
1
Forth stage
Medicine
Lec-2
.د
حسين محمد
1/1/2014
Dyslipidemia
Dietary fats and their food sources
Lipid Metabolism
Cholesterol and triglyceride(TG) are insoluble
Plasma lipoproteins : large complexes composed of hydrophobic core of TG and cholesterol
ester(CE), enveloped by
hydrophilic surface coat of phospholipid (PL), unesterified ("free") cholesterol, and
apolipoproteins (with detergent-like properties
,)
play an essential role in the absorption of hydrophobic lipids: dietary cholesterol, fat-soluble
vitamins; TG, and their transport from the liver to peripheral tissues; and vice versa.
Transport of Dietary Lipids
Exogenous Pathway:
Dietary triglycerides emulsified by bile
Bile salts attach to TG to emulsify them, which aids
access by pancreatic lipase
)
and hydrolyzed by lipases
((
monoglycerides and fatty acids are liberated
))
within the intestinal lumen and form micelles.
Enterocytes extract monoglyceride and free fatty acids from micelles and
re-esterify them into TG. TG is combined with (Apoprotein B48) ,CE, PL and cholesterol to
produce chylomicrons, secreted into the intestinal lymph ,delivered via the thoracic duct to
the systemic circulation.
Intestinal cholesterol (dietary and biliary sources) ,fatty acids, and fat-soluble vitamins are
absorbed in the proximal small intestine.
(a specific transport protein (Niemann-Pick C1-Like 1 NPC1L1) has been identified that
ferries cholesterol from the intestinal lumen into the enterocyte). A bulk of the cholesterol
is esterified, incorporated into chylomicrons .
Lacteals(lymphatic capillary) :
Chylomicrons pass into the lacteals, forming a milky substance known as chyle. The lacteals
merge to form larger lymphatic vessels that transport the chyle to the thoracic duct where
it is emptied into the subclavian vein.
Chylomicron is hydrolysed by lipoprotein lipase
(
Released free fatty acids are taken up by adjacent myocytes or adipocytes and either
oxidized to generate energy or reesterified and stored as triglyceride.
The residual 'remnant' chylomicron particle cleared by (LDL)-receptors in the liver.

2
Complete absorption of dietary lipids takes about 6-10 hours, so chylomicrons are
undetectable in the plasma after a 12-hour fast.
Chylomicrons and VLDL are TG rich, carrying dietary and liver produced TG respectively.
Most plasma cholesterol is carried as cholesteryl esters in LDLs and HDLs.
The density of a lipoprotein Is determined by the amount of lipid per particle. HDL is the
smallest and most dense because they contain the highest proportion of protein to
cholesterol.
Chylomicrons and VLDLs are the largest and least dense lipoprotein particles.
HDL High-density lipoprotein
LDL Low-density lipoprotein
IDL Intermediate-density lipoprotein
VLDL Very low-density lipoprotein
CETP Cholesteryl ester transfer protein enzyme
LCAT Lecithin:cholesterol acyltransferase enzyme
HMGCoAR = hydroxy-methyl-glutaryl-coenzyme A reductase.
Endogenous lipid:
In the fasting state, the liver is the major source of plasma lipids, Lipoprotein lipase
converts VLDL to remnant particles called IDL.
(
The released free fatty acids are taken up by adjacent myocytes or adipocytes and either
oxidized to generate energy or reesterified and stored as triglyceride, Most IDL cleared by
LDL receptors in the liver, some are processed by hepatic lipase to an LDL).
Also VLDL transfer phospholipids and TGs to HDL in exchange for CE via CETPenzyme and
eventually VLDL are converted to cholesterol-rich LDL molecules.
07
%
of circulating LDL is cleared by liver LDL receptor . Delivery of cholesterol via this
pathway down-regulates further expression of the LDL receptor gene and reduces the
synthesis and activity of the rate-limiting enzyme for cholesterol synthesis, HMGCoA
reductase. These negative feedback pathways, control the intracellular free cholesterol
level within a narrow range.
Cholesterol derived from LDL regulates several processes and can be used for the synthesis
of bile acids, steroid hormones, and cell membranes.
Reverse cholesterol transport:
HDL particles are synthesized and catabolized in the liver and intestines
Peripheral tissues are guarded against excessive cholesterol accumulation by HDL . Nascent
HDL obtains free cholesterol from peripheral tissues. Acirculating enzyme, LCAT promotes
the uptake of free cholesterol by HDL (esterification).
