Adrenal gland
Samir AlSaffarAdrenal Anatomy
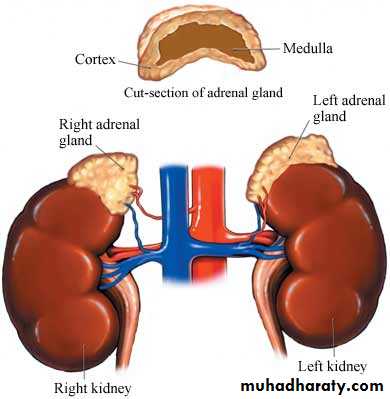
The adrenal glands (also known as suprarenal glands) are endocrine glands that sit atop the kidneys; in humans, the right suprarenal gland is triangular shaped, while the left suprarenal gland is semilunar shapedweights about four grams
Adrenal Anatomy
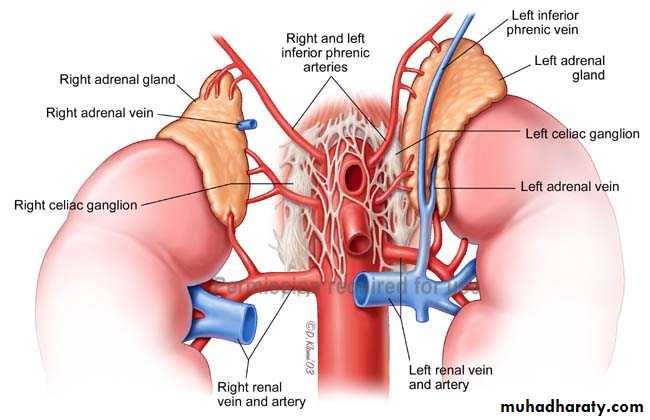
The arterial blood supply from the aorta, phrenic and renal arteries. A large adrenal vein drains on the right side into the inferior vena cava and on the left side into the renal vein.Adrenal Histology
Each adrenal gland has two distinct structures, the adrenal cortex and the medulla, both of which produce hormones
adrenal cortex is a mesodermal cells
adrenal medulla is a neuroectodermal cells
Adrenals Physiology
Zona ReticularisSex steroids (androgens)
Zona Fasciculata
Glucocorticoids (Cortisol)
Glucose homeostasis and many others
Zona Glomerulosa
Mineralocorticoids (Aldosterone)
Na+, K+ and water homeostasis
Medulla: “Catecholamines”
Epinephrine, Norepinephrine, dopamine
CORTEX
Adrenal cortex
Kidney
Posterior
Pituitary GlandHypothalamus
Anterior
Pituitary Gland
ACTH
Stress
Circadianrhythm
CRH
(-)
Glucocorticoids,
Muscle:
Protein catabolismLiver:
Deamination of proteins into amino acids, gluconeogenesis (glucose)Adipose tissue
lipolysisImmune system:
suppressedHypothalamopituitary adrenal (HPA) axis
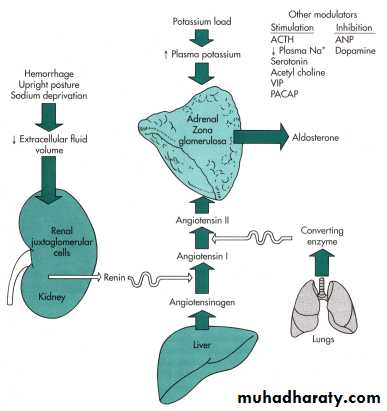
Regulation of adrenal cortexRegulation of Aldosterone Secretion
Sodium retention and potassium excretion
increase plasma volumeKidneys
Sweat glands
Salivary glands
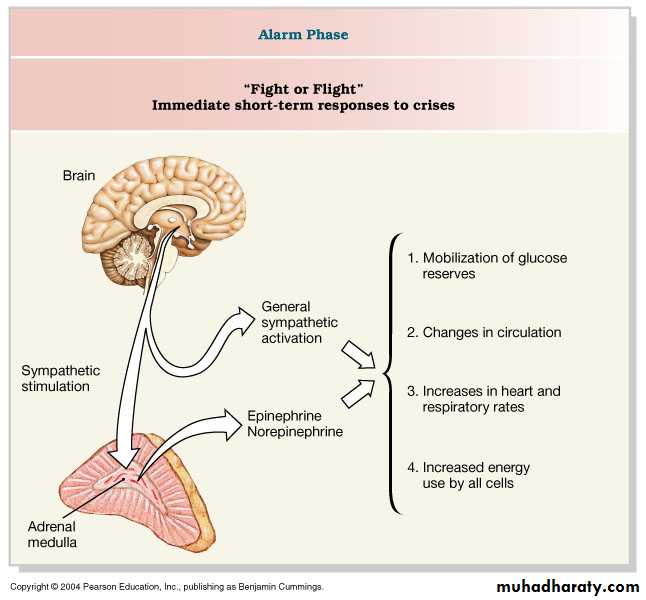
Regulation of adrenal medulla
DISORDERS OF THE ADRENAL CORTEX
IncidentalomaDefinition
A clinically unapparent mass detected incidentally by imaging studies conducted for other reasons
Incidentalomas
detected on imaging studies in 4% of patients.
