Session One
Minerals, trace elements and toxicityRadioactivity
Aims of the session
The aim of this session is that you understand the the chemical elements required by living organisms, and how this is met by nutrition. Also you will understand the balance level of minerals in every organ, tissue and cell of the human body to maintaining a healthy existence, and to know what are toxic metals, related disease and how to remove them safely. On the other hand you will be able to know the radiation elements and type of radiation and how it could be useful in medical treatment.
Structure of the Session:
08:30-09:30 Lecture: Minerals, trace elements and toxicity
09:30-10:30 Lecture: Radioactivity
11:30-02:00 Group Work: essential elements, toxicity, and difference between radiation
and radioactivity
Intended learning outcomes
After this session and yourself study you should be able to:describe the major elements that are essential for life.
describe the normal and abnormal value of Dietary minerals and trace elements.
describe the disease that cause by Deficiency of elements.
describe the toxic elements and how to remove them safely.
describe the type of radiation and the uses of each type.
The difference between radiation and radioactivity.
the affect of radioactivity on the body
Reading:
Jacques Osthuizen, Environmental Health - Emerging Issues and Practice, InTech, China,2012. Chapter 10
Radiation Biology: A Handbook for Teachers and Students, International Atomic Energy Agency, Vienna, 2010. Chapter 2
Lecture notes
Minerals and trace elementsMinerals are the building blocks of our bodies. They are required for body structure, fluid balance, protein structures and to produce hormones. They are a key for the health of every body system and function.
They act as co-factors, catalysts or inhibitors of all enzymes in the body. Copper and iron, for example, along with other minerals, are required for the electron transport system, and thus needed for all cellular energy production.
Minerals are classified into four groups:
The macrominerals, or those found in large quantity in our bodies. They include calcium, magnesium, sodium, potassium, phosphorus, Chloride and sulfur.
Trace minerals include Iron, Zinc, Iodine, Selenium, Copper, Manganese, Fluoride, Chromium, Molybdenum.
Possibly required trace minerals may include rubidium, tin, niobium, gold, silver and others.
Toxic metals include beryllium, mercury, lead, cadmium, aluminum, antimony, nickel, bismuth, barium, uranium and others.
These categories overlap slightly because assessing minerals that are required by humans is not a clear cut science. Some may be needed in minuscule amounts. Clinical studies to prove this by depriving people of vital minerals would be cruel and possibly disastrous.
Also, some forms of the required minerals can be highly toxic. Examples are some forms of copper, iron, manganese, hexavalent chromium selenium and others. Too much of even the most needed minerals can also become toxic.
Trace element
In analytical chemistry, a trace element is an element in a sample that has an average concentration of less than 100 parts per million (PPM) measured in atomic count or less than 100 micrograms per gram.
In biochemistry, a trace element is a dietary mineral that is needed in very minute quantities for the proper growth, development, and physiology of the organism.
Whereas the shortage of trace elements in the body may result in stunted growth or even death, their presence in higher amounts is also harmful.
The body requires different amounts of each mineral; people have different requirements, according to their age, sex, physiological state (e.g. pregnancy) and sometimes their state of health.
The Department of Health has published Dietary Reference Values (DRVs) for minerals for different groups of healthy people.
Toxic Metal
Toxic metals comprise a group of minerals that have no known function in the body and, in fact, are very harmful to plant, animal and human bodies. Mankind today is exposed to the highest levels of these metals in recorded history.
This is due to their industrial use for the past 300 years, the burning of fossil fuels without scrubbers, and improper incineration of waste materials worldwide. Toxic metals are now everywhere, and affect everyone on planet earth. They have become a major cause of illness, aging and even genetic defects.
The study of toxic metals is part of nutrition and toxicology, areas not emphasized in medical schools. For this reason, this important cause of disease is given little attention in conventional mainstream medicine. This article focuses on the extent of toxic metal problems – sources of toxic metals, symptoms, and how to remove them safely, quickly and deeply.
Testing for poisoning
People are continually exposed to metals in the environment. Medical tests can detect metals often, but this is to be expected and alone is not evidence that a person is poisoned. Metal screening tests should not be used unless there is reason to believe that a person has had excessive exposure to metals. People should seek medical testing for poisoning only if they are concerned for a particular reason, and physicians should consider a patient's history and physical examination before conducting tests to detect metals.
People who have metal tests when such testing is not indicated often have results higher than the typical range, even when they are not experiencing metal toxicity. People who get such results may be overly concerned, then seek further unnecessary health care.
Lecture notes
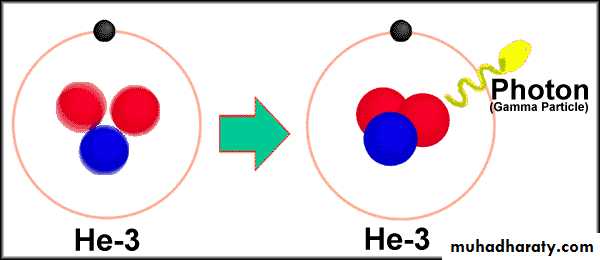
Radioactivity In medical science
Nucleus: It’s where the Protons and Neutrons are located in
an Atom.Protons: Positively Charged Particles in the Nucleus of the atom. Mass = (approx) 1 AMU
Neutrons: Neutrally charged particles in the nucleus of an atom Mass = (approx) 1 AMU
Electrons: These move around the nucleus. They have virtually no mass and have a negative charge.
Z = The Atomic Number. It’s the Number of Protons in the nucleus of an Atom.
A = Mass Number: Number of Protons + Number of Neutrons in the nucleus of an atom.
N = Number of Neutrons
Nuclear Stability
Only certain combinations of neutrons and protons in nucleus are stable.
Line of stability
N/Z ≈ 1 for low Z nuclides
N/Z ≈ 1.5 for high Z nuclides
Nuclides with odd number N and Z tend to be unstable!
Stability achieved by conversion of a N to Z or which accompanied by emission of energy
As the nucleus of an unstable atom breaks down, it gives out rays and particles called emissions.