HDL release their cholesterol to the liver via the scavenger receptor B1 (SRB1).
CETP mediates the transfer of CE from the HDL or LDL to VLDL or chylomicrons in exchange
for TG.

3
The proteins associated with lipoproteins, called apolipoproteins, are required for the
assembly, structure, and function of lipoproteins.
ApoA-I, which is synthesized in the liver and intestine, is found on virtually all HDL particles.
ApoA-II is on approximately two-thirds of all HDL particles.
ApoB is the major structural protein of chylomicrons, VLDLs, IDLs, and LDLs; one molecule
of apoB, either apoB-48 (chylomicron) or apoB-100 (VLDL, IDL or LDL), is present on each
lipoprotein particle.
Lipids and cardiovascular disease
Plasma lipoprotein levels are major modifiable risk factors for cardiovascular disease.
Increased levels of atherogenic lipoproteins ((especially LDL, IDL, lipoprotein (a) and
possibly chylomicron remnants)) contribute to the development of atherosclerosis.
Increased plasma concentration and reduced diameter favour subendothelial accumulation
of these lipoproteins. Following oxidation, Apo B-containing lipoproteins are no longer
cleared by normal mechanisms.
They trigger a self-perpetuating inflammatory response during which they are taken up by
macrophages to form foam cells, a hallmark of atherosclerotic lesions.
Conversely, HDL removes cholesterol from the tissues to the liver, where it is metabolised
and excreted in bile. HDL may also counteract some components of the inflammatory
response.
Consequently, low HDL cholesterol levels, which are often associated with triglyceride
elevation, also predispose to atherosclerosis.
Primary lipid abnormalities:
The Fredrickson classification (types I-V) adds little to clinical decision-making.
Alternatively, primary lipid abnormalities can be classified according to the
predominant lipid problem:
1.Hypercholesterolaemia
2.Hypertriglyceridaemia
3.Mixed hyperlipidaemia
Classification of primary hyperlipidaemia:
.
1.Predominant hypercholesterolaemia
Polygenic (majority
)
Familial hypercholesterolaemia
Hyperalphalipoproteinaemia
.
2.Predominant hypertriglyceridaemia
Polygenic (majority
)
Familial hypertriglyceridaemia
Lipoprotein lipase deficiency

4
.
3.Mixed hyperlipidaemia
Polygenic (majority
)
Familial combined hyperlipidaemia
Dysbetalipoproteinaemia
Predominant hypercholesterolaemia
Polygenic hypercholesterolaemia
The most common cause increase in LDL-C. Physical signs, such as corneal arcus and
xanthelasma, The risk of cardiovascular disease(CVD) is proportional to the degree of LDL-C
(or Apo B) elevation, but is modified by other major risk factors, particularly low HDL-C.
Familial hypercholesterolaemia (FH)
Autosomal dominant , premature CVD. Xanthomas of the Achilles or extensor digitorum
tendons. Corneal arcus before age 40.
Hyperalphalipoproteinaemia
Increased levels of HDL-C. does not cause CVD.
Primary mixed hyperlipidaemia
Hypertriglyceridaemia , increase in LDL or IDL, is usually polygenic, often in association with
type 2 diabetes, central obesity, increase risk of CVD.
Familial combined hyperlipidaemia
Is a dominantly inherited. The overproduction of atherogenic Apo B-containing
lipoproteins.It results in elevation of cholesterol, TG or both in different family members at
different times. It is associatedwith an increased risk of CVD but it does not produce any
pathognomonic physical signs.
Dysbetalipoproteinaemia type 3, broad-beta or remnant hyperlipidaemia). Accumulation of
roughly equimolar levels of cholesterol and TG.
Premature cardiovascular disease is common and it may also result
in the formation of palmar xanthomas, tuberous xanthomas
or tendon xanthomas.

5
Causes of secondary hyperlipidaemia
-Secondary hypercholesterolaemia:
Moderately common:
1.Hypothyroidism
2.Pregnancy
3.Cholestatic liver disease
4.Drugs (diuretics, ciclosporin, corticosteroids, androgens, antiretroviral agents).