More than 75% are non-functioning adenomas
but Cushing’s adenomas, phaeochromocytomas and even adrenocortical carcinomas may be present
Diagnosis
complete history and clinical examinationbiochemical work-up for hormone excess
additional imaging studies
Hormonal evaluation includes
a 1-mg overnight dexamethasone suppression test;• 24-hour urinary cortisol excretion (optional);
• 24-hour urinary excretion of catecholamines, metanephrines
or plasma-free metanephrines;
• serum potassium, plasma aldosterone and plasma renin activity;
• serum DHEAS, testosterone or 17β-hydroxyestradiol (virilising
or feminising tumour).
Adrenal gland biopsy
■ Never biopsy an adrenal mass until phaeochromocytoma has been biochemically excluded■ The indication for adrenal gland biopsy is to confirm adrenal gland metastasis
Treatment
Any non-functioning adrenal tumour greater than 4 cm in diameter and smaller tumours that increase in size over time should undergo surgical resection
Primary hyperaldosteronism
Primary hyperaldosteronism (PHA) is defined by hypertension,hypokalaemia and hypersecretion of aldosteroneThe incidence of hypokalaemic PHA is approximately 2%.
Primary hyperaldosteronism
PathologyThe most frequent cause of PHA with hypokalaemia is a unilateraladrenocortical adenoma (Conn’s syndrome)
Primary hyperaldosteronism
Clinical featuresMost patients are between 30 and 50 years of age with a female predominance.
non-specific symptoms
Primary hyperaldosteronism
