhythmic discharge of medullary neurons concerned with respiration is
spontaneous, it is modified by neurons in the pons and afferents in the vagus from
receptors in the airways and lungs. A specific nuclei in the pons contains neurons
active during inspiration and neurons active during expiration. When these nuclei is
damaged, respiration becomes slower and tidal volume greater, and when the vagi are
also cut in an anesthetized animals, there are prolonged inspiratory spasms that
resembles breath holding (apneusis). The normal function of these nuclei is unknown
but it may play a role in switching between inspiration and expiration.

19
REGULATION OF RESPIRATORY ACTIVITY
A rise in the PCO2 or H+ concentration of the arterial blood or a drop in its PO2
increases the level of respiratory neurons activity in the medulla, and a change in the
opposite direction have a slight inhibitory effect. The effects of variations in the blood
chemistry on the ventilation are mediated via respiratory chemoreceptors- the carotid
and the aortic bodies and collection of cells in the medulla and else where that are
sensitive to changes in the chemistry of blood. They initiate impulses that stimulate
the respiratory center.
CHEMICAL CONTROL OF BREATHING
The chemical regulatory mechanism adjust ventilation in such away that the alveolar
PCO2 is normally held constant, the effect of excess H+ in the blood are combated,
and the PO2 is raised when it falls to a potentially dangerous level. The respiratory
minute volume is proportionate to the metabolic rate, but the link between
metabolism and ventilation is CO2, not O2. the receptors in the carotid and aortic
bodies are stimulated by a rise in the PCO2 or H+ concentration of arterial blood or a
decline in its PO2.
Carotid and Aortic Bodies
There is a carotid body near the carotid bifurcation on each side, and there are usually
two or more aortic bodies near the arch of the aorta. Each carotid and aortic body
contain islands of two types of cells, type I and type II cells, surrounded by
fenestrated sinusoidal capillaries. The type I cells are closely associated with cup-like
endings of the afferent nerves. These cells resemble adrenal chromffin cells and has
dense core granules containing catecholamines that are released upon exposure to
hypoxia and cyanide. The cells are excited by hypoxia, and the principal transmitter
appears to be dopamine, which excites the nerve endings. The type II cells are
sustentacular cells each surrounds four to six type I cells.
Afferents from the carotid bodies ascends to the medulla via the carotid sinus and
the glossopharyngeal nerves, and fibers from the aortic bodies ascend in the vagi.
In the carotid bodies, blood flow is enormous, the O2 needs of the cells can be met
largely by dissolved O2 alone. Therefore the receptors are not stimulated in
conditions such as anemia and carbon monoxide poisoning, in which the amount of
dissolved O2 in the blood reaching the receptors is generally normal even though the
combined O2 in the blood is markedly decreased. The receptors are stimulated when
the arterial PO2 is low, or when, because of vascular stasis, the amount of O2
delivered to the receptors per unite time is decreased. Powerful stimulation is also
produced by cyanide, which prevents O2 utilization at the tissue level. In sufficient
doses, nicotine activate the chemoreceptors. It has been reported that infusion of K+
increases the discharge rate in the chemoreceptors afferents, and since the plasma K+
level is increases during exercise, the increase may contribute to exercise induced
hyperpnea.
Chemoreceptors in the Brainstem

20
The chemoreceptors that mediates hyperventilation produced by increases arterial
PCO2 after the aortic and the carotid bodies are denervated are located in the medulla
oblongata and called medullary chemoreceptors.
Ventilatory Responses to Changes in Acid-Base Balance
Changes example response result
----------------- ---------------- ------------------------- ------------
1- metabolic acidosis diabetes mellitus hyperventilation fall H+
(ketoacidosis) Decrease alveolar PCO2
2-metabolic alkalosis protracted vomiting ventilation depressed raisin H+
(HCl loss) arterial PCO2 raises
3-respiratory acidosis hypoventilation
4-respiratory alkalosis hyperventilation
Ventilatory Response to CO2
The arterial PCO2 is normally maintained at 40 mm Hg.
Increase arterial PCO2 (increase tissue metabolism or increase inhaled CO2) ------
ventilation stimulated------- increase pulmonary excretion of CO2--- arterial
PCO2 falls to normal.
This mechanism has upper limit. When the PCO2 of the inspired gas is close to the
alveolar PCO2, elimination of the PCO2 become difficult. When the CO2 content of
the inspired gas more than 7% the alveolar and the arterial PCO2 begin to rise
abruptly in spite of hyperventilation. The result is accumulation of CO2 in the body
(hypercapnia) depresses the central nervous system, including the respiratory center,
and produces headache, confusion, and eventually coma (CO2 narcosis).
Ventilatory response to Oxygen lack
When the O2 content of the inspired air in decreased, respiratory minute volume is
increased. The stimulation is slight when the PO2 of the inspired air is more than 60
mm hg, and mark stimulation of respiration is occurs only at a lower PO2 values.
However, any decline in arterial PO2 below 100 mm Hg produces increase discharge
in the nerves from the carotid and the aortic chemoreceptors.