Less common:
1.Nephrotic syndrome
2.Anorexia nervosa
3.Porphyria
4.Hyperparathyroidism
-Secondary hypertriglyceridaemia:
1.Diabetes mellitus (type 2)
2.Chronic renal disease
3.Abdominal obesity
4.Excess alcohol
5.Hepatocellular disease
6.Drugs (β-blockers, retinoids, corticosteroids, antiretroviral agents).
Clinical manifestations of hyperlipidaemia:
Note that xanthelasma and corneal arcus may be non-specific, especially in later life.
What are xanthomas?
Skin lesions caused by the accumulation of fat in macrophage in the skin and more rarely in
the layer of fat under the skin.
Tuberous and Tuberoeruptive Xanthomas
Firm , nontender cutaneous and subcutaneous nodules,on extensor surfaces of the joints,in
areas of prior trauma.

6
Eruptive Xanthomas
Crops of small, red-yellow painless papules, on an erythematous base on the torso, elbows
,chest, and buttock regions. may be tender and itchy.
Plane xanthomas
Flat papules anywhere on the body,on the creases of the palms ( palmar xanthoma ) are
indicative of a type III dysbetalipoproteinaemia.
Tendinous xanthoma papules and nodules associated with Type II hyperlipidaemia , found
in the tendons of the hands, feet, and Achilles tendon.
Lipid measurement
performed for
1.Screening for primary or secondary prevention of CVD disease.
2.Investigation of patients with clinical features of lipid disorders.
3.Testing relatives of patients with dyslipidaemia.
Levels of (TC), (TG) and (HDL-C) need to be obtained after12-
hour fast to permit accurate calculation of (LDL-C) according to the
Friedewald formula (LDL-C = TC - HDL-C - (TG/2.2) mmol/L). (mg/dL can be converted to
mmol/L by dividing by 38 for cholesterol and 88 for triglycerides).
The formula unreliable when TG levels exceed 4 mmol/L (350 mg/dL). (requires
ultracentrifugation techniques or direct assays for LDL-C).
Hypertriglyceridaemia interferes with the serum amylase assay ,produce a falsely low
result.
Urine amylase to creatinine ratio measured to diagnose acute pancreatitis, the result less
likely affected by hypertriglyceridaemia.
Alternatives :removal of lipids before serum amylase measurement by using
ultracentrifugation.
Non-fasting samples are often used to guide therapeutic decisions since they are
unaffected in terms of TC and measured LDL-C, albeit that they differ from fasting samples
in terms of TG, HDL-C and, to some extent, calculated LDL-C.
Direct measurement of VLDL and LDL is also possible; however, due to high cost and
technical complexity, these are performed primarily in reference lab.

0
Management of dyslipidaemia:
Lipid-lowering therapies have a key role in the secondary and primary prevention of CVD.
Assessment of absolute risk, treatment of all modifiable risk factors and optimisation of
lifestyle, especially diet and exercise, are central to management in all cases.
The benefit of lipid lowering is proportional to the risk of coronary heart disease (CHD) in
the individual patient.
The greatest benefit is obtained in patients with established CHD.
In general, patients who already have CHD, diabetes mellitus, chronic renal impairment or
an absolute risk of cardiovascular disease of greater than 20% in the ensuing 10 years are
arbitrarily regarded as having sufficient risk to justify drug treatment.
Target levels for patients receiving drug treatment.
High-risk patients should aim for HDL-C > 1 mmol/L (38 mg/dL) and fasting TG < 2 mmol/L
(approximately 180 mg/dL), whilst target levels for LDL-C have been reduced from 2.5 to
2.0 mmol/L (76 mg/dL) or less.
In general, total cholesterol should be < 5 mmol/L (190 mg/dL), and < 4 mmol/L
(approximately 150 mg/dL) in high-risk patients and in secondary prevention of CVD.
Non-pharmacological management:
Dietary counselling to reduce intake of saturated and trans-unsaturated fat to less than 7-
10% of total energy , cholesterol to < 250 mg/day.
Replace sources of saturated fat and cholesterol with alternative foods ,lean meat, low-fat
dairy products, low glycaemic index carbohydrates.
.
Increase consumption of cardioprotective , fruit, vegetables, fish, pulses, nuts, legumes.
Reduce energy-dense foods such as fats, soft drinks, whilst increasing activity and exercise
to maintain or lose weight.