The breaking down of unstable nuclei happens spontaneously – it is unaffected by heat, pressure, or whether the element is solid, liquid or gas.
What is Radiation?
Radiation is emission of energy from unstable nuclei which are trying to become stable
Radiation can cause ionisation (removal of electrons) of the atoms in our cells which can cause mutations in DNA.
Isotopes (atoms of the same element with differing numbers of neutrons) whose nuclei break down at random are referred to as radioactive.
They are also known as radioisotopes.
There are three different types of atomic radiation – alpha (α), beta (β), or gamma (γ).
Radioactive Emissions
Emission
What?
Penetration
Alpha (α)
2 protons
2 neutrons
Few cm in air. Stopped by paper.
Beta (β)
electron1 meter in air. Stopped by thin aluminium.
Gamma (γ)
Electromagnetic waveFew meter of concrete will reduce their energy. Difficult to stop.
Alpha Radiation
2 protons and 2 neutrons (= helium nucleus) ejected from the nucleusPositive charge of +2
Very high ionising power – this means it collides with lots of atoms and knocks electrons off them, making them ions
Short range in air – a few centimetres
Stopped by a piece of paper.
Alpha particles not typically used in medical imaging
Often followed by gamma and characteristic x-ray emission.
238U 234Th + 4He92 90 2
Beta Radiation
An neutron that breaks down into a proton and an electron
The electron is ejected from the nucleus; the atomic number of the atom changes because there is an extra proton in the nucleus thus turns atom into different element of atomic number
Negative charge of –1
Low ionising power
Beta particle identical to ordinary electron
Antineutrinos have infinitesimal mass and no charge, so hard to detect
Beta decreases N/Z ratio, therefore the daughter closer to stability
14C 14N + 0e 6 7 -1
Gamma Radiation
Not made of protons or electrons
A high-energy electromagnetic wave
Emitted from nuclei changing from a high energy level to a lower one
Frequently accompanies α and β emissions
No charge, so very low ionisation power
Background Radiation
Radioactive isotopes occur naturally:Cosmic rays – we are protected from these by the atmosphere, but airline pilots receive a higher dose.
Granite – contains uranium, people in Cornwall receive a higher dose than usual because there is a lot of granite there.
Food, our bodies, buildings etc.
There are also made artificially:
Medical Uses – cancer treatment, medical tracers
Nuclear Industry – weapons industry, power industry
Half-Life
The time needed for one half of the original nuclei of an isotope to decay to other substances is called the half- life.Half-life is fixed – no matter how big the sample, what the temperature or pressure is, it is always the same length of time.
A sample of a radioisotope will never completely disappear……its radioactivity always disappears by half, even in the tiniest amounts.
Dangers of Radiation
The main danger from radioactivity is the damage it does to the cells in your body.
Most of this damage is due to ionisation when the radiation passes.
If levels of radiation are high there can be damage due to heating effects as your body absorbs the energy from the radiation, rather like heating food in a microwave oven. This is particularly true of gamma rays.
The affect of radioactivity on the body
When radiation interacts with the tissue of our bodies (whether it is from radioactive atoms inside the body or from an external source such as an x-ray machine) it can cause damage to cells. This damage stems from the process of ionization. Ionization is simply the "knocking off" of electrons from the atoms they are normally said to "orbit." These electrons act as the "glue" that holds atoms together in chemical bonds. So, if some of the electrons get knocked loose by ionizing radiation, some of the chemical bonds get broken. This can result in damage to the cells.
Our bodies are marvelously well adapted to repair this damage. But high levels of radiation can cause illness, injury or death.
Radiotherapy Mechanism
Radiation therapy works by damaging the DNA of cancerous cells. This DNA damage is caused by one of two types of energy, photon or charged particle. This damage is either direct or indirect ionization of the atoms which make up the DNA chain. Indirect ionization happens as a result of the ionization of water, forming free radicals, notably hydroxyl radicals, which then damage the DNA.
Cells have mechanisms for repairing single-strand DNA damage and double-stranded DNA damage.
Targeting double-stranded breaks increases the probability that cells will undergo cell death.
Cancer cells are generally less differentiated and more stem cell-like; they reproduce more than most healthy differentiated cells, and have a diminished ability to repair sub-lethal damage.
Single-strand DNA damage is then passed on through cell division; damage to the cancer cells' DNA accumulates, causing them to die or reproduce more slowly.
Group work
Essential Elements, ToxicityWrite the Causes, Signs and symptoms, Pathophysiology and Treatment for the following state:
Hypokalemia, Hypocalcaemia, Hypophosphatemia and Iron deficiency anemia.
Enumerate trace element analyses methods in clinical chemisty.
What are the atmospheric and water Pollution of Inorganic pollutants fron industry sources.
Write the Signs and symptoms for acute and chronic toxicity of the following metals, and what are the typical treatment that should be given:
Arsenic, Lead and Mercury
Radiation and Radioactivity
What is the radiation effect of Alpha and Beta on human for each type?
What are the uses of each type of radiation?
If polonium-210 emits an alpha particle, what the other product will be? Write the equation.
What is the meaning of Positron emission
Classify radiation according to its original?
What's the difference between radiation and radioactivity?
Session Two
The Chemistry of Acids and Bases& Isomerism
Aims of the session
The aim of this session is that you should understand the important features of acids and bases and to determine the parameters of acid-base balance. Also you should understand the effect of isomers in our body and other organism on the planet.Structure of the Session:
08:30-09:30 Lecture: The Chemistry of Acids and Bases
09:30-10:30 Lecture: Isomerism
11:30-02:00 Group Work: Problem solution, acid/base balancestere-oisomers of steroid and The biological importance of chirality
Intended learning outcomes
Understand the ionization of strong and week acid/ base in solution
Calculate the PH and Equilibrium Involving Weak Acids and BasesTo demonstrate the acid-base properties of salts of weak acids and bases
to prove the buffering action of a solution of a weak acid and its salt or of a weak base and its salt.
To understand the effect of acid-base data on the blood and cell function.