21
Breath Holding
Respiration can be voluntarily inhibited for some time, but eventually the voluntary
control is overridden. The point at which breathing can no longer be voluntarily
inhibited is called the breaking point. Breaking is due to rise in arterial PCO2 and
fall in PO2. individuals can hold their breath longer after removal of the carotid
bodies. Delay the break point could be done in the following:
1- breathing 100% oxygen before breath holding raises alveolar PO2 initially, so the
break point is delayed.
2- Hyperventilating room air, because CO2 is blown off and the PCO2 is lower at
the start.
3- Psychological factors, subjects can hold their breath longer when they are told
their performance is very good than when they are not.
NONCHEMICAL INFLUNCES ON RESPIRATION
Coughing and Sneezing
Coughing begins with deep inspiration followed by forced expiration against a closed
glottis. This increase the intrapleural pressure to 100 mm Hg or more. The glottis is then
suddenly opened, producing an explosive outflow of air at a velocity up to 965 Km per
hour. Sneezing is a similar expiratory effort with a continuously open glottis. The reflex
help expel irritants and keep airways clear.
Afferents from higher centers and Proprioceptors
Pain and emotional stimuli affects respiration, so there must be afferents from the limbic
system and hypothalamus to the respiratory neurons in the brain stem. In addition, even
though breathing is not usually a conscious event, both inspiration and expiration are
under voluntary control. The pathways for voluntary control pass from neocortex to the
motor neurons innervating the respiratory muscles, bypassing the medullary neurons.
Active and passive movement movements of joints stimulates respiration, presumably
because impulses in afferent pathways from proprioceptors in muscles, tendons and joints
stimulate the respiratory neurons. This effect probably helps increase ventilation during
exercise.
Respiratory Components of Visceral Reflexes
Inhibition of respiration and closure of glottis during vomiting and swallowing not only
prevent the aspiration of food or vomitus into the trachea but, in case of vomiting, fix the
chest so that contraction of the abdominal muscles increase the intra-abdominal pressure.
Similar glottic closure and inhibition of respiration occur during voluntary and
involuntary straining.
Hiccup is spasmodic contraction of the diaphragm and other inspiratory muscles that
produces inspiration during which the glottis suddenly closes. The glottic closure is
responsible for the characteristic sensation and sound. Hiccup occur in the fetus in utero
as well as throughout the extrauterine life. Their function is unknown. Most attacks of

22
hiccups are usually of short duration, and often respond to breath holding or other
measures that increase arterial CO2. intractable hiccup , which can be debilitating,
sometime responds to dopamine agonists and perhaps to some centrally acting analgesic
compounds.
Yawning is a peculiar respiratory act whose physiological basis and significance are
uncertain. Like hiccups, it occurs in utero, and it occurs in fish and tortoises as well as
mammals. Underventilated alveoli have a tendency to collapse, and it has been suggested
that the deep inspiration and the stretching open them and prevent the development of
atelectasis. However, in actual experiments, no atelectasis preventing effect of yawing
could be demonstrated. Yawing increases the venous return to the heart, which may befits
the circulation. It has been suggested that yawning is nonverbal signals used for
communication between monkeys in a group, and one could argue that on a different
level, the same thing is true in humans.
Effects of sleep
Respiration is less rigorously controlled during sleep than in the waking state, and brief
periods of apnea occurs in normal sleeping adults. Changes in the ventilatory response to
hypoxia vary. If the PCO2 falls during the waking state, various stimuli from
proprioceptors and environments maintain respiration, but during sleep, the stimuli are
decreased and a decrease in PCO2 can cause apnea.
Respiratory Adjustments in Health and Disease
EXERCISE