Therapeutic Lifestyle Changes (TLC)
Even minor weight loss can substantially reduce cardiovascular risk, especially in centrally
obese patients, adjust alcohol consumption.
Additional benefits with intake of foods containing lipid-lowering nutrients such as n-3 fatty
acids, dietary fibre and plant sterols.
If possible, drug that adversely affect the lipid profile should be replaced.
8
Dietary fats and their food sources:
Raises LDL
("bad"
cholesterol)
Little effect on
HDL ("good"
cholesterol) or
triglycerides
Fatty meats (beef, pork) Poultry skin
Butterfat (in whole milk, cream, ice
cream, cheese) Tropical oils (coconut,
palm)
Chocolate
Saturated fat
Lowers LDL if
substituted for
saturated fat
Keeps HDL up
Olive oil
Peanut oil
Canola oil
Monounsaturated
fat
Linoleic acid in
moderation can
lower LDL
Sunflower oil
Sesame oil
Corn oil
Soybean oil
Polyunsaturated
fat
Lowers
triglycerides
"Thins" the
blood
All fish, especially fatty fish, such
as salmon and mackerel
Plant sources, such as walnuts,
canola, and flaxseed oils
Omega-3 fats
Raises LDL
Little effect on
HDL but at high
levels can lower
HDL
Hydrogenated fats, margarine,
vegetable shortening, nondairy
creamer and whipped toppings Snack
foods (potato chips, cookies, cakes)
Peanut butter that contains
hydrogenated fat
Trans fatty acids
Very low-fat diets
Although may indeed lower cholesterol levels, they are not recommended.
A diet with less than 25% of its calories from fat can increase triglycerides and decrease
HDL. Such a diet may deplete your body of other important nutrients and vitamins.In
comparison, a cholesterol-reducing diet allows 25% to 35% of calories to come from total
fat, with 7% from saturated fat.
9
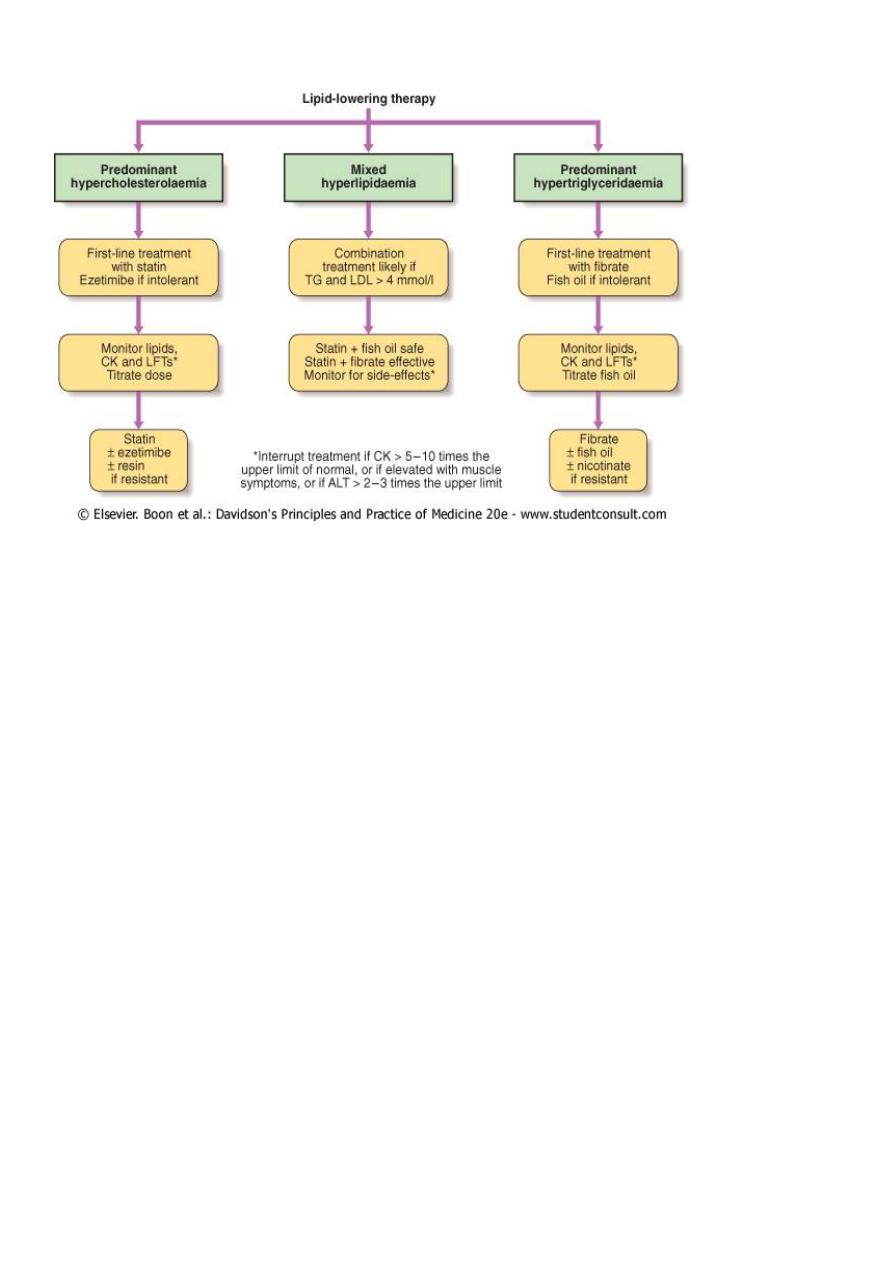
Pharmacological management:
Predominant hypercholesterolaemia
Statins inhibit cholesterol synthesis, up-regulating activity of the LDL receptor. This
increases clearance of LDL and its precursor, IDL, resulting in a secondary reduction in LDL
synthesis.
Reduce LDL-C by up to 60%, TG by up to 40% and increase HDL-C by up to 10%. There is
clear evidence of protection against total and coronary mortality, stroke and cardiovascular
events in high-risk patients.
Meta-analysis of major RCTs involving over 90 000 subjects receiving statins for an average
of 5 years showed reduced mortality from coronary artery disease, 19%, stroke, 17%) per 1
mmol/L reduction in LDL-C.
Statins are generally well tolerated and serious side-effects are rare (below 2%).