The relationship between diet and acid-base homeostasis
Understand the major clinical causes for acid-base disturbances
Interpret blood gas data in terms of acid-base status
Differentiate acidosis from alkalosis and to determine if the primary cause is respiratory or metabolic
Describe the major causes of increased and decreased serum bicarbonate
Differentiate between the all kind of isomers
Understand the effect if isomers on biological system
Reading:
Richard F. Daley and Sally J. Daley, Organic Chemistry, Daley & Daley, 2005. Chapter 5 & 11
Lecture notes
The Chemistry of Acids and Bases
The pH of a solution is defined as follows"
pH = - log [H+]
The symbol log stands for logarithm. The logarithm of a is the power to which the number 10 must be raised to equal the number. We can write the previous equation in the following form:
[H+] = 1 × 10-10
[H+] is the molar concentration of hydrogen ion so, the pH of a solution is the negative power to which the number 10 must be raised to equal the molar concentration of hydrogen ions in the solution. For example, the hydrogen ion concentration of pure water is 1× 10-7 M. Its pH will be:
[H+] = 1 × 10-10 M=1 × 10-pH
pH= 7
The relationship between the molar concentration of hydrogen ion and its pH is inversely. The higher the value of the pH of a solution, the lower is its hydrogen ion concentration and vice versa. Thus an acidic solution has a pH value less than 7, and a basic solution has a pH value greater than 7.
A difference of 1 in pH value represents a tenfold difference in strength. That is, an acid of pH 4.5 is ten times as strong as one of pH 5.5. likewise, a base of pH 10.7 is 100 times as strong (10×10) as one of pH 8.7. therefore, a small change in pH indicates a definite change in acid or base strength.
Hydroxide and hydrogen ion concentratios are related by Kw, the ion product constant of water. If we know the molar concentration of either the hydroxide ion or the hydrogen ion, we can easily calculate the other. We can do the same with pH by defining the pOH as follows:
[OH-] = 1 × 10-pOH
Substituting values of [H+] and [OH-] into the expression for Kw, we obtain:
Kw = [H+] [OH-] = 1.00 x 10-14 at 25 oC
Kw = [1 × 10-pH ] [1 × 10-pOH] = 1.00 x 10-14 at 25 oC In a neutral
Another way of writing this equation is the following:
pH + pOH = 14
Thus, if we know the pH of a solution, we can easily determine its pOH.
The molar concentrations of all substances, and hydrogen ions are written in the following way:
[H+] = n × 10m
The value of m is always a whole number that can be either positive or negative. The above equation can be used only if the value of n is 1.
pH= -m
if n is any other number and not equals 1, the equation:
pH = - log [H+]
must be used to convert hydrogen ion concentrations to pH.
The pH of Some Common Body Fluids
The various body fluids differ both in their acidity and their range of acidities. Stomach acid is the most acidic, and it has a narrow pH range (1 to 3). Blood plasma is slightly basic, and it has a very narrow pH range. If the pH of blood plasma change to a value outside this range, the ability of the blood to transport oxygen is reduced. Therefore, maintaining the pH of blood plasma within a narrow range is important to human life. Urine has a wide pH range. It can be acidic, basic, or neutral. This wide range of the pH of urine is due to many acidic and basic substances are removed from the body through the urine to help maintain the pH of blood plasma. A list of the pH values of body fluids given in the following table.
Fluid
pH range
Blood
7.35-7.45
Gastric juices
1.6-1.8
Bile
7.8-8.6
Urine
5.5-7.0
Saliva
6.2-7.4
pH Measurement
To measure pH in a laborato1y, a pH-meter is used. This instrument is standardized by placing its pair of special electrodes into a solution of known pH to see that it is functioning and recording properly. Then the electrodes are placed in a solution of unknown pH and the pH is determined by reading the value on the pH-meter. A quicker but less accurate method is to touch a drop of the liquid to a specially prepared piece of indicator paper and then determine the pH by comparison with a pH color scale.Buffer Solutions
A buffer solution is a mixture of either a weak acid plus a salt of this weak acid or a weak base plus a salt of this weak base. Such a mixture reacts with both acids and bases, so small additions of either strong acids or strong bases cause little change in its pH.
A mixture of acetic acid and sodium acetate dissolved in water is an exa1nple of a buffer1 solution. Tle mixture has a large reservoir of both weak acid, moluecules (C2H3O2H), and the conjugate base of the acid, the acetate ion of sodium acetate (C2H3O2-). Now if small amount of a strong acid added to the buffer solution, it will react with the conjugate base as follows:
H3O+ + C2H3O2- LINK Word.Document.12 "D:\\كلية الطب\\محاضرات\\محاضرات المرحلة الاولى طب-موديول 1\\acid base and organic stereochemisty\\Work Book.docx" OLE_LINK1 \a \r \* MERGEFORMAT C2H3O2H + H2O
Strong acid
The equilibrium lies to the right because water is the weaker base and acetic acid is the weaker acid. As a result, most of the added hydrogen ion is removed from the solution and the pH hardly changes.
If hydroxide ions added to the buffer solution, they will react with molecules of acetic acid to form acetate ions and water as follows:
OH- + C2H3O2 LINK Word.Document.12 "D:\\كلية الطب\\محاضرات\\محاضرات المرحلة الاولى طب-موديول 1\\acid base and organic stereochemisty\\Work Book.docx" OLE_LINK1 \a \r \* MERGEFORMAT C2H3O2- + H2O
Strong base
Again, the equilibrium lies to the right. Then, most of the hydroxide ions are removed from solution and the pH is only changed slightly.
The pH of a buffer solution is determined by the pKa of the weak acid and the log of the ratio of the concentration of the conjugate base to the concentration of the acid.
[conjugate base]pH = pKa + log –––––––––––––– [weak acid]
A buffer solution has a limited ability to react with acids and bases without drastically changing its pH.
A solution acts as a buffer when it contains both members of a conjugate acid-base pair.
Buffer Capacity and Buffer Range
There is a limit to the ability of a buffer solution to neutralize added acid or base.
This buffer capacity is reached before either buffer component has been consumed.