23
Many cardiovascular and respiratory mechanisms must operate in an integrated fashion if
the O2 needs of the active tissue are to be met and the extra CO2 and heat removed from
the body during exercise.
Changes in Ventilation
1-the amount of O2 entering the blood in the lungs is increased .
2-the PO2 of blood flowing into the pulmonary capillaries falls from 40 to 25 mm Hg or
less, so that the alveolar-capillary PO2 gradient is increased.
3-blood flow per minute is increased from 5.5 L/min to as much as 20-35 L/min.
4-the total amount of O2 entering the blood therefore, increases from 250 mL/min at rest
to a value as high as 4000 mL/min.
5-CO2 excretion increases from 200 mL/min to as high as 8000 mL/min.
6-O2 uptake reach a maximum level, above this level, blood lactate level continue to rise
until oxygen debt is being incurred.
7-ventelation increases abruptly with the onset of exercise, followed after a brief pause by
a further, more gradual increase. The abrupt increase at the start of exercise is
presumably due to psychic stimuli and afferent impulses from proprioceptors in muscles,
tendons, and joints. The more gradual increase is presumably humoral even though
arterial pH, PCO2, and PO2 remain constant during moderate exercise
8- with moderate exercise, there is increase in the depth of the respiration, this is
accompanied by an increase in the respiratory rate when the exercise is more strenuous.
9-ventelation is abruptly decreases when the exercise ceases.
10-the respiratory rate after exercise does not reach basal levels until the O2 debt is
repaid. This may take as long as 90 min.
11-the stimuli to ventilation after exercise is the elevated H+ concentration due to lactic
acidemia.
12-the amount of O2 debt is the amount by which O2consumption exceeds basal
consumption from the end of exercise until the O2 consumption has return to pre exercise
level.
Changes in the Tissue
1- maximum O2 uptake during exercise is limited by the maximum rate at which O2
is transported to the mitochondria in the exercising muscles.
2- The PO2 in the venous blood from exercising muscles falls nearly to zero.
3- Capillary bed of contracting muscles is dilated and many previously closed
capillaries are open.
4- More O2 is removed from hemoglobin.
5- Additional O2 is supplied because, as a result of the accumulation of CO2 and the
rise in the temperature in active tissue-and perhaps because of a rise in red blood
cells 2,3BPG-the dissociation curve shifts to the right.
6- The net result is a three folds increase in O2 extraction from each unite of blood,
30 fold or more increase in blood flow, the metabolic rate of muscle rise as much
as 100-fold during exercise.
Exercise Tolerance and fatigue
The limiting factors in exercise performance were the rate at which O2 could be
delivered to the tissues or the rate at which O2 could enter the body in the lungs.