Liver function test abnormalities , myalgia, asymptomatic increase in CK, myositis and,
infrequently, rhabdomyolysis.
Side-effects are more likely in Patients who are elderly Debilitated or
Receiving other drugs
that interfere with statin degradation, which usually involves cytochrome P450 3A4 or
glucuronidation.

17
Cholesterol absorption inhibitors:
Ezetimibe inhibit the intestinal mucosal transporter NPC1L1 that absorbs dietary and biliary
cholesterol. This action is synergistic with the effect of statins. 10 mg/day dose reduces LDL-
C by 15-20%.
well tolerated, but its effect on cardiovascular disease endpoints is yet to be determined.
Plant sterol-also reduce cholesterol absorption, lower LDL-C by 7-15%.
Bile acid sequestering resins, such as colestyramine, colestipol and colesevalam
Prevent the reabsorption of bile acids, thereby increasing de novo bile acid synthesis from
hepatic cholesterol, the resultant depletion of hepatic cholesterol up-regulates LDL
receptor activity and reduces LDL-C in a manner that is synergistic with the action of statins.
High doses can achieve substantial reductions in LDL-C and modest increases in HDL-C, but
TG may rise. Resins are safe, but they may interfere with bioavailability of other drugs.
Colesevalam may cause fewer gastrointestinal effects than older preparations.
Plant sterols and stanols have a structure very similar to that of cholesterol.
Sterols are found naturally in small quantities in many fruits, vegetables, nuts, seeds,
legumes
Stanols are found in trace levels in similar foodstuffs but are produced by hydrogenation of
plant sterols for commercial use.Foods enriched with stanols or sterols lower serum
cholesterol levels by reducing intestinal absorption of cholesterol.
Predominant hypertriglyceridaemia"
Fibrates"
Stimulate peroxisome proliferator activated receptor (PPAR) alpha, which controls the
expression of gene products that mediate the metabolism of TG and HDL. Act upon
lipoprotein lipase by increasing its activity, resulting in a reduction in TG by up to 50% and
increase HDL-C by up to 20%, but LDL-C changes are variable. well tolerated, share a similar
side-effect profile to statins, myalgia, abnormal liver function tests,may increase the risk of
cholelithiasis and prolong the action of anticoagulants.
If target levels are not achieved, the fibrates or nicotinic acid and fish oil can be combined.
Insulin deficiency should be corrected for optimal activity of lipoprotein lipase.