DiagnosisThe key feature of the biochemical diagnosis is the assessment of potassium level and the aldosterone to plasma renin activity
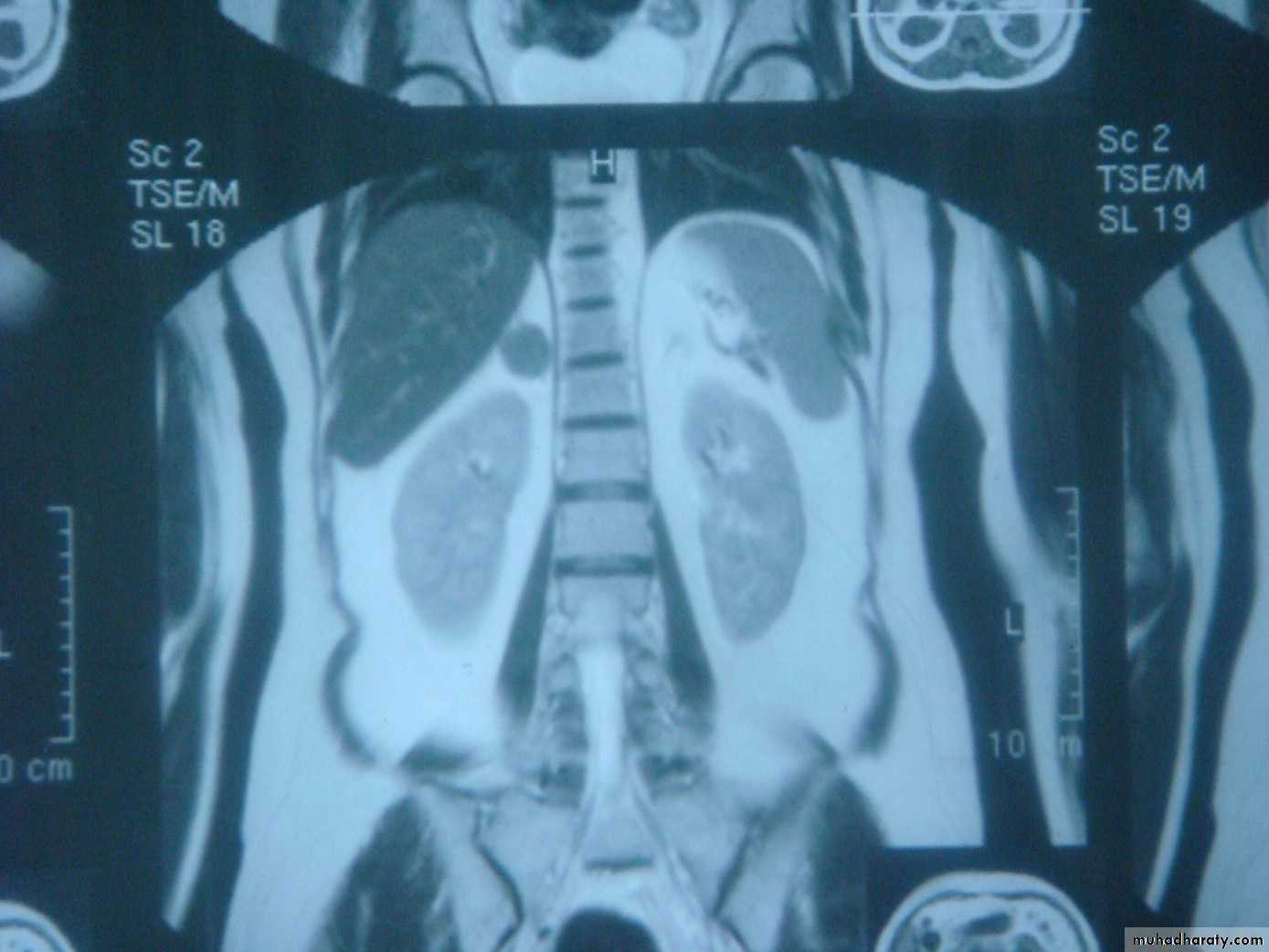
MRI or CT should be performed to distinguish unilateral from bilateral disease
Primary hyperaldosteronism
Treatment
The first-line therapy for PHA with bilateral hyperplasia is
medical treatment with spironolactone.
In most cases supplemental antihypertensive medication is necessary.
Unilateral laparoscopic adrenalectomy is an effective therapy in patients with clear evidence of unilateral or asymmetrical bilateral disease.
In 10–30% of patients who undergo an adrenalectomy, hypertension persists despite adequate diagnostic work-up and treatment.
• Excessive cortisol caused by
endogenous production orexcessive use of corticosteroids.
endogenous production
It either ACTH-dependent (85%) or ACTH-independent(15% )in origin.
Cushing’s syndrome
The most common cause of ACTH-dependent Cushing’s syndrome is Cushing’s disease resulting from a pituitary adenoma secreting ACTH.Or due to Ectopic ACTH-producing tumors (small cell lung cancer, foregut carcinoid)
ACTH-independent Cushing’s syndrome (low ACTH levels) is caused by
Unilateral adrenocortical adenoma.
Adrenocortical carcinoma .
Bilateral macronodular or micronodular hyperplasia
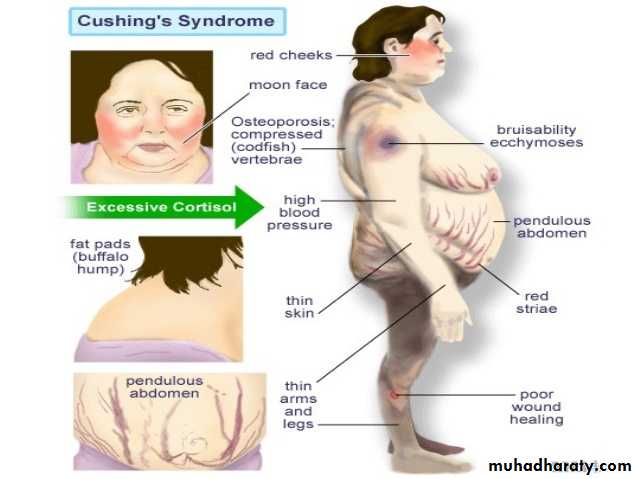
Clinical features of Cushing’s syndrome
Weight gain – particularly around the belly."Moon face" – a rounded shape of the face.