24
These factors play a role, but it is clear that other factors also contribute and that
exercise stops when sensation of fatigue progresses to the sensation of exhaustion.
Fatigue is produced in part by bombardment of the brain by neural impulses from
muscles, and decline in blood pH produced by lactic acidosis also makes one feel
tired.
HYPOXIA
Hypoxia is O2 deficiency at the tissue level. hypoxia has been divided into four types:
1- hypoxic hypoxia: in which the PO2 of the arterial blood is reduced.
2- Anemic hypoxia: in which the arterial PO2 is normal but the amount of
hemoglobin available to carry O2 is reduced.
3- Stagnant or ischemic hypoxia: in which the blood flow to a tissue is so low that
adequate O2 is not delivered to it despite a normal PO2 and hemoglobin
concentration.
4- Histotoxic hypoxia: in which the amount of O2 delivered to a tissue is adequate
but, because of the action of the toxic agent, the tissue cells cannot make use of
the O2 supplied to them.
Effect on cells
Hypoxia cause production of transcription factors (hypoxia-inducible factors; HIFs).
These are made up of
A and B subunits. In normally oxygenated tissues, the a subunits
are rapidly ubiquitinated and destroyed. In hypoxic cells, the a factors dimerize with the
B and the dimers ate genes that produce angiogenic factors and erythropoietin.
Effects on brain
In hypoxic hypoxia and the other generalized forms of hypoxia, the brain is affected first.
A sudden drop in the inspired PO2 to less than 20 mm Hg cause loss of consciousness in
10-20 seconds and death in 4-5 minutes. Less sever hypoxia cause a variety of mental
aberrations not unlike not unlike those produced by alcohol: impaired judgment,
drowsiness, dulled pain sensibility, excitements, loss of time sense, and headache. Other
symptoms include anorexia, nausea, vomiting, tachycardia, and when hypoxia is severe,
hypertension.
Cyanosis
Reduced hemoglobin has a dark color, and dusky bluish discoloration of the tissues,
called cyanosis, appears when the reduced hemoglobin concentration of the blood in the
capillaries is more than 5g/dL. Its occurrence depends on the total amount of hemoglobin
in the blood, the degree of hemoglobin unsaturation, and the state of capillary circulation.
Cyanosis is most easily seen in the nail beds and mucus membranes and in the earlobes,
lips and fingers where the skin is thin. Cyanosis does not occur in anemic hypoxia,
because the total hemoglobin contents is low; in histotoxic hypoxia, blood gas content is
normal.
HYPOXIC HYPOXIA
Hypoxic hypoxia is a problem in normal individuals at a high altitudes and in
complication of pneumonia and in variety of other diseases of respiratory systems.

25
Effects of decreased Barometric Pressure
The composition of the air stays the same, but the total barometric pressure falls with
increasing altitude. Therefore, the PO2 also fall. At 3000 m above sea level, the alveolar
PO2 is about 60 mm Hg and there is enough hypoxic stimulation of the chemoreceptors
to definitely increase ventilation.
Effects of High Altitude
In unacclimatized subjects, mental symptoms such as irritability appears at about 3700 m.
at 5500 m, the hypoxic symptoms are severe, and at altitude above 6100 m,
consciousness is lost.
When they first arrive at a high altitude, many individuals develops transient “mountain
sickness” this symptoms develops 8-24 hours after arrival at altitude and last 4-8 days. It
characterized by headache, irritability, insomnia, breathlessness, nausea and vomiting. It
cause is unsettled, but it appears to be associated with cerebral edema. The low PO2 at
high altitude causes arterial dilatation, and if cerebral autoregulation does not
compensate, there is an increase in capillary pressure that favors increase transudation of
fluid into brain tissue. Individuals who do not develops mountain sickness have a diuresis
at high altitude, and urine output decreases in individuals who develops the condition.
High altitude illness includes not only mountain sickness but also two more serious
symptoms that complicates it: high altitude cerebral edema and high altitude
pulmonary edema.
All forms of high altitude illness are benefited by descent to lower altitude and by
treatment with the diuretics acetazolamide. This drug inhibit carbonic anhydrase,
producing increase HCO3- excretion in urine, stimulating respiration, increasing PaCO2,
reducing the formation of CSF. In high altitudes pulmonary edema, prompt treatment
with O2 is essential, Nifedipine, a Ca cannel blocker that lowers pulmonary artery
pressure, is also useful.
Acclimatization
Acclimatization to high altitude due to the operation of a variety of compensatory
mechanisms.
1- the respiratory alkalosis produced by hyperventilation shifts the oxygen-
hemoglobin dissociation curve to the left, but a concomitant increase in red blood
cell 2,3BPG tend to decrease the O2 affinity of hemoglobin. The net effect is a
small increase in P50. the decrease in O2 affinity makes more O2 available to the
tissue.
2- The initial ventilatory response to increase altitude to increase altitude is
relatively small, because the alkalosis ten to counteract the stimulating effect of
hypoxia. However, ventilation steadily increase over the next 4 days because the
active transport of H+ into CSF, cause a fall in CSF pH that increase the response
to hypoxia. After 4 days, the ventelatory response start to decline slowly, but this
takes years of residence at higher altitudes for it to decline to initial level.