In general, the more concentrated the buffer components in a solution, the more added acid or base the solution can neutralize.
As a rule, a buffer is most effective if the concentrations of the buffer acid and its conjugate base are equal or nearly so.
Therefore, a buffer is most effective when the desired pH of the buffer is very near pKa of the weak acid of the buffer.
Blood Buffers
The blood retains its fairly constant pH because of the presence of buffers. These buffers are present both in the blood plasma and in the red cells. Those in the plasma are primarily sodium buffers; those in the red blood cells are mainly potassium buffers. The blood buffers consist of the following:
1. Bicarbonate buffers.
2. Phosphate buffers.
3. Protein buffers (including hemoglobin and oxhemoglobin).
Bicarbonate Buffers
The bicarbonate buffer system in the red blood cells consists of carbonic acid (H2CO3), and potassium bicarbonate (KHCO3). The bicarbonate buffer system in the blood plasma consists of carbonic acid and sodium bicarbonate (NaHCO3). If we assume that a strong acid (such as HCl) is added to a sample of blood, it will react with the salt part of the buffer and undergo the following reactions:
HCl + KHCO3 → H2CO3 + KCl (in blood cells)
HCl +NaHCO3 → H2CO3 + NaCl (in blood plasma)
The carbonic acid produced (H2CO3) is part of the original buffer. Note that the strong acid (HC1) has been replaced by a very weak one (H2CO3). The other products (KC1 and NaCl) are neutral salts and will not affect the pH of the system.
If we assume that a strong base such as (KOH or NaOH) is added to a sample of blood, the following reactions will occur with the bicarbonate buffer systems:
KOH + H2CO3 → KHCO3 +H2O (in blood cells)
NaOH + H2CO3 → NaHCO3 + H2O (in blood plasma)
The salts ( KHCO3 and NaHCO3), are part of the original buffer system and the water produced is neutral. So that the pH again. is unaffected.
In both cases (reaction with a strong acid or a strong base) more of the buffer is produced plus a neutral compound.
The bicarbonate buffers and the blood protein buffers play major part in the control of the pH, the phosphate buffers have an important role inside the cell and in the urine.
Phosphate Buffers
The phosphate buffers consist of mixtures of K2HPO4 and KH2PO4 (also Na2HPO4 and NaH2PO4), which function similarly to the bicarbonate buffers in neutralizing excess acid and base:
HCl +K2HPO4 → KH2PO4 + KCl
KOH + KH2PO4 → K2HPO4 + H2O
More buffer Neutral compound
Hemoglobin Buffers
The hemoglobin buffers account for more than one half of the total buffering action in the blood. There are hemoglobin buffers and oxyhemoglobin buffers.
Hemoglobin Buffer
Oxyhemoglobin Buffer
HHb
HHbO2
KHb
KHbO2
These buffers, as well as other proteins that act as buffers in the blood-stream, pick up excess acid or base to keep the pH of the blood within its normal range.
Acid-Base balance in Blood
Carbonic acid-bicarbonate ion (conjugate acid-base pair) acts as a buffer in the control of the pH of blood.
Carbonic acid is formed by dissolving carbon dioxide in aqueous body fluids. It is a weak acid that ionizes to bicarbonate ion. The equation for these two equilibrium reactions is as follows:
CO2 + H2O H2CO3 HCO3- + H+
Normally, in body fluids such as blood, there is 24 mEq/L of bicarbonate ion to 1.2 mEq/L of carbonic acid.
[HCO3-][H2CO3]=24 mEq/L1.2 mEq/L=20
The pH of blood is within its normal range of 7.35 - 7.45 when this ratio, 20 parts carbonate ion to 1 part carbonic acid.
The pH of blood becomes more acidic when the ratio [HCO3-]/[H2CO3] becomes less than 20/1, say 16/1 or 12/1. The acidic condition of the blood signified by a pH less than 7.35 is called acidemia.
The pH of blood becomes more basic when the ratio [HCO3-]/[H2CO3] becomes more than 20/1, say 25/1 or 30/1. The alkaline condition of the blood signified by a pH greater than 7.45 is called alkalemia.
Death occurs if the pH of the blood is more acidic than 6.8 or more basic than 7.8.
Buffers in the body differ in one important respect from those in the laboratory. The body can supply components of the buffer solution as they are used up or can remove from the body any excess component. To explain this, let us consider how the body uses the carbonic acid-bicarbonate ion buffer system to overcome the increase in either the acid or the base concentration in the blood.
Acidosis
Consider a patient who has an illness that causes an increase in the concentration of acidic products in the blood. The physiologic processes causing academia are called acidosis. The acidic products react with bicarbonate ions to produce carbonic acid.
H3O+ + HCO3- LINK Word.Document.12 "D:\\كلية الطب\\محاضرات\\محاضرات المرحلة الاولى طب-موديول 1\\acid base and organic stereochemisty\\Work Book.docx" OLE_LINK1 \a \r \* MERGEFORMAT H2CO3 + H2O
Acidic products
This causes a decrease in the ratio [HCO3-]/[H2CO3], so acidosis will occur. One of the functions of both the lungs and the kidneys is to maintain the pH of the blood by supplying the buffer components that are used up or removing any excess components from the body.
The circulation of air into and out of the lungs, called ventilation. An increase in the amount of carbonic acid in the blood causes increase in the amount of carbon dioxide (CO2) formed from the decomposition of carbonic acid. To lose this excess (CO2), deeper and faster breathing, called hyperventilation occurs.
This causes a decrease in the acidity of the blood because the carbon dioxide formed is lost through the lungs. The kidneys can help by releasing more bicarbonate ion into the blood and removing hydrogen ions. In these ways, the body returns the [HCO3-]/[H2CO3] ratio to its normal value of 20 and maintain the acid-base balance in the blood.
Alkalosis
Consider another patient who has an illness causes an increase in the concentration of basic products in the blood. The physiologic processes causing alkalemia are called alkalosis.
The lungs and the kidneys will both involved in this case. These basic products react with carbonic acid to form bicarbonate ions. This time the ratio [HCO3-]/[H2CO3] increases.