Easy bruising skin, with thinning of the skin.
Acne volgaris.
Ruddy complexion (plethora) – a reddening of the
face or cheeks.
"Buffalo hump" a mound of fat at the back of the neck.
Abnormal hair growth on the face or abdomen.
Edema due to excess fluid buildup in the lower legs.
Cushing’s syndrome
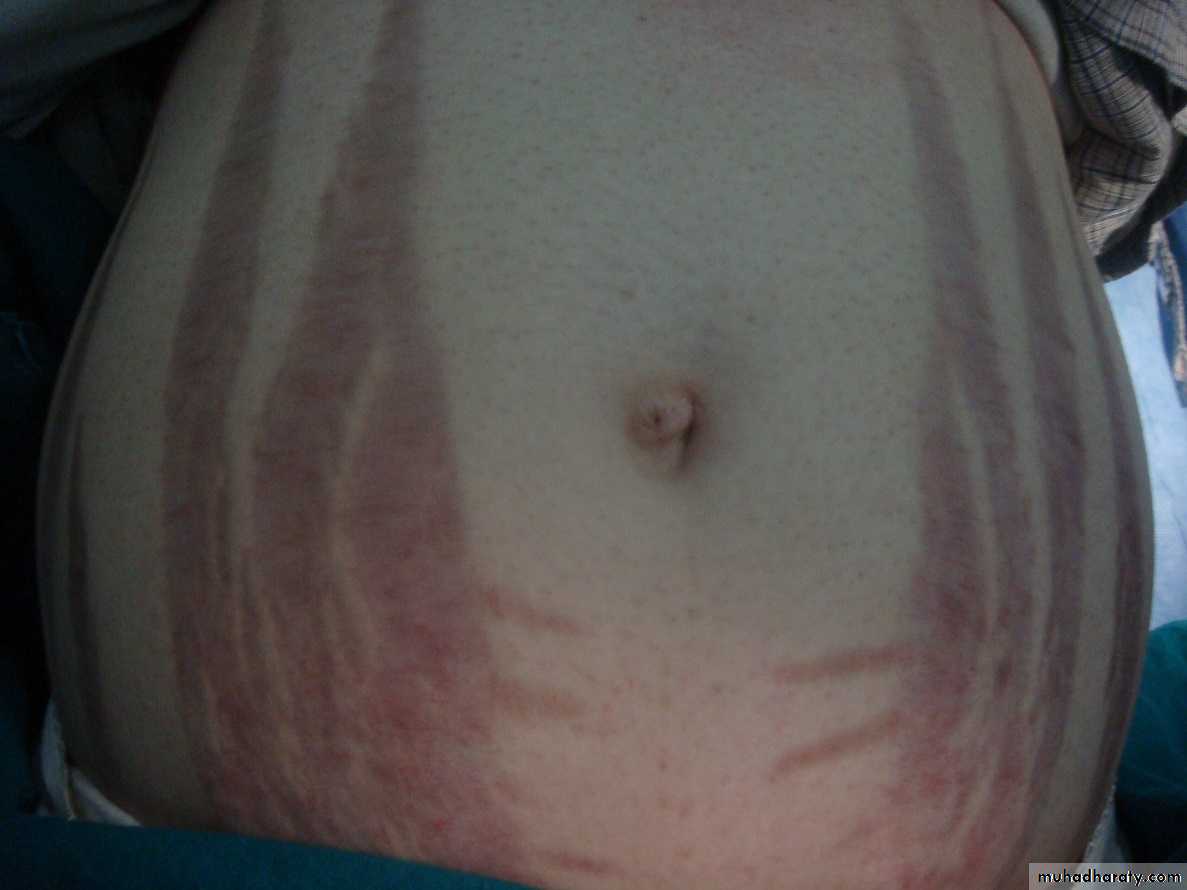
Stretch marks(purple striae) at lower abdomen and thigh.