Nicotinic acid (vitamin B3)
In pharmacological doses, this reduces peripheral fatty acid release with the result that
cholesterol and TG decline whilst HDL increases.

11
Side-effects : flushing, gastric irritation, liver function disturbances, exacerbation of gout
and hyperglycaemia.
Slow-release formulations and low-dose aspirin may reduce flushing. Combination therapy
with the prostaglandin D2 receptor inhibitor laropiprant to further reduce flushing is being
evaluated.
At higher doses decrease lipolysis in the peripheral tissues, also inhibit synthesis and
esterification of fatty acids in liver. and therefore it decreases lipid level in blood.
at lower doses it used as vitamin and in the treatment of Pellagra.
Highly polyunsaturated long-chain n-3 fatty acids:
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) comprise approximately 30%
of the fatty acids in fish oil.
EPA and DHA are potent inhibitors of VLDL TG formation. Intakes of greater than 2 g n-3
fatty acid (equivalent to 6 g of most forms of fish oil) per day lower TG in a dose-dependent
fashion. Up to 50% reduction in TG may be achieved with 15 g fish oil per day.
Changes in LDL-C and HDL-C are variable. Fish oil fatty acids have also been shown to inhibit
platelet aggregation and improve cardiac arrhythmia in animal models. Dietary and
pharmacological trials indicate that n-3 fatty acids reduce mortality from coronary heart
disease.
Mixed hyperlipidaemia
Can be difficult to treat. Statins alone are less effective first-line therapy once fasting TG
exceeds around 4 mmol/L (350 mg/dL). Fibrates alone are ---first-line therapy for
dysbetalipoproteinaemia, but they may not control the cholesterol component.
Combination therapy is often required.
-
-Effective combinations include: statin plus fish oil; fibrate plus ezetimibe; statin plus
nicotinic acid; or statin plus fibrate.
-Fibrates are effective in combination with statins but the risk of myopathy is increased.
-There is some evidence that fenofibrate is safer than gemfibrozil in this regard.
Monitoring of therapy:
The effect of drug therapy can be assessed after 6 weeks (12 weeks for fibrates), and it is
prudent to review side-effects, lipid response, CK and liver function tests at this stage.
Follow-up should encourage continued compliance (especially diet and exercise), and
include monitoring for side-effects and cardiovascular symptoms or signs, and
measurement of weight, blood pressure and lipids, as well as review of absolute
cardiovascular disease risk status.

12
If myalgia or weakness is associated with CK elevation > 5-10 times the upper limit of
normal, or if sustained alanine aminotransferase (ALT) elevation > 2-3 times the upper limit
of normal is detected, treatment should be interrupted and alternative therapy sought.
In combined therapy, fibrates should be given in the morning and
statins at night so that
the peak dosages do not overlap.
Dyslipidaemia in pregnancy:
Cardiovascular disease is very unlikely amongst women of child-bearing age, but is possible
in women with severe risk factor profiles or familial hypercholesterolaemia.
Lipid metabolism:
lipid and lipoprotein levels increase during pregnancy. Increase in LDL-C which resolves
post-partum. hypertriglyceridaemia may be exacerbated
.
Treatment:
Dyslipidaemia is rarely thought to warrant treatment. Teratogenicity has been reported
with systemically absorbed agents, and non-absorbed agents may interfere with nutrient
bioavailability.
Management of hyperlipidaemia in the elderly
Benefit of statin therapy: maintained up to the age of 80.
Myocardial infarction
Maximal postinfarction reductions in total cholesterol occur at days 4 to 5 with levels 47%
below baseline.
L &HDL decrease to their nadir on day 7 to 48% and 32% below baseline, respectively.
Triglyceride increase 58% above baseline on day 7.
These alterations generally stabilize by 2 months after the event. cholesterol levels are no
longer valid after 24 h from presentation. Nevertheless, several studies have shown that
the total /HDL and the LDL /HDL ratio are also strong predictors of coronary events, ratios
remained unchanged.
Therefore, most experts recommend measuring the serum cholesterol levels within the first
24 h after the onset of MI or measure the ratios.
The ratios of total to HDL cholesterol and LDL to HDL cholesterol that correlate with the
development of coronary events are 4.5 (Ideally, one should strive for ratios of 2 or 3 and
2.5, respectively.