OH- + LINK Word.Document.12 "D:\\كلية الطب\\محاضرات\\محاضرات المرحلة الاولى طب-موديول 1\\acid base and organic stereochemisty\\Work Book.docx" OLE_LINK1 \a \r \* MERGEFORMAT H2CO3 HCO3- + H2O
Basic products
The simplest way to prevent this ratio from increasing is to keep the carbon dioxide in the blood and use it to produce carbonic acid. To do this, loss of carbon dioxide through the lungs is minimized by slower and shallow breathing, cal1ed hypoventilation.
As before, the kidneys can help by removing bicarbonate ions and adding hydrogen ions to the blood. Thus, the lungs and kidneys can function to maintain the pH of the blood within its normal range of 7.35 to 7.45.
Lecture notes
Isomerism
Isomerism
Certain compounds possessing the same molecular formula, exist in different forms, account of having different arrangement of atoms.
Classification of Isomerism
Structural isomerism
Mesmerism
Positional isomerism
Automatism
Stereoisomerism
Geometrical isomerism
Optical isomers
Enantiomers
meso
Diastereomers
Structural isomerism:
In which the atoms are linked together in different ways.
n-BUTANEiso-BUTANE Note: 1 H here
Stereoisomerism:
compounds in which the atoms have different spatial arrangements.Types of Stereoisomerism
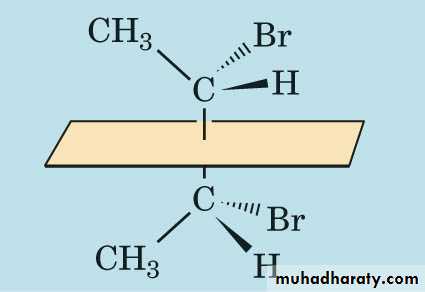
Geometrical isomerism:
Fumaric acid
Maleic acid
Physical properties
287°C
139°C
M.pt
1.64
1.59
Density
0.7 gm\100ml
78.8 gm\100ml
Solubility in water
occurs in compounds in which free rotation is prevented by the presence of double bond, ring structure or steric factors.
Therefore geometrical isomers differ only in configuration (spatial arrangement) giving rise to a cis isomer which has identical or similar atoms on the same side of the double bond, and a trans isomer, which has them on opposite sites.
HHCOOHCOHOCis- isomerMaleic acidtrans- isomerFumaric acid
It has been found that many geometric isomers also have different biological activities. One example is the compound retinal (oxidized form of vit. A), present in the retinal of the eye. Retinal combines with proteins called opsins to form visual pigments. The initial chemical event responsible for the vision is the isomerism of Cis – retinal to trans – retinal.
CCHHH21C14H4C5OCCHHH21C14H4C5Olighttrans-cis-
Optical isomers:
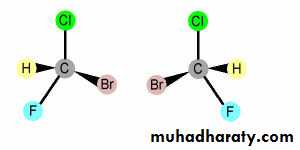
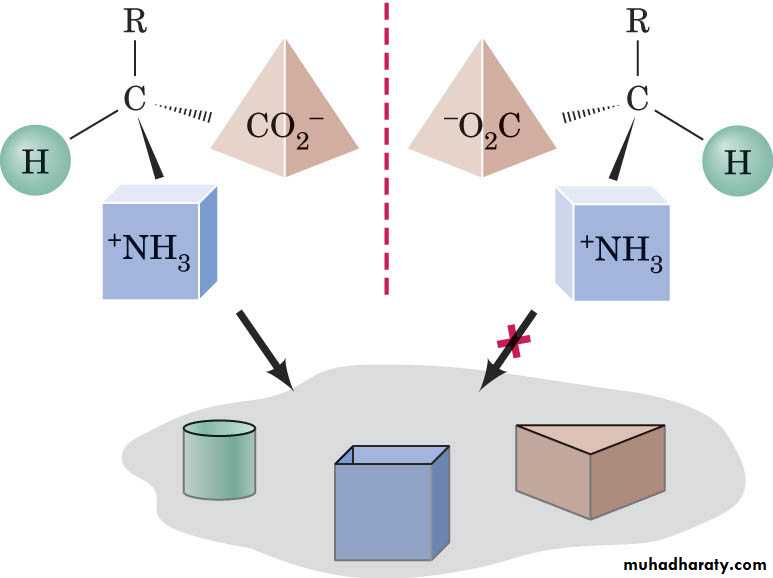
If 4 different atoms or groups are attached to the same carbon, there are two isomers which are not superimposable on their mirror images. These isomers are called Enantiomers
Mirror plane
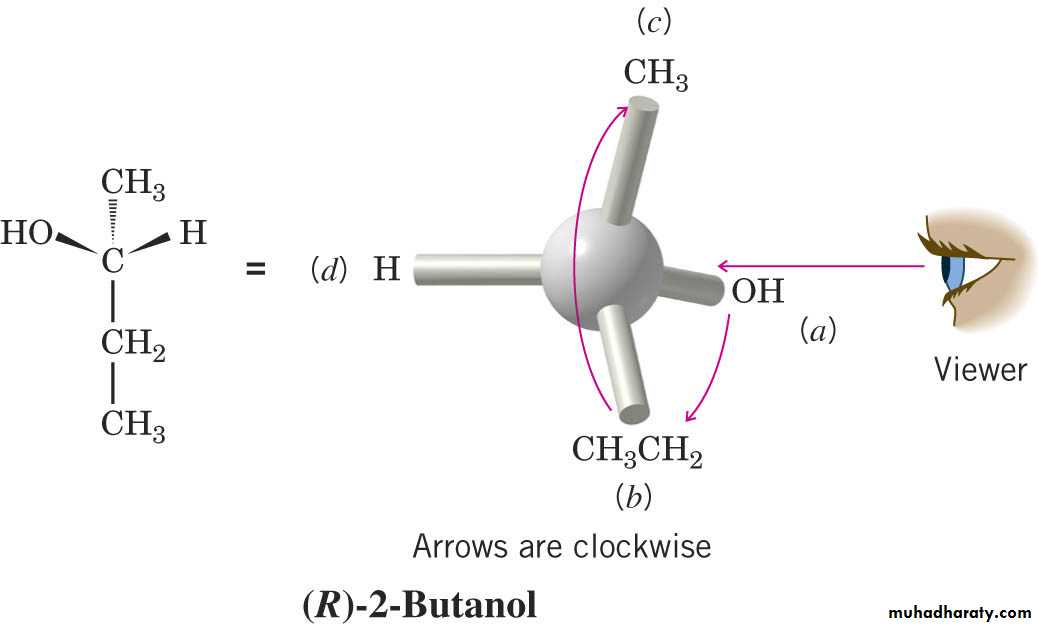
The central C atom to which four different atoms or groups are attached is called asymmetrical carbon atom (chiral center).