Muscle weakness the arms and legs may become skinny like twigs from muscle wasting.Menstrual disturbances.
Osteoporoses. Hypertension, Diabetes, Mood changes. Hypokalaemia.
Morning and midnight plasma cortisol levels are elevated, possibly with loss of diurnal rhythm.
2- Dexamethasone fails to suppress 24-hour urinary cortisol excretion.
3- Serum ACTH levels discriminate ACTH-dependent from ACTH-independent disease.
Investigations
Elevated or normal ACTH levels provide evidence for an ACTH producing pituitary tumour (85%) or ectopic ACTH production.
Therefore, in patients with elevated ACTH, MRI of the pituitary
gland must be performed.
If MRI is negative, a CT scan of the chest and abdomen is warranted to detect an ectopic cortisol-producing tumour.
In patients with suppressed ACTH levels, a CT or MRI scan is performed to assess the adrenal glands.
Treatment
Medical therapy with metyrapone or ketoconazole reducessteroid synthesis and secretion and is used in patients with severe
hypercortisolism or if surgery is not possible.
ACTH-producing pituitary tumours are treated by trans-sphenoidal resection or radiotherapy
TreatmentIf an ectopic ACTH source is localized, resection will cure hypercortisolism.
Patients with an ectopic ACTH-dependent Cushing’s syndrome and an irresectable or un localized primary tumor treated by bilateral adrenalectomy.Treatment
A unilateral adenoma is treated by unilateral adrenalectomy.In cases of bilateral ACTH-independent disease, bilateral adrenalectomy is the primary treatment.
Treatment
adrenocortical adenoma
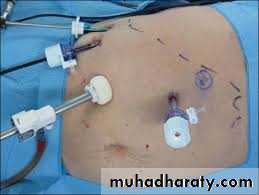
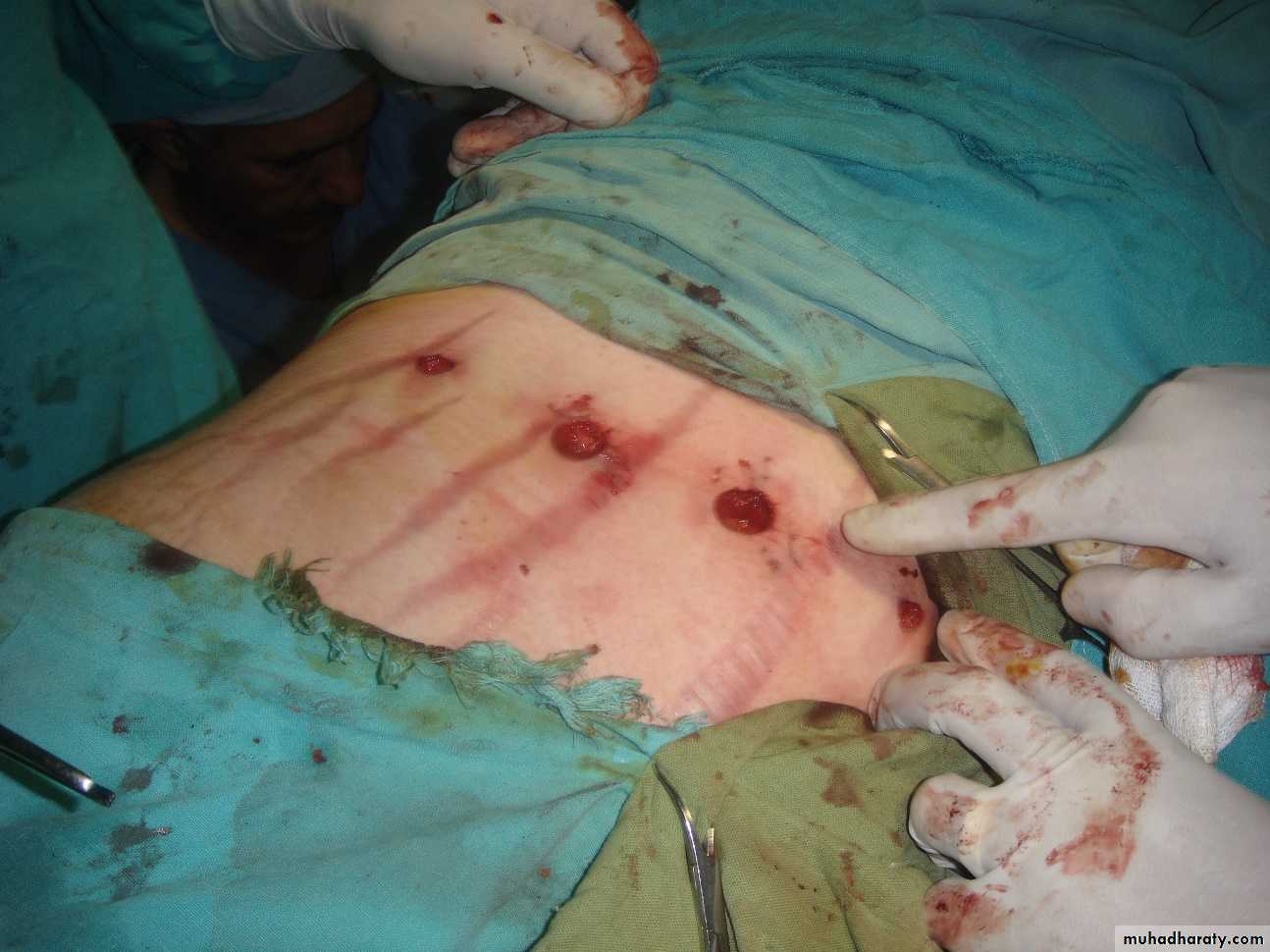
4 ports for Lap adrenalectomyPreoperative management
Patients with Cushing’s syndrome are at an increase risk of1-Hospital-acquired infection.
2- Thromboembolic condition.
3- Myocardial complications.
4- Post operative Addison crises
Therefore, prophylactic anti-coagulation and the use of prophylactic antibiotics are essential with post operative steroid supplement.
Postoperative management