26
3- Erythropoietin secretion increase promptly on ascent to high altitude. This will
leads to increase in circulating red blood cells from 2-3 days and sustained as
long as the individual remains at high altitude.
4- In the tissue, mitochondria, which are the site of oxidative reactions, increase in
number, and myoglobin increases, which facilitates the movement of O2 into the
tissues.
Diseases Causing Hypoxic Hypoxia
The diseases that causes Hypoxic Hypoxia can be roughly divided into:
1- disease in which the gas exchange apparatus fails (lung failure) e.g. pulmonary
fibrosis or ventilation-perfusion imbalance.
2- Diseases causes large amount of blood shunted from the venous to the arterial
side of the circulation e.g. congenital heart diseases.
3- Respiratory pump failure (ventilatory failure)e.g. respiratory muscles fatigue,
peumothorax, bronchial obstruction or depression in the respiratory neurons in the
medulla by drugs like morphine or others.
Ventilation-Perfusion Imbalance
Patchy ventilation-perfusion imbalance by far the most common cause of hypoxic
hypoxia in clinical situations. In disease processes that prevent ventilation of some of the
alveoli, the ventilation-blood flow ratios in different parts of the lung determine the
extent to which systemic arterial PO2 decline. As the nonventilated alveoli are perfused,
the nonventiltated but perfused portion of the lung act as a left-to-right shunt, pumping
unoxygentated blood to the left side of the heart. Lesser degrees of ventilation-perfusion
imbalance are more common. The CO2 content of the arterial blood is generally normal
in such situations, since extra loss of CO2 in overventilated regions can balance
diminished loss in underventilated areas.
Venous-to-,Arterial Shunts
When cardiovascular abnormality such as an interatrial septal defect permits large
amounts of unoxygenated venous blood to bypass the pulmonary capillaries and dilute
the oxygenated blood in the systemic arteries (right-to-left shunt), chronic hypoxic
hypoxia and cyanosis (cyanotic congenital heart disease) results. Administration of 100%
rises the O2 content of the alveolar air and improves the hypoxia due to hypoventilation,
impaired diffusion, or ventilation perfusion imbalance, by increasing the amount of O2 in
the blood leaving lungs. However, in patients with venous-to-arterial shunts and normal
lungs, any beneficial effect of 100% O2 is slight and is due to solely to increase in
amount of dissolved O2 in the blood.
Collapse of the Lung

27
When a bronchus or bronchiole is obstructed, the gas in the alveoli beyond the
obstruction is absorbed and the lung segment collapse. Collapse of the alveoli is called
atelectasis. The atelectatic area may range in size from a small patch to a whole lung.
Some blood is diverted from collapsed area to better ventilated portions of the lung, and
this reduces the magnitude of decline in arterial PO2.
Pneumothorax
When air entered to the pleural space, through either rupture in the lung or a hole in the
chest wall, the lung on the affected side collapses because of its elastic recoil. Since the
intrapleural pressure on the affected side is atmospheric, the mediastinum shifts towards
the normal side.
If there is a flap of tissue over the hole in the lung or chest wall that acts as a flutter
valve, permitting air to enter during inspiration but preventing its exit during expiration,
the pressure in he pleural space rises above atmospheric pressure (tension
pneumothorax). The hypoxic stimulus to respiration cause deeper inspiratory efforts,
which further increase the pressure in the pleural cavity, kinking the great veins and
causing further hypoxia and shock. Intrapleural pressure in such cases may rise to 20-30
mm Hg. The peripheral veins become distended and intense cyanosis occur. The
condition is potentially fatal if the pneumothorax is not decompressed by removing the
air in the intrapleural space.
On the other hand, if the whole through which air enter the pleural space seals off
(closed pneumothorax), respiratory distress is not great because, with each inspiration,
air flows into the lung on the unaffected side rather than into the pleural space. Because
the vascular resistance is increased in the collapsed lung, blood is diverted to the other
lung. Consequantlly, unless the pneumothorax is very large, it dose not cause much
hypoxia.
The air in the closed pneumothorax is absorbed, since it is at atmospheric pressure, its
total pressure, PO2 and PN2 are greater than the corresponding values in venous blood.
Gas diffuses down these gradients into the blood, and after 1-2 weeks all of gas
disappears.
Asthma
Asthma is characterized by episodic or chronic wheezing, cough, and feeling of tightness
in the chest as a result of bronchoconstriction. Its fundamental causes is still unknown
despite intensive researches. Three abnormalities are presents: airway obstruction that is
at least partially reversible, airway inflammation, and airway hyperresponsiveness to a
variety of stimuli. A link to allergy has long been recognized, and plasma IgE levels are
often elevated. Leukotrienes are realsed from eosinophils and mast cells, and leukotrienes
cause bronchoconstriction. Numerous other amines, neuropeptides and interleukins have
effects on bronchial smooth muscle cells, or produce inflammation, and they may be
involved in asthma.