Enantiomers have identical physical properties like melting point, boiling point etc. may be distinguished from each other by their ability to rotate the plane or polarized light in opposite direction (optical isomers).
Therefore any compound that rotates plane-polarized light is said to be optically active.
If the rotation of the plane is in a clock wise direction (to the Wright) the substance is said to be Dettro rotatory indicated by D or a + sign, and if the rotation is in an anti- clock wise direction, Laevo rotatory indicated by L or a – sign.
When enantiomers are mixed to gether in equal proportion, the rotatory capacity of one isomer is cancelled by that of the other such mixture is called a racemic mixture (opticaly inactive).
If the compound contain two asymmetric atoms like tartaric acid which have the following structure can exist as more than two stereoisomers. The general rule is that:
No. of stereoisomers= 2n where n= no. of cheral center
+COOHCOOHOHHHOH( - ) - tartaric acidCOOHCOOHHOHOHH( ) - tartaric acid
Diastereomers
Compounds that are optical isomers but they are not mirror- images i.e. not enantiomers like D- Erythrose and D- threose.
Diastereomers: compounds that contain more than one chiral center, therefore diastereomers can exist in more than two isomers
If the configuration of both of these groups a dextrorotatory, then they reinforcing each other and the whole molecule is dextrorotatory. The same thing for both group, are laevorotatory. The molecule as a whole will be laevorotatory. The third possible configuration is when one group is dextro and the other group is laevo, in this case, the total rotation is the subtraction product of the rotatory capacity of each group.
If the molecule composed of two similar groups linked together by two asymmetric carbon atom each group cancel the other. and the molecule is meso (optically in active), like meso tartaric acid.
COOHCOOHHOHHOH
Stereoisomerism and living system
Living system usually react with only one of apair of enantiomers, hence they are called chiral.
The part of the living system that is chiral is the enzyme.
An enzyme has the ability to react with only one of apair of enantiomers this ability is called stereospecificity.
The enzyme reacts with only one enantiomers of the substrate.
D-enantiomer can bind to a maximum (3) sites while L-enantiomer can bind to a maximum of (2) sites.
Group work
The Chemistry of Acids and Bases
Problem #1: Aspirin has a pKa of 3.4. What is the ratio of A¯ to HA in:
(a) the blood (pH = 7.4)
(b) the stomach (pH = 1.4)
2- Define the Buffer capacity?
3- What the meaning of acid–base homeostasis
4- Which organ are responsible for acid-base balance.
5- Classifying the type acid- base imbalance
6- What are the symptoms of:
Respiratory acidosis and alkalosis
Metabolic acidosis and alkalosis
Isomerism
Enumerate the biological importance of chirality
Streptomycin is an antibiotic that is especially useful against penicillin-resistant bacteria.
Draw the structure of streptomycin.
Identify all of the stereocenters in the structure of streptomycin.
D-Galactitol is one of the toxic compounds produced by the disease galactosemia. Accumulation of high levels of D-galactitol causes the formation of cataracts. A Fischer projection for D-galactitol is shown here.
Draw the mirror image of D-galactitol.
What is the stereochemical relationship between D-galactitol and its mirror image?Cortisone is a natural steroid that can be isolated from the adrenal cortex. It has antiinflammatory properties and is used to treat a variety of disorders (e.g., as a topical application for common skin diseases).
Draw the structure of cortisone.
Identify all of the stereocenters in cortisone.
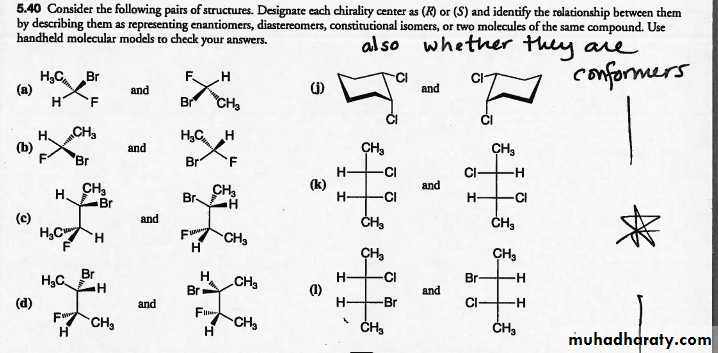
Identify the relationship between by describing them as representing enantiomers, diastereomers, constitutional isomers, or two molecules of the same compound.
Session Three
The Chemistry of Alcohols& Organic Sulfur Compounds
Aims of the session
The aim of this session is that you should understand the important features of alchohols and its effect on human body. Also you should understand the effect of organic sulfur compounds in our body and toxicity of each one.Structure of the Session:
08:30-09:30 Lecture: The Chemistry of Alcohols
09:30-10:30 Lecture: Organic Sulfur Compounds
11:30-02:00 Group Work: Nomenclature, reaction and medical knowledge on Alcohols and Organic Sulfur Compounds
Intended learning outcomes
Candidates should be able to explain why alcohols are not hydrocarbons and to interpret displayed and molecular formula of alcohols.
Candidates should be learn the physical and chemical properties of alcohols
Learn about chemical reaction of alcohol in living system
Students need to understand the dangers of substance abuse.
Identify thiols (mercaptans) by the presence of an SH group.
The mild oxidation of thiols gives disulfides.
Discuss several examples of sulfur compounds.