Supplemental cortisol should be given after surgery. In total, 15 mg/ h IV for the first 12 hours followed by a daily dose of 100 mg for 3 days, which is gradually reduced thereafter.After unilateral adrenalectomy, the contralateral suppressed gland needs up to 1 year to recover adequate function.
In 10% of patients with Cushing’s disease who undergo a bilateral adrenalectomy, the pituitary adenoma converts into an aggressive
tumor (Nelson’s syndrome).
Adrenal insufficiency
Primary adrenal insufficiency is caused by the loss of function of the adrenal cortex. Symptoms are only evident when about 90% of the adrenal cortex is destroyed.Diseases associated with adrenal insufficiency:
■ Tuberculosis
■ After bilateral adrenalectomy
■ Hemorrhage into the glands
■ secondary metastases
■ Systemic diseases (Boeck’s disease, amyloidosis, Wilson’s disease)
■ Adrenogenital syndrome
■ HIV infection
■ Polyglandular autoimmune syndrome
Adrenal insufficiency
Secondary adrenal insufficiency is defined as a deficiency of pituitary ACTH secretion.
Tertiary adrenal deficiency is due to loss of hypothalamic CRH secretion , therapeutic glucocorticoid administration, brain tumor or irradiation.
Chronic adrenal insufficiency
Primary type:Anorexia, weakness and nausea. Hypotension,
hyponatraemia, hyperkalaemia and hypoglycaemia.
As a result of negative feedback, ACTH level
increase and cause hyperpigmentation of the skin and mucosa.
INVESTEGATION
Basal ACTH levels are found to be highwith cortisol levels decreased.
Treatment
Replacement therapy with daily oral hydrocortisone (10 mg m–2 body surface area) and fludrocortisone (0.1 mg).To prevent an Addisonian crisis, patients must be aware of the need to adjust the dose in case of illness or stress.
If patients with adrenal insufficiency are scheduled for surgery, appropriate steroid cover must be administrated
Acute adrenal insufficiency
Acute adrenal insufficiency usually presents as shock with fever, nausea, vomiting, abdominal pain, hypoglycaemia and electrolyte imbalance (Addisonian crisis).The Waterhouse–Friderichsen syndrome is a bilateral adrenal infarction associated with meningococcal sepsis and is rapidly fatal unless immediately treated.