28
Asthma attacks are more severe in the late-night and early-morning hours, because this
is the period of maximal constriction in the circadian rhythm of bronchial tone. Cool air
and exercise, both of which normally cause bronchoconstriction, also trigger asthma
attacks, and in about 5% of asthmatics attacks are triggered by aspirin. However,
innumerable other substances have been found to trigger asthma attacks.
Beta2-adrenergic agonists have long been the main stay for treatment for mild to
moderate asthma attacks, because beta2-adrenergic receptors mediate bronchodilatation.
Inhaled and systemic steroids are used in even in mild to moderate cases, they are very
effective, but there side effects are a problem.
Emphysema
In the degenerative and potentially fatal pulmonary disease called emphysema, the lung
loss their elasticity as a result of destruction of elastic tissue and the wall between alveoli
break down so that the alveoli are replaced by large air sacs. The physiological dead
space is greatly increased. And because inadequate and uneven alveolar ventilation and
perfusion of underventelated alveoli, severe hypoxia develops. Late in the disease,
hypercapnia also develops, inspiration and expiration are labored, and the work of
breathing is greatly increased. The chest become enlarge and barrel shaped because the
chest wall expands as the opposing elastic recoil of the lungs declines. The hypoxia leads
to polycythemia. Pulmonary hypertension develops and the right side of the heart
enlarges (cor pulmonale) and then fails.
The most common cause of emphysema is heavy cigarette smoking. The smoke cause an
increase in the number of pulmonary alveolar macrophage, and these macrophages
release a chemical substances that attracts leukocytes to the lungs. The leukocytes in turn
release proteases including elastase, which attacks the elastic tissue in the lung. At the
same time
a1-antitrypsin, a plasma protein that normally inactivate elatase and other
proteases, itself is inhibited. The
a1-antitrypsin is inactivated by oxygen free radicals, and
these are relased by the leukocytes. The final result is a protease-antiprotease imbalance
with increase destruction of the lung tissue. In about 20% of cases of emphysema, a
congenital deficiency of active
a1-antitrypsin is present.
OTHER FORMS OF HYPOXIA
Anemic Hypoxia
Hypoxia due to anemia is not severe at rest unless the hemoglobin deficiency is marked,
because the red blood cells 2,3-BPG increases. However, anemic patients may have
considerable deficiency during exercise because of limited ability to increase O2 delivery
to the active tissue.
Stagnant hypoxia
Hypoxia due to slow circulation is a problem in organs such as the kidneys and hearts
during shock. The liver and possibly the brain are damaged by stagnate hypoxia in
congestive heart failure. The blood flow to the lungs is normally very large, and it takes
prolonged hypotension to produce significant damage
Histotoxic Hypoxia