Reading:
Richard F. Daley and Sally J. Daley, Organic Chemistry, Daley & Daley, 2005. Chapter 5 & 11
Organic Chemistry, by Solomons and Fryhle. Either the 9th, 10th or 11th edition is acceptable.
Lecture notes
Alcohol
Alcohol
In chemistry, an alcohol is any organic compound in which a hydroxyl group (-OH) is bound to a carbon atom of an alkyl or substituted alkyl group. The general formula for a simple acyclic alcohol is CnH2n+1OH.
Generally, the word alcohol, when used alone, usually refers to ethanol, also known as grain alcohol or (older) spirits of wine. Ethanol is a very strong and unique smelling, colorless, volatile liquid formed by the fermentation of sugars. It also often refers to any beverage that contains ethanol. It is the most widely used depressant in the world, and has been for thousands of years. This sense underlies the term alcoholism (addiction to alcohol).
Other forms of alcohol are usually described with a clarifying adjective, as in isopropyl alcohol (propan-2-ol) or wood alcohol (methyl alcohol, or methanol). The suffix -ol appears in the official chemical name of all alcohols.
Structure
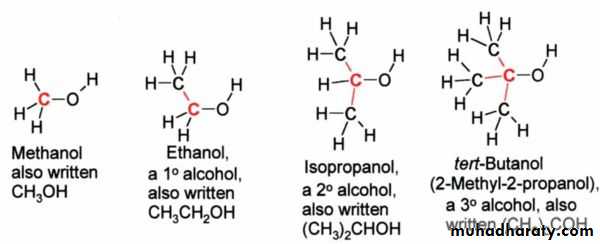
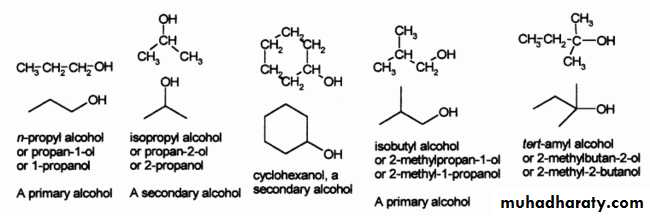
There are three major, subsets of alcohols: 'primary' (1°), 'secondary' (2°) and 'tertiary' (3°), based upon the number of carbons the C-OH carbon is bonded to. Methanol is the simplest 'primary' . The simplest secondary alcohol is isopropyl alcohol (propan-2-ol), and a simple tertiary alcohol is tert-butyl alcohol (2-methylpropan-2-ol).
Nomenclature
Systematic namesIn the IUPAC system, the name of the alkane chain loses the terminal "e" and adds "ol", e.g. "methanol" and "ethanol". When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the "ol": propan-1-ol for CH3CH2CH2OH, propan-2-ol for CH3CH(OH)CH3. Sometimes, the position number is written before the IUPAC name: 1-propanol and 2-propanol. If a higher priority group is present (such as an aldehyde, ketone or carboxylic acid), then it is necessary to use the prefix "hydroxy", for example: 1-hydroxy-2-propanone CH3COCH2OH.
Common names
For alcohols usually takes name of the corresponding alkyl group and add the word "alcohol", e.g. methyl alcohol, ethyl alcohol or tert-butyl alcohol. Propyl alcohol may be n-propyl alcohol or isopropyl alcohol depending on whether the hydroxyl group is bonded to the 1st or 2nd carbon on the propane chain. Isopropyl alcohol is also occasionally called sec-propyl alcohol.As mentioned above alcohols are classified as primary (1°), secondary (2°) or tertiary (3°), and common names often indicate this in the alkyl group prefix. For example (CH3)3COH is a tertiary alcohol is commonly known as tert-butyl alcohol. This would be named 2-methylpropan-2-ol under IUPAC rules, indicating a propane chain with methyl and hydroxyl groups both attached to the middle (#2) carbon.
Physical properties
Alcohols are present as liguids and solids.
The hydroxyl group generally makes the alcohol molecule polar.
Those groups can form hydrogen bonds to one another and to other compounds.
This hydrogen bonding means that alcohols can be solubility in water, methanol, ethanol, and propanol are miscible in water, butanol, with a four-carbon chain, is moderately soluble, Alcohols of five or more carbons (Pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance.
Because of hydrogen bonding, alcohols tend to have higher boiling points than comparable hydrocarbons and ethers
Chemical properties
Alcohols, like water, can show either acidic or basic properties at the -OH group. They are generally slightly weaker acids than water, but they are still able to react with strong bases such as sodium hydride or reactive metals such as sodium, lithium, etc. The salts that result are called alkoxides with the general formula RO-M+.
The basicity of the conjugate base depends on the class of parent alcohol.
Tertiary alkoxide > Secondary alkoxide > Primary alkoxide
Dehydration of Alcohols
RCH2CH2OHH2SO4heatRCH=CH2+ H2OIn some cases, dehydration of alcohols produces a mixture of products.
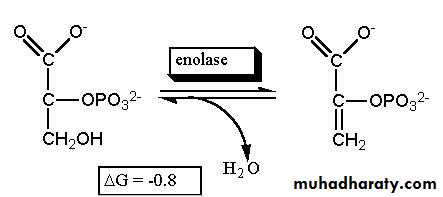
In Living Systems:
The dehydration of 2-phosphoglycerate to phosphoenolpyruvate is a critical step in the metabolism of the glucose sugar.Oxidation of Alcohols
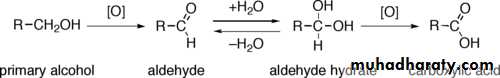
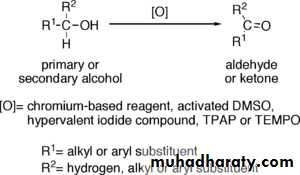
Primary alcohols (R-CH2-OH) can be oxidized either to aldehydes (R-CHO) or to carboxylic acids (R-CO2H).The oxidation of secondary alcohols (R1R²CH-OH) normally terminates at the ketone (R1R²C=O) stage.
Tertiary alcohols (R1R²R³C-OH) are resistant to oxidation.