Treatment
1- Intravenous administration of hydrocortisone, 100 mg every 6 hours, 2- 3 liters of saline is given in 6 hours under careful cardiovascular monitoring.
3- Concomitant infections, which are frequently present, require aggressive treatment.
Phaeochromocytoma (adrenal paraganglioma)
A tumour of adrenal medulla, derived from chromaffin cells and produces catecholamines.It represents 0.1–0.6% of hypertensive patients.
It is known as the ‘10% tumor’ as 10% are inherited, 10% are extra-adrenal, 10% are malignant, 10% are bilateral and 10% occur in children.
its either hereditary which associated with several tumor syndromes and diagnosed earlier or sporadic which occur after the fourth decade
Clinical features
The cardinal pictures are headache, palpitations and sweating. Paroxysms may be precipitated by physical training, general anesthesia and some drugs and agents (contrast media, tricyclic antidepressive, metoclopramide and opiates).Hypertension may occur continuously, be intermittent or absent.
Investigation
1- elevation of adrenaline, noradrenaline, metanephrine and normetanephrine levels in a 24-hour urine collection.
2- imaging study for the localization of the phaeochromocytoma and/or metastases. MRI is preferred because contrast media used for CT scans can provoke paroxysms.
FNA biopsy is contraindicated.
Treatment
Laparoscopic resection is the treatment of phaeochromocytoma.
If the tumor is larger than 8–10 cm or radiological signs of malignancy are detected an open approach should be considered.
Preoperative evaluation:
α- adrenoreceptor blocker (phenoxybenzamine) is used to block catecholamine excess during surgery.Additional β- blockade is required if tachycardia or arrhythmias develop; this should not be introduced until the patient is α-blocked.
Neuroblastoma
It is a malignant tumor that is derived from the sympathetic nervous system. It arises from adrenal medulla in (38%).Clinical features
Predominantly newborn infants and young children (< 5 years of age) are affected. Symptoms are caused by a mass in the abdomen or by metastases )70%(with proptosis, bone pain, painless bluish skinPrognosis depends on the tumor stage and the age at diagnosis. Patients are classified as low, intermediate or high risk.
Investigation
Urinary excretion (24-hurine) of vanillylmandelicacid (VMA),levels are present in about 80%.
(The catecholamine excess is asymptomatic) .
CT/MRI of the chest and abdomen, a bone scan.
Bone marrow aspiration and core biopsies in suspicions of secondary.
Treatment
Low-risk patients are treated by surgery alone (the addition of
6–12 weeks of chemotherapy is optional) whereas intermediate risk patients are treated by surgery with adjuvant chemotherapy (carboplatin, cyclophosphamide, etoposide, doxorubicin).
High-risk patients receive high-dose chemotherapy followed by surgical resection in responding tumors and myeloablative stem cell rescue.
Ganglioneuroma
It is a benign adrenal neoplasm arises from neural crest tissue characterized by mature sympathetic ganglion cells and Schwann cells in a fibrous stroma.Clinical features
It can be found in all age groups , more common before the age of 60.
it is occur anywhere along the paravertebral sympathetic plexus and in the adrenal medulla (30%).
Most often they are identified incidentally by CT or MRI performed for other indications.
Treatment
Treatment is by surgical excision.
Adrenocortical carcinoma
A rare malignancy with an incidence of 1–2 cases per 1 000 000 with generally poor prognosis.A slight female predominance is observed (1.5:1).
The age distribution is bimodal with a first peak in childhood and a second between the fourth and fifth decades.
Clinical presentation
Approximately 60% of patients present with evidence of steroid hormone excess (Cushing’s syndrome). Patients with nonfunctioning tumours complain of abdominal or back pain caused by large tumours.Diagnosis
1-measurements of DHEAS, cortisol and catecholamines to exclude a phaeochromocytoma
2- dexamethasone suppression test.