29
Hypoxia due to inhibition of tissue oxidative processes is most commonly due to cyanide
poisoning. Cyanide inhibits cytochrome oxidase and possibly other enzymes. Methelene
blue or nitrates are used to treat cyanide poisoning. They act by forming methemoglobin,
which then reacts with cyanide to form cyanmethemoglobin, a nontoxic compounds.
The extent of treatment with these compounds is limited by the amount of
methemoglobin that can be safely formed. Hyperbaric oxygen may also be useful.
OXYGEN TREATMENT
Value
Administration of oxygen rich gas mixtures is very limited value in stagnant, anemic, and
histotoxic hypoxia because all that can be accomplished in this way is an increase in the
amount of dissolved O2 in the arterial blood. This is also true in hypoxic hypoxia when it
is due to shunting of unoxygnated venous blood past the lungs. In other forms of hypoxic
hypoxia O2 is of great benefit.
Oxygen Toxicity
The toxicity seems to be due the production of superoxide anion (O2-), which is free
radical, and H2O2. when 80-100% O2 is administered to human for periods of 8 hours or
more, the respiratory passages become irritated, causing substernal distress, nasal
congestion, sore throat, and coughing.
Some infants treated with O2 for respiratory distress syndrome develop a chronic
condition characterized by lung cysts and densities (bronchopulmonary dysplasia). This
syndrome may be a manifestation of O2 toxicity. Another complication in these infants is
retinopathy of prematurity (retrolental fibroplasia),the formation of opaque vascular
tissue in the eyes, which can lead to serious vascular defects.
Hyperbaric Oxygen Therapy
Exposure to 100% O2 at 2-3 atmospheres can increase dissolved O2 in arterial blood to
the point that arterial O2 tension is greater than 2000 mmHg and tissue O2 tension is 400
mmHg. If exposure is limited to 5 hours or less at these pressures, O2 toxicity is not
problem. Therefore, hyperbaric O2 therapy in closed tanks is used to treat diseases in
which improved oxygenation of the tissue cannot be achieved in other ways. It is of value
in carbon monoxide poisoning, radiation-induced tissue injury, gas gangrene, very severe
blood loss anemia, diabetic leg ulcer and other wounds that are slow to heal.
HYPERCAPNIA AND HYPOCAPNIA
Hypercapnia
Retention of CO2 in the body (hypercaonia) initially stimulates respiration. Retention of
larger amounts produces symptoms due to depression of the central nervous system,
confusion, diminished sensory acuity, and eventually coma with respiratory depression
and death. In patients with these symptoms, the PCO2 is markedly elevated, severe
respiratory acidosis is present, and the plasma HCO3- is markedly elevated. Large
amounts of HCO3- are excreted, but more HCO3- is reabsorbed, raising the plasma
HCO3- and partially compensate for the acidosis.

30
Hypercapnia may occur ventilation-perfusion inquilinity and when for any reason
alveolar ventilation inadequate in the various forms of pump failure. It is exacerbated
when CO2 production is increased. For example, in febrile patients there is 13% increase
in CO2 production for each 1 C rise in temperature, and high carbohydrate intake
increase CO2 production. Normally alveolar ventilation increases and extra CO2 is
expired, but it accumulates when ventilation is compressed.
Hypocapnia
Hypcapnia is the result of hyperventilation, during voluntary hyperventilation, the arterial
PCO2 falls from 40 to 15 mm Hg while the alveolar PO2 rises to 120-140 mm Hg.
The more chronic effect effects of hypocapnia are seen in neurotic patient who
chronically hyperventilated. Cerebral blood flow may be reduced 30% or more because
of direct constrictor effect of hypocapnia on the cerebral vessels. The cerebral ischemia
causes light-headedness, dizziness, and parasthesias.
Other consequences of hypocapnia are due to associated respiratory alkalosis, the
blood PH being increased to 7.5 or 7.6. the plasma HCO3- level is low, but HCO3-
reabsorption is decreased because of the inhibition of the renal acid secretion by the low
PCO2. the plasma total calcium level does not change, but the plasma Ca+2 level falls
and hypocapnic individuals develop carpopedal spasm, and tetany.
DISEASES AFFECTING THE PULMONARY CIRCULATION
Pulmonary Hypertension
Sustain primary pulmonary hypertension can occur at any age. Like systemic arterial
hypertension. The causes include hypoxia, inhalation of cocaine, appetite suppressing
drugs that increase extracellular serotonin, and systemic lupus erythematosus. Some cases
are familial.
All these conditions lead to increase pulmonary vascular resistance. If appropriate
therapy is not initiated, the increase right ventricular afterload leads eventually to right
heart failure and death. Treatment with vasodilators such as prostcyclin analogs is
effective.
Pulmonary Embolization
One of the normal functions of the lung is to filter out small blood clots, and this occur
without any symptoms. When emboli block larger branches of the pulmonary artery, they
provoke rise in the pulmonary artery pressure, and rapid shallow respiration (tachypnea).
The rise in the pulmonary artery pressure may be due to reflex vasoconstriction via the
sympathetic nerve fibers, but reflex vasoconstriction appears to be absent when large
branches of pulmonary artery are blocked. The tachypnea is reflex response to activation
of vagally innervated pulmonary receptors close to the vessel walls, these appears to be
activated at the site of the embolization.

31