In Living Systems:
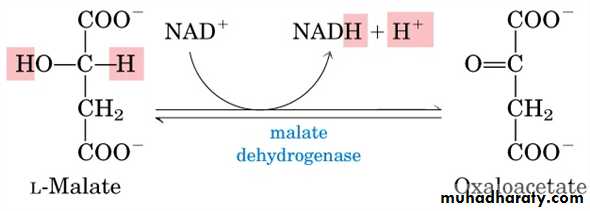
Malate is oxidized to oxalacetate which occurs in the citric cycle using an enzyme dehydrogenase that catalyses such reactions
Thise reaction is known to reguire the presence of nicotinamide, which links itself to the enzyme. The enzyme is then called pyridine – linked dehydrogenase.
Toxicity
Alcohols often have an odor described as 'biting' that 'hangs' in the nasal passages. Ethanol in the form of alcoholic beverages has been consumed by humans since pre-historic times. The consumption of large doses result in drunkenness or intoxication may lead to a hangover as the effect wears off) and, depending on the dose and regularity of use, can cause acute respiratory failure or death and with chronic use has medical repercussions.such as deterloration of liver, and loss of memory, and it may act as mild hypnotic.Aqueous solution of 60 -70% ethanol are often used to clean skin before an injection or minor surgery.
Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized, and often their metabolism produces even more toxic substances. Methanol, or wood alcohol, for instance, is oxidized by alcohol dehydrogenase enzymes in the liver to the poisonous formaldehyde, which can cause blindness or death.
An effective treatment to prevent formaldehyde toxicity after methanol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a substrate. Any remaining methanol will then have time to be excreted through the kidneys. Remaining formaldehyde will be converted to formic acid and excreted.
Toxicity of multifunctional alcohols
Ethylene glycol is toxic compound, its tioxicity is due to an oxidation product. Liver enzyme oxidase ethylene glycol to oxalic acid.
Oxalic acid crystallized as calcium salt (CaC2O4) in the kidney leading to renal damage, which might lead to kidney failure and death. Propylene glycol is non toxic, and can be used as solvent for drugs, it can be oxidized by liver enzyme to pyruvic acid which is an intermediate in carbohydrate metabolism in the body.
Lecture notes
Organic Sulfur CompoundsSulfur is a group VIA element which has a bonding characteristics similar to those of oxygen and forms a number of organic compounds that are analogous to those of oxygen.
Two representative classes of organic sulfur compounds are thiols and sulfides.
Thiols
Thiols are sulfur alcohols in which the hydroxyl oxygen atom is replaced by a sulfur atom, as in the general formula: R-S-H.
Thiols are also called mercaptans because they can react with mercury and are known as “mercury captures”.
The sulfur alcohols are named as alkanethiols in the IUPAC system but they are commonly referred to as alkyl mercaptans. For example,
Thiols are more volatile than alcohols.
They have disagreeable odor.
They are highly reactive.
They react with carboxylic acid to form thioesters.
The most significant difference between the reaction of thiols and alcohols in the case with which thiols are oxidized.
Mild oxidizing agents e.g. oxygen (in presence of metal catalyst), iodine, and hydrogen peroxide (H2O2) form disulfides from thiols
Notice:
Oxidation of thiols occurs at the sulfur atom, where as oxidation of alcohols occur at the carbon atom.Sulfides
Organic sulfides are analogous to ethers (R-O-R) in which the O atom is replaced by an S atom.
R-S-R’ sulfide: Sulfides can be oxidized to form sulfoxides.
R-S-S-R` Disulfides: have the formula R-S-S-R’. They can be produced by the oxidation of mercaptans, as in the reaction shown below.
R-S-H + H-S-R + [O] R-S-S-R + H2O
Oxidation of thiols to disulfide in living system
Cysteine (on of the amino acids in proteins) is oxidized by oxygen in presence of iron salts to cystine.
Cystine is important in protein structure because its disulfide bond serves as a covalent cross- link between two polypeptide chains.
Sulfa Drugs
Primary and secondary alkylamines react with arylsulfonyl chlorides to form N- substituted and N,N-di substituted sulfonamides (one of the sulfa drugs from aniline.
Analogous reactions have been used to synthesize many other sulfa drugs, such as:
Sulfa drugs are effective in combating infections diseases because their structure resemble the structure of 4- aminobenzoic acid. Many bacteria need 4- aminobenzoic acid synthesize the coenzyme folic acid.
In presence of sulfa drugs, the bacteria use the sulfa drug instead of 4-aminobenzoic acid in the synthesis of folic acid to form an altered folic acid. Part of the structure of altered folic acid is obtained from the sulfa drug instead of 4- aminobenzoic acid as in the following figures:
Altered folic acid (biologically in active)
Part of altered folic acid in whichsulfanilamide replaces 4- aminobenzoic acidThe altered folic acid, however, is ineffective as a coenzyme in the bacteria, so the infection organism dies. Humans are not affected by sulfa drugs because we cannot synthesize folic acid (we obtain folic acid from food).
That is way sulfa drug is used as an anti metabolite which is a compound that is a competitive inhibitor of an important enzyme- catalyzed reaction of the invading organism.
Group work
The Chemistry of AlcoholsWhat happens when ethyl alcohol is treated with K2Cr2O7 and H2SO4?
What is 'denatured' alcohol?
Draw the Structural Formula for:
2,4-dimethyl-3-pentanol
5-methyl-4-hexen-2-ol
3,3-dibromo-1-methylcyclohexane-1-ol
3-phenyl-1,3-butanediol
4-cyclobutyl-3,5-cycloheptadien-1-ol
What the products of the reaction the following alcohols with CrO3 in aqueous H2SO4 or with H2SO4 in heat ( >150 ºC)
1-propanol
2-butanol
2-methyl-2-propanol
Cyclohexanol
benzyl alcohol
What is Cholesterol?
Organic Sulfur Compounds
enumerate the importance of sulfur compounds in human body.
What effect does sulfur have on human health when found in the atmosphere?
What is glutathione: Uses and Side Effects?