3-MRI and CT are equally effective in distinguishing adrenocortical adenoma from carcinoma
Treatment
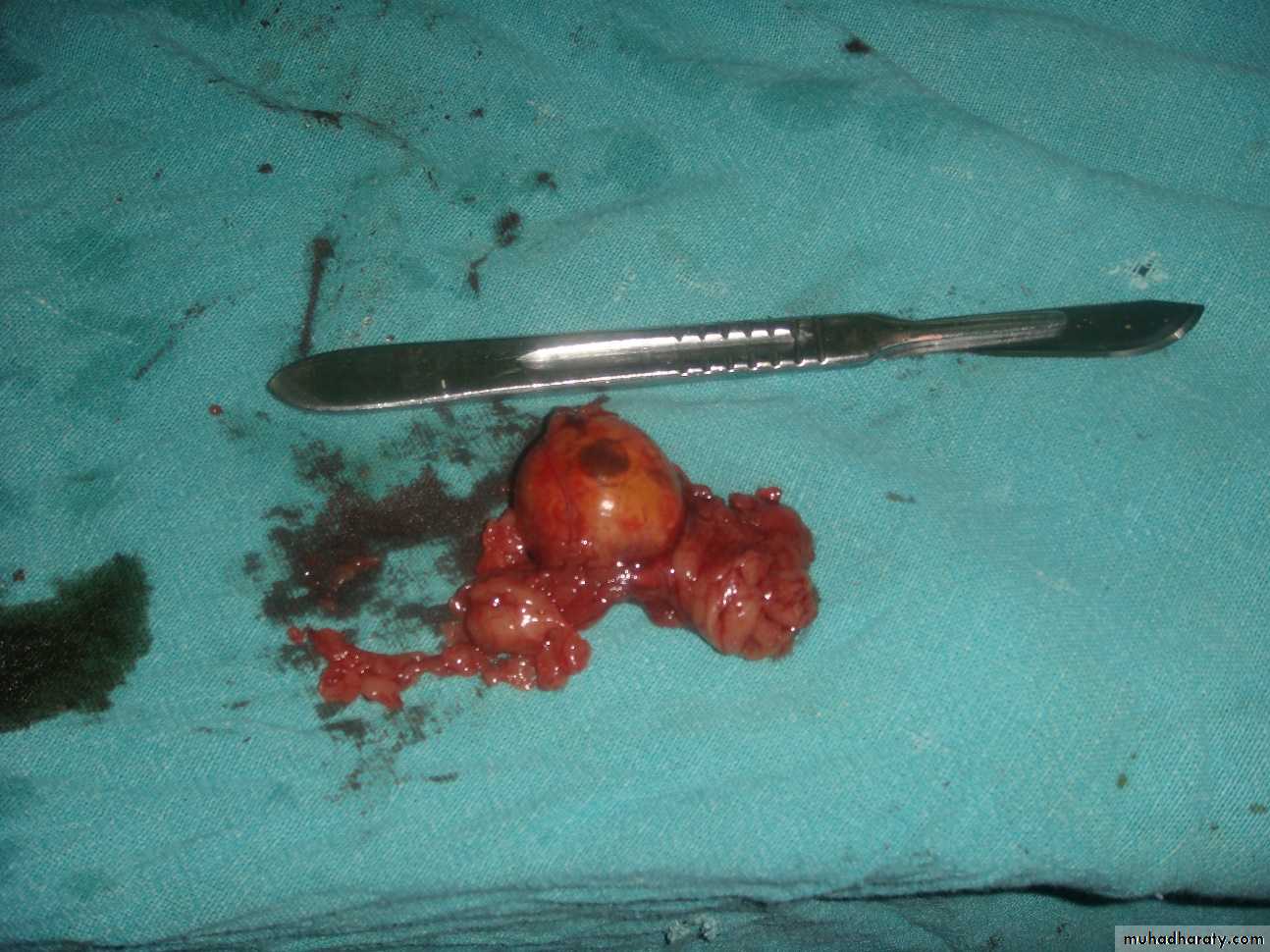
Complete tumor resection should be attempted whenever possible.Laparoscopic adrenalectomy is associated with a high incidence of local recurrence and not recommended.
Tumor debulking plays a role in functioning tumours to control hormone excess.
Patients should be treated postoperatively with mitotane alone or in combination with other cytotoxic.
Adjuvant radiotherapy may reduce the rate of local recurrence
Adrenal metastases
Adrenal metastases are discovered at autopsy in one-third of patients with malignant disease.The most common primary tumours are breast, lung, renal, gastric, pancreatic, ovarian and colorectal cancer.
In selected cases an adrenalectomy can be performed